Melt Electrospinning of Polymers: Blends, Nanocomposites, Additives and Applications
Abstract
1. Introduction
2. Melt Processing of Polymers with Additives
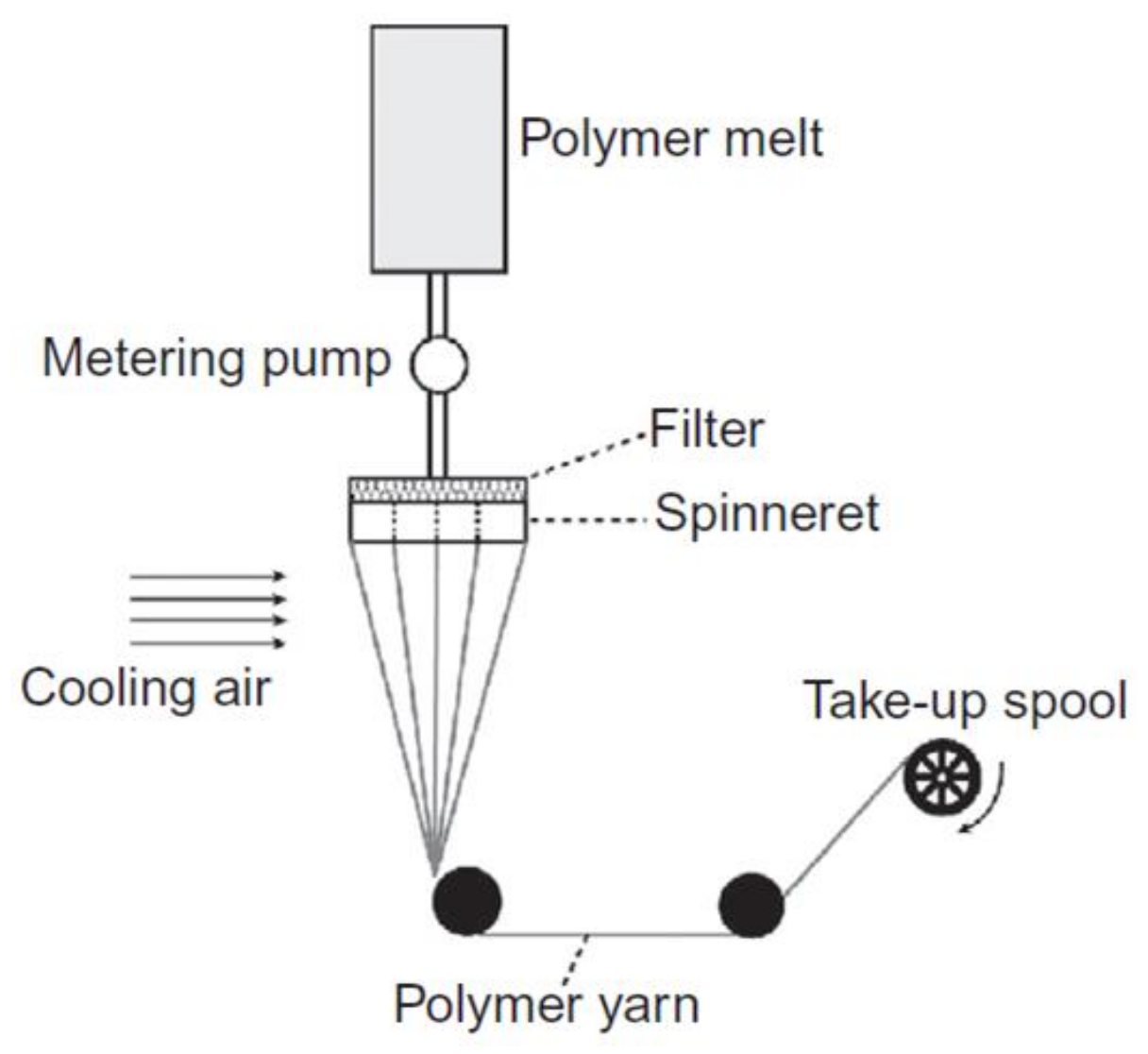
3. Conventional Melt Spinning
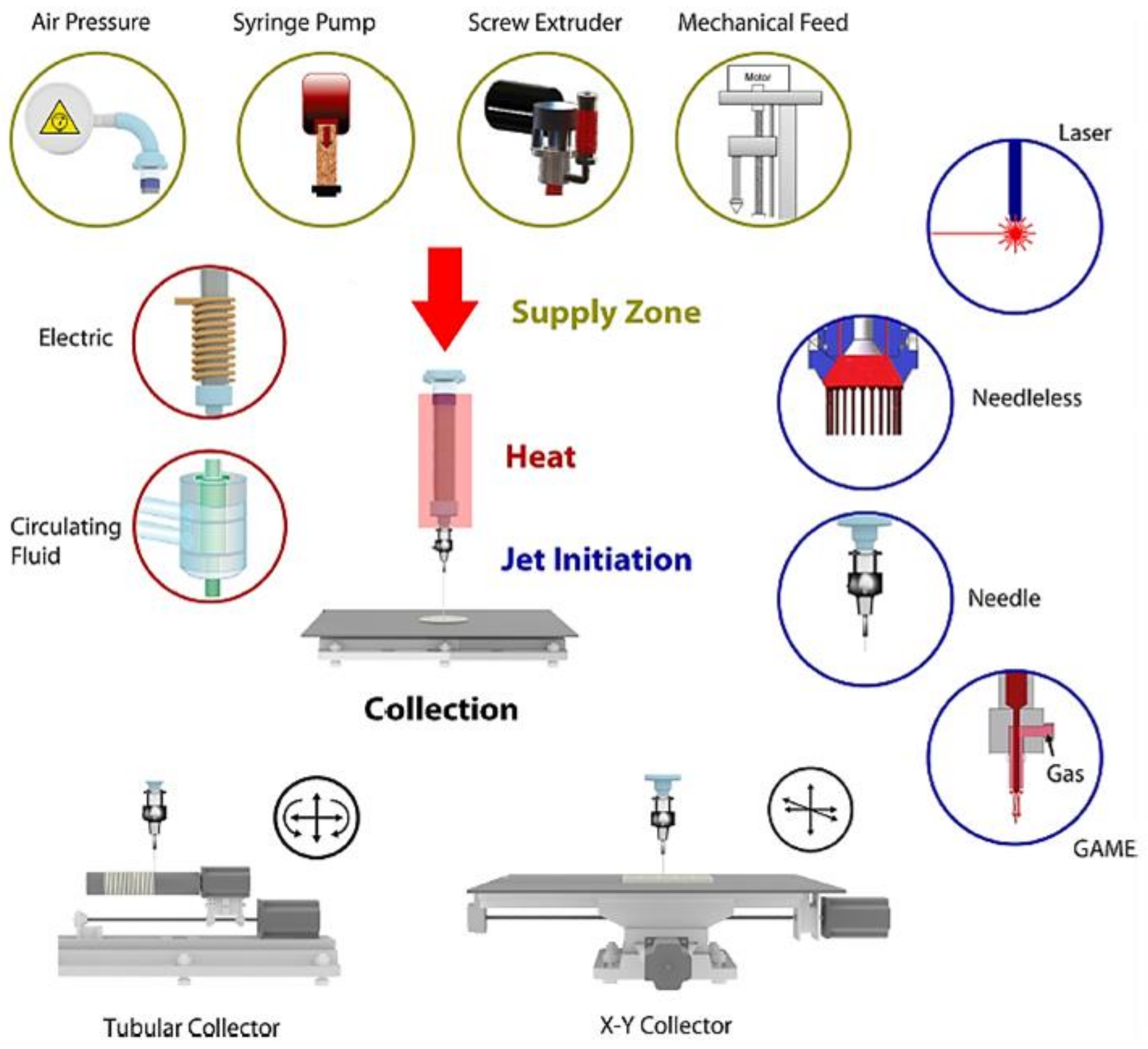
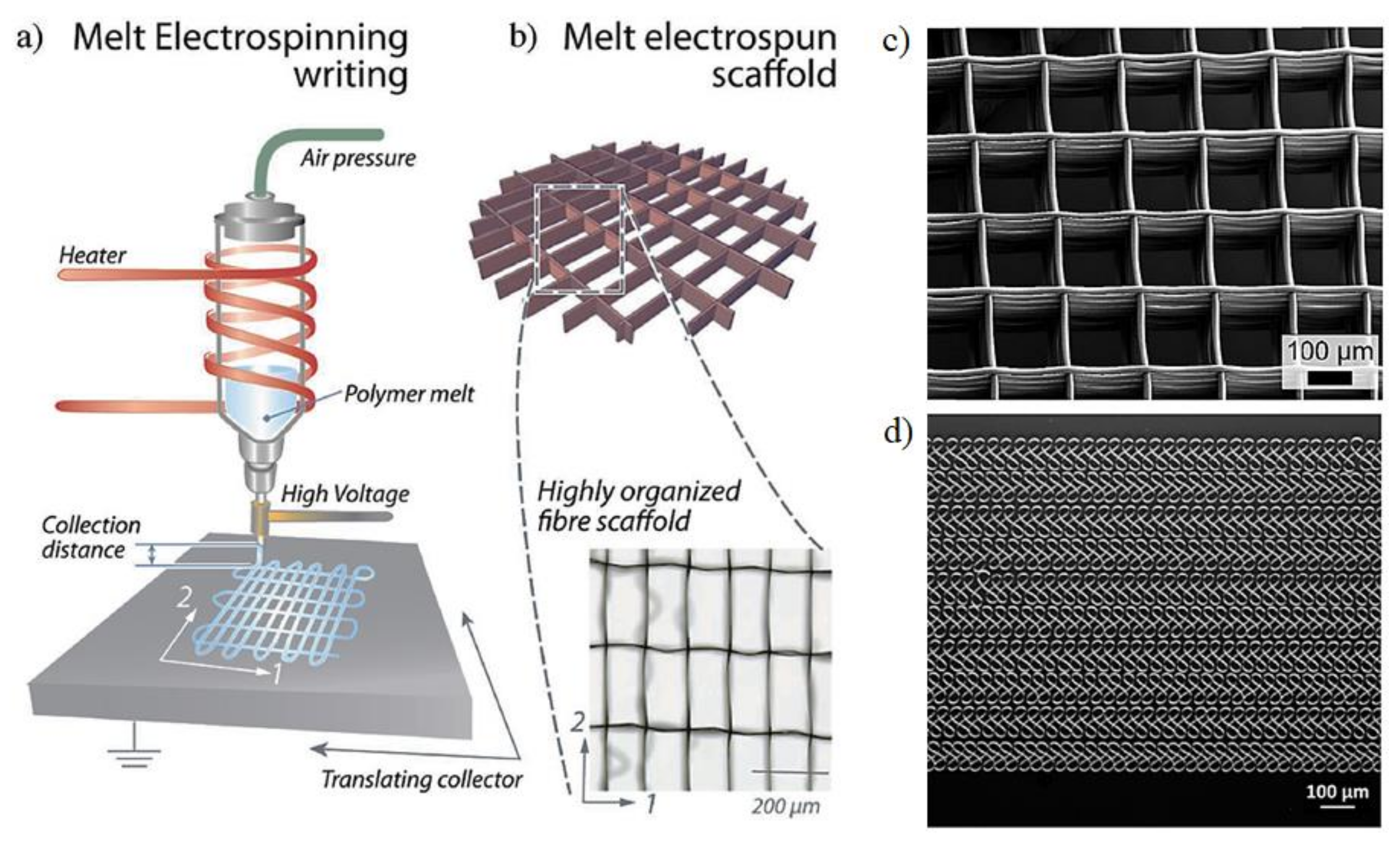
4. Melt Electrospinning
4.1. Melt Electrospinning Conditions
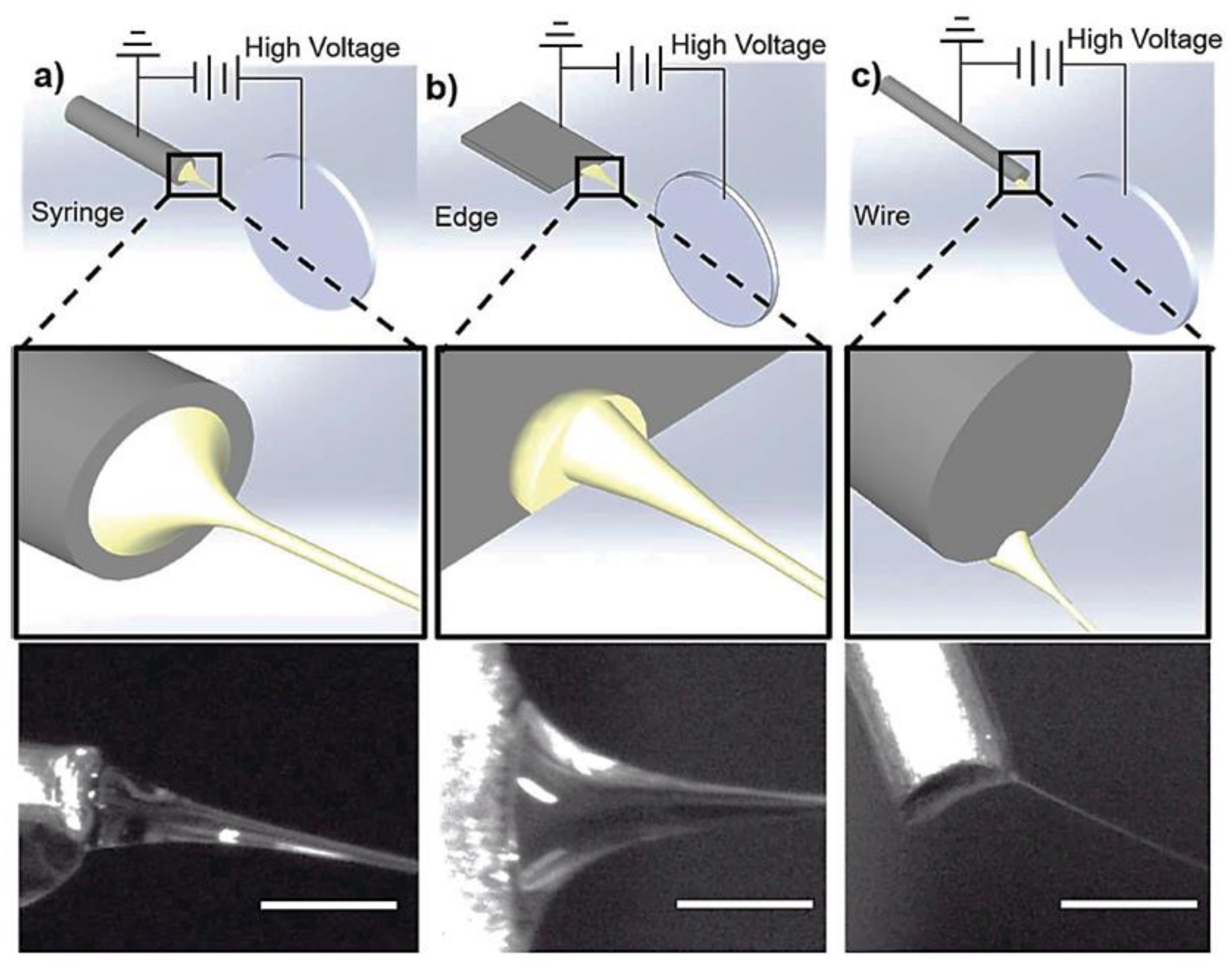
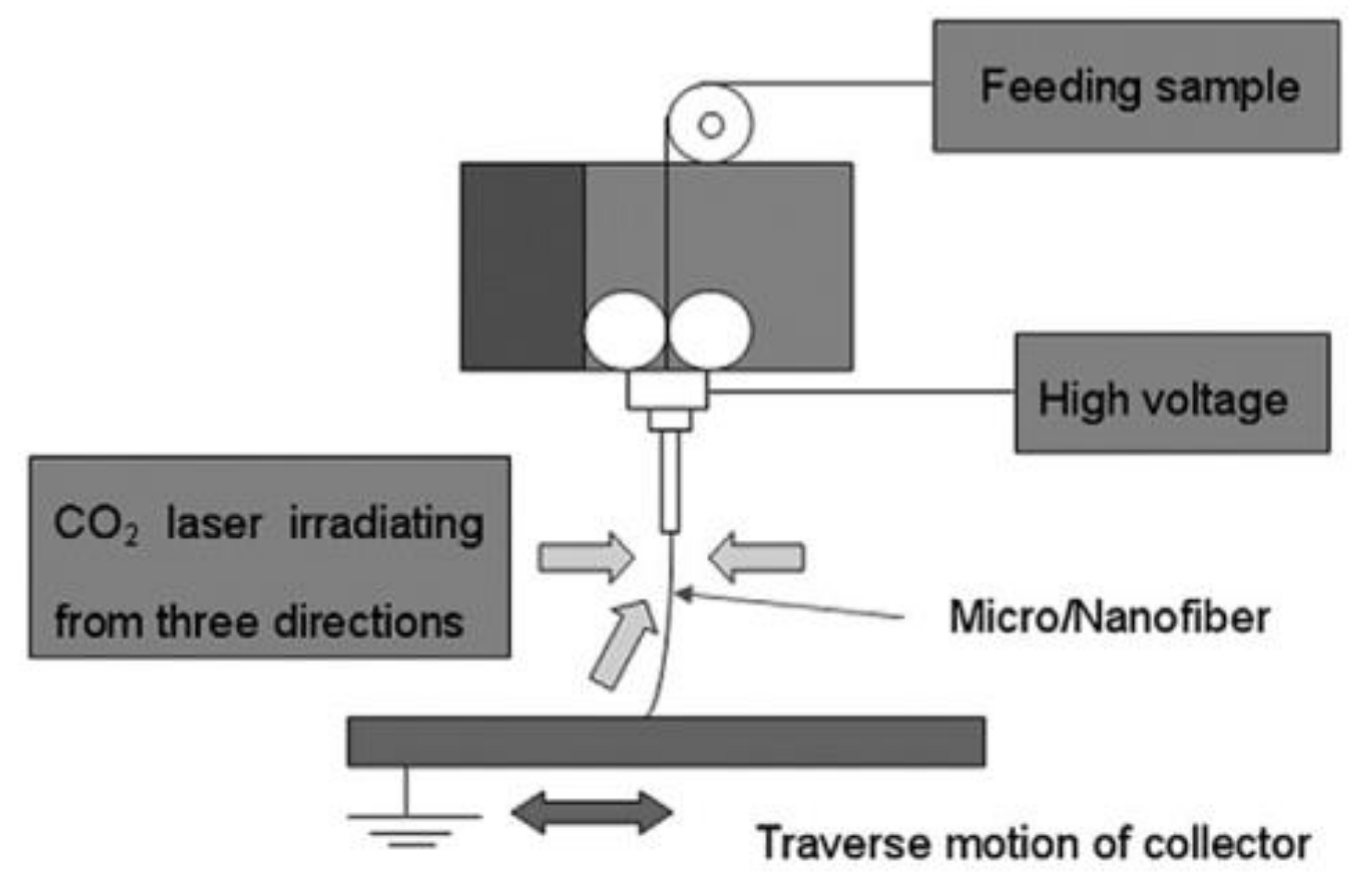
4.2. New Techniques and Configurations
5. Additives in Melt Electrospinning
5.1. Salt, Viscosity Modifiers, Stabilizers and Dyes
5.2. Pharmaceutical Additives
| Polymer | Additive | Effects in the Process * | Fibers Diameter | Ref. |
|---|---|---|---|---|
| PP | Sodium oleate (7 wt.%) | ↑ electrical conductivity, ↓ hydrophobicity | 371 nm | [43] |
| NaCl (5 wt.%) | ↑ electrical conductivity | 310 nm | [43] | |
| Poly(ethylene glycol) (PEG) | ↓ melt viscosity | [66] | ||
| Poly(dimethyl siloxane) (PDMS) | ↓ melt viscosity | [66] | ||
| Calcium stearate (5 wt.%), Zinc stearate (5 wt.%), Magnesium stearate (5 wt.%) | ↓ melt viscosity, ↑ electrical conductivity | 1–5 µm | [67] | |
| Sodium stearate (5 wt.%) | ↓ melt viscosity | 1.3 µm | [67] | |
| Sodium stearate, Stearic acid | Plasticizer | [69] | ||
| Irgastat® P16 (6 wt.%), Sodium stearate (2 wt.%) | Antistatic (insulator character), ↑ electrical conductivity | 210 nm | [40,68] | |
| β-Nucleating agent (La+3 and Ca+2) crystallization in β form | ↓ shear stress, ↑ tensile strength, ↑ toughness, ↑ fluidity | <5 µm | [66] | |
| PMMA | Di-2-ethylhexyl terephthalate (DOTP) (40 wt.%) | Plasticizer, ↓ melt viscosity (facilitate the jet formation) | 4 µm | [38] |
| PA6 | Sodium stearate, sodium oleate, sodium myristate (10 wt.%) | ↓ melt viscosity, ↑ electrical conductivity | <1.5 µm | [76] |
| PCL | NaCl (8 wt.%) | ↓ melt viscosity, ↑ electrical conductivity | ~3 µm | [80] |
| NaCl (25 wt.%) | ↑ melt viscosity, ↓ electrical conductivity | ~25 µm | [80] | |
| PLA | Sodium stearate (2–4 wt.%) | ↓ melt viscosity | 16–20 µm | [82] |
| Polyethylene succinate (PES) | Plasticizer, ↓ melt viscosity | [82] | ||
| Haematoxylin (2 wt.%) | Plasticizer, ↑ polymer degradation, ↓ electrical resistance | 16.04 µm | [83] | |
| Acetyl tributyl citrate (ATBC) (6 wt.%) | Plasticizer, ↓ melt viscosity | <236 nm | [85] |
| Polymer | Additive | Drug Activity | Effects in the Process and/or Application | Ref. |
|---|---|---|---|---|
| Eudragit® E | Carvedilol | Beta-blocker | Plasticizers, polymer melting reduced and prevented the drug degradation. | [97] |
| Soluplus® | Indomethacin | Anti-inflammatory | Plasticizers, fast drug release. | [98] |
| PCL | Curcumin | Anticancer | Not affect the fiber morphology, low drug release rate. | [90] |
| Daunorubicin hydrochloride | Anticancer | Fiber diameters barely fluctuated and smooth surfaces. Localized tumour therapy with slow release rate. | [115] | |
| Triclosan/PLA nanoparticles | Antimicrobial | Fibers diameter of 40–60 µm. Soft connective tissue engineering and long-term drug delivery. Slow and controlled release. | [128] | |
| PCL/PEG | Ciprofloxacin | Antibiotic | Wound dressing. | [116] |
| HDPE | Chlorhexidine dihydrochloride | Antimicrobial | Fibrous membrane for medical and filtration applications with porosity of 77% and pore size of 10 µm. | [21] |
| PLA/PHB | Dipyridamole (DPD) | Antithrombotic, antithrombogenic | The drug reduced the melting temperature. Fibers had a rough surface with varying diameter. Drug delivery system. | [134] |
| Black plaster/PEG | Sanguis Draconis resin | Prevent osteoporosis | Traditional medicine. | [137] |
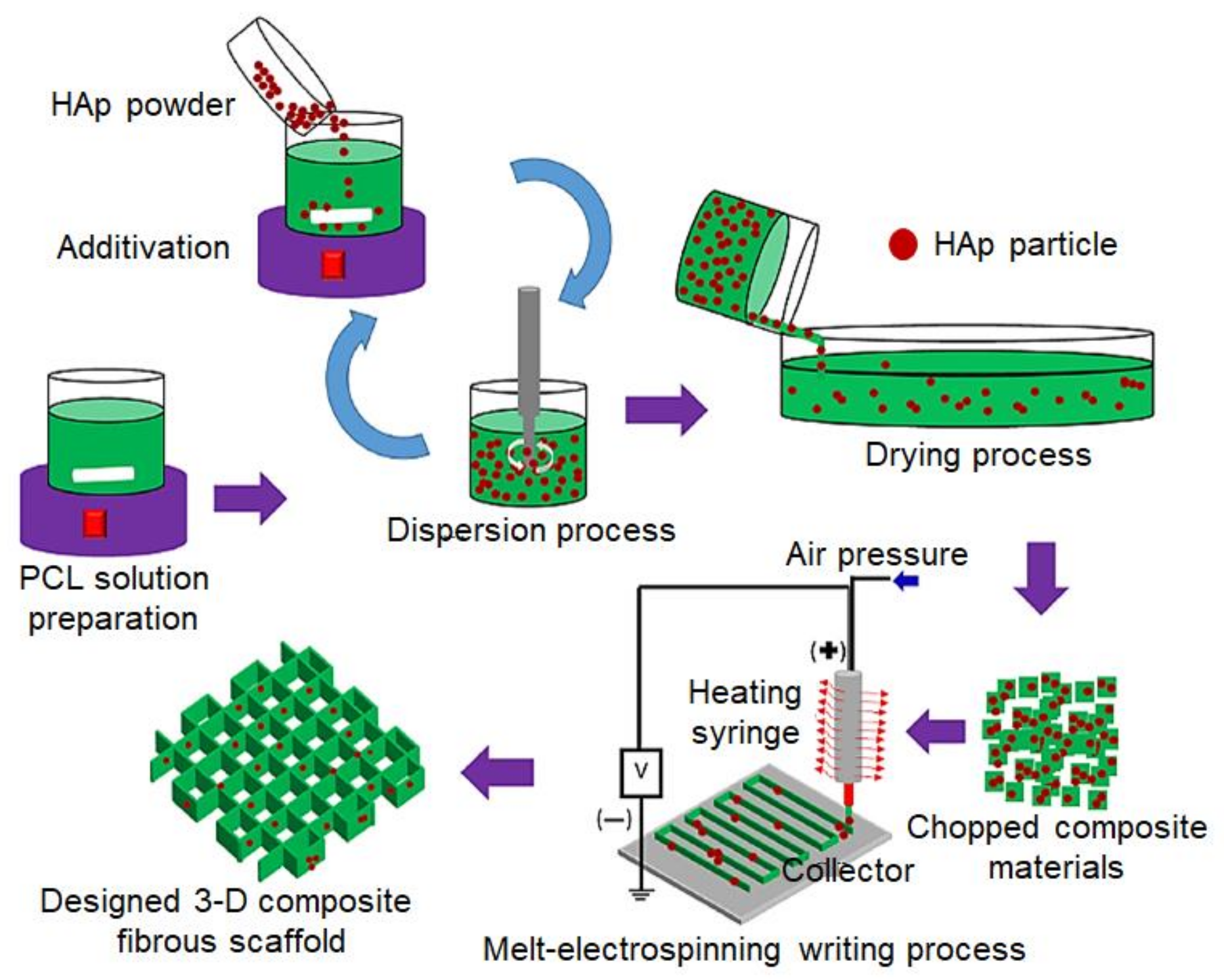
6. Melt Electrospun Nanocomposites
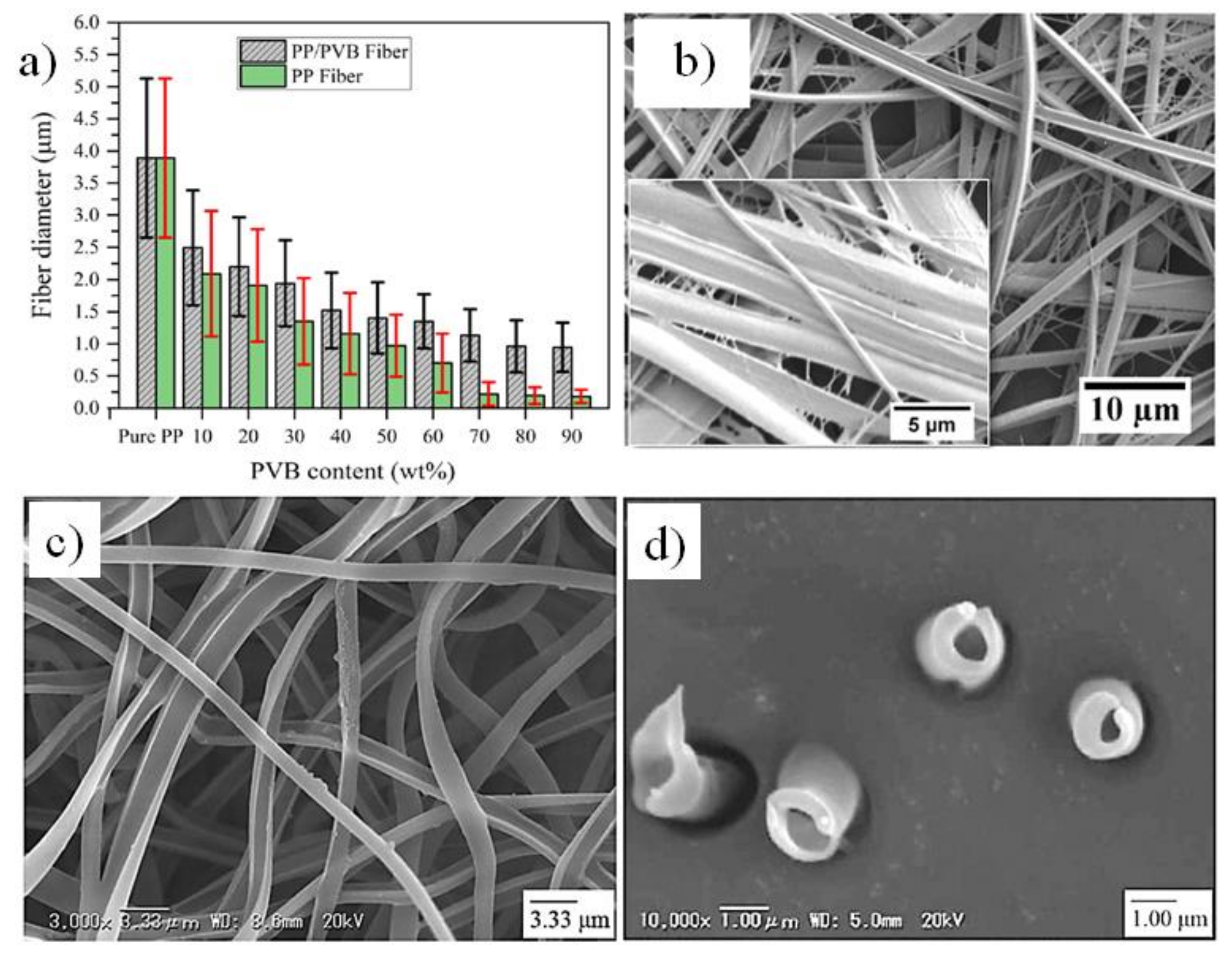
7. Melt Electrospun Polymer Blends
| Polymer | Nanoparticle | Effects in the Process and/or Application | Ref. |
|---|---|---|---|
| PLLA | nHAp (3 wt.%) | Hydrophilic matrix. Biomedical applications due to its biocompatibility and osteoconductivity. | [59] |
| PLA | WO3 (7 wt.%) | Efficient light-to-heat conversion, great NIR absorption, high hydrophobicity. | [146] |
| PP | TiO2/Sodium stearate nanoparticles | Dyeing-wastewater decolourization, photocatalytic applications. | [152] |
| PET | SiO2 nanoparticles | Good thermal and chemical stability, excellent mechanical properties, and well dye ability. | [153,154] |
| PCL | Fe3O4 magnetic nanoparticles | Magnetic hyperthermia treatments (cancer treatment), wound dressing and haemostasis. | [160] |
| nHAp | Increase roughness and diameter of the fibers. Tissue engineering applications. | [163] | |
| PA6 | MMT (montmorillonite) (1–10 wt.%) | Viscosity, Young’s modulus and electrical conductivity increased. | [156,165] |
8. Relevant Applications of Melt Electrospinning
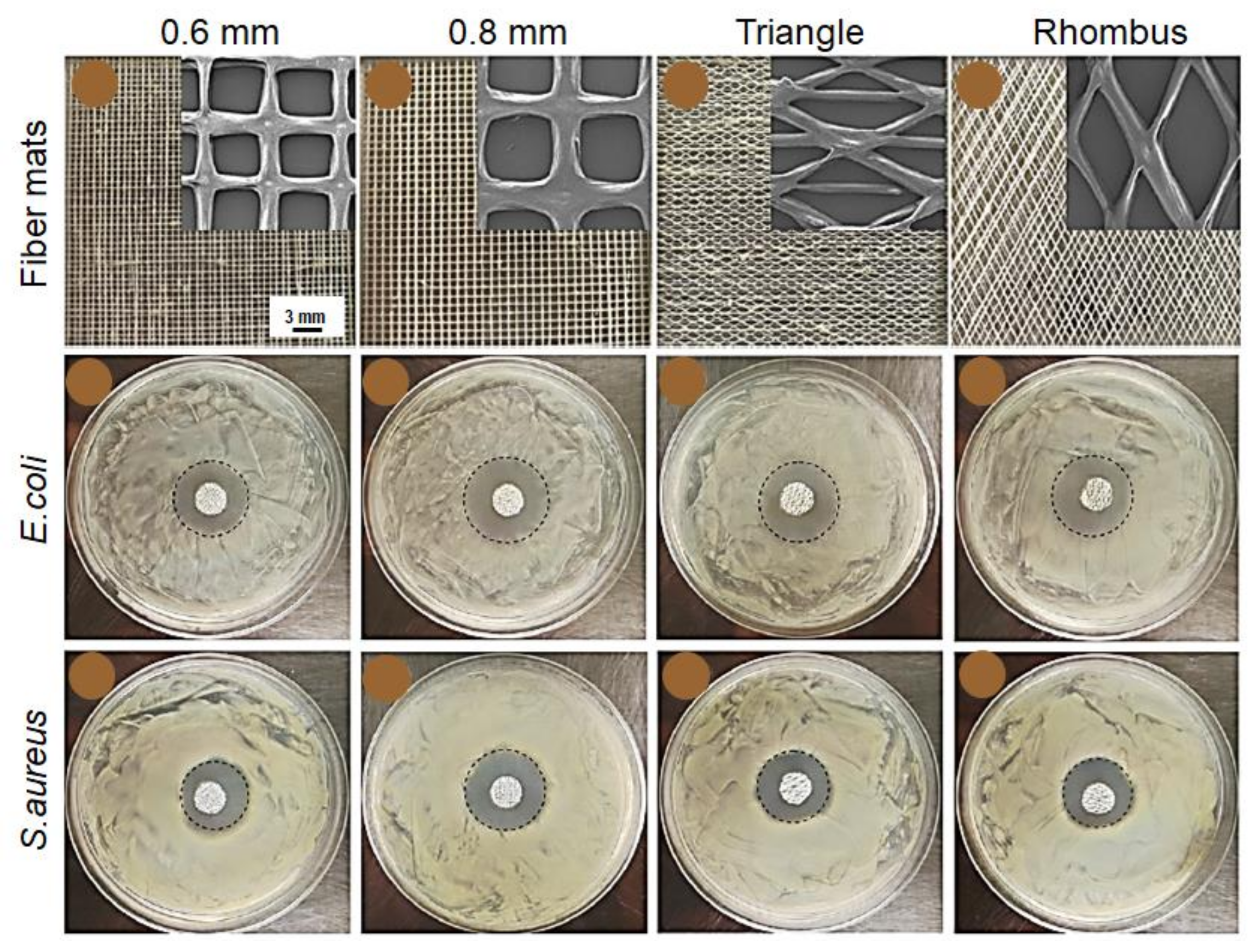
8.1. Filtration and Separation
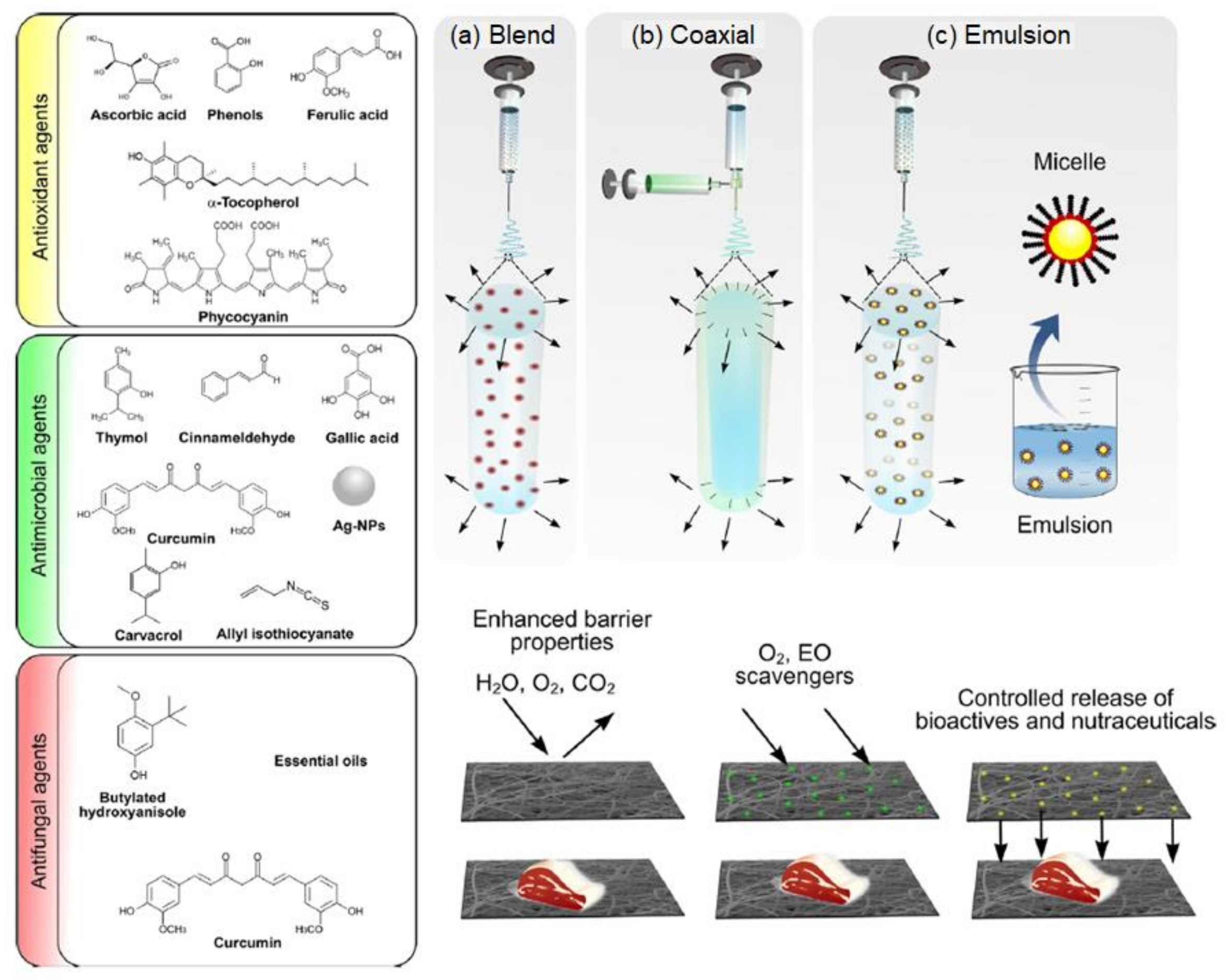
8.2. Food Packaging
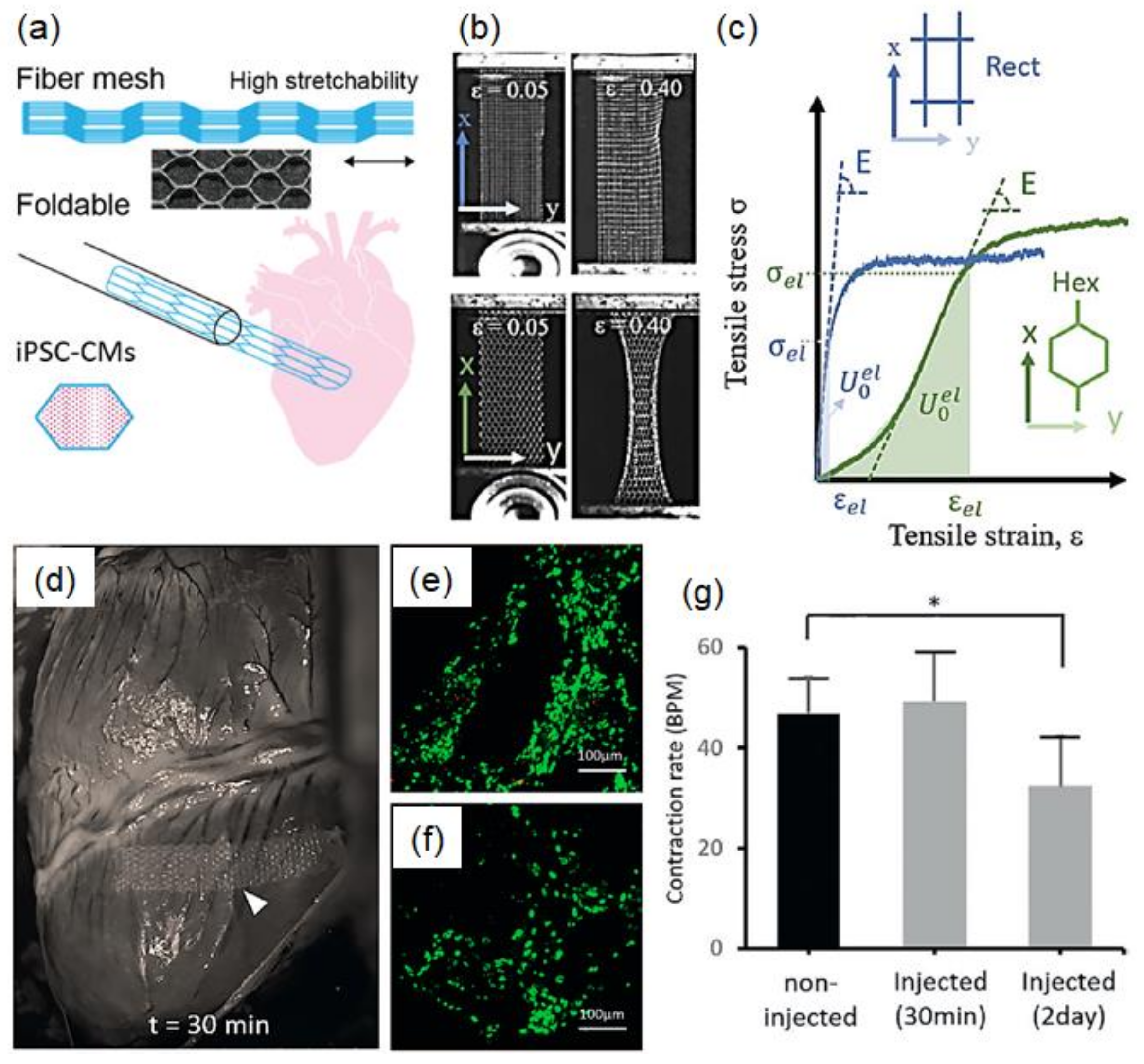
8.3. Biomedical Applications and Tissue Engineering
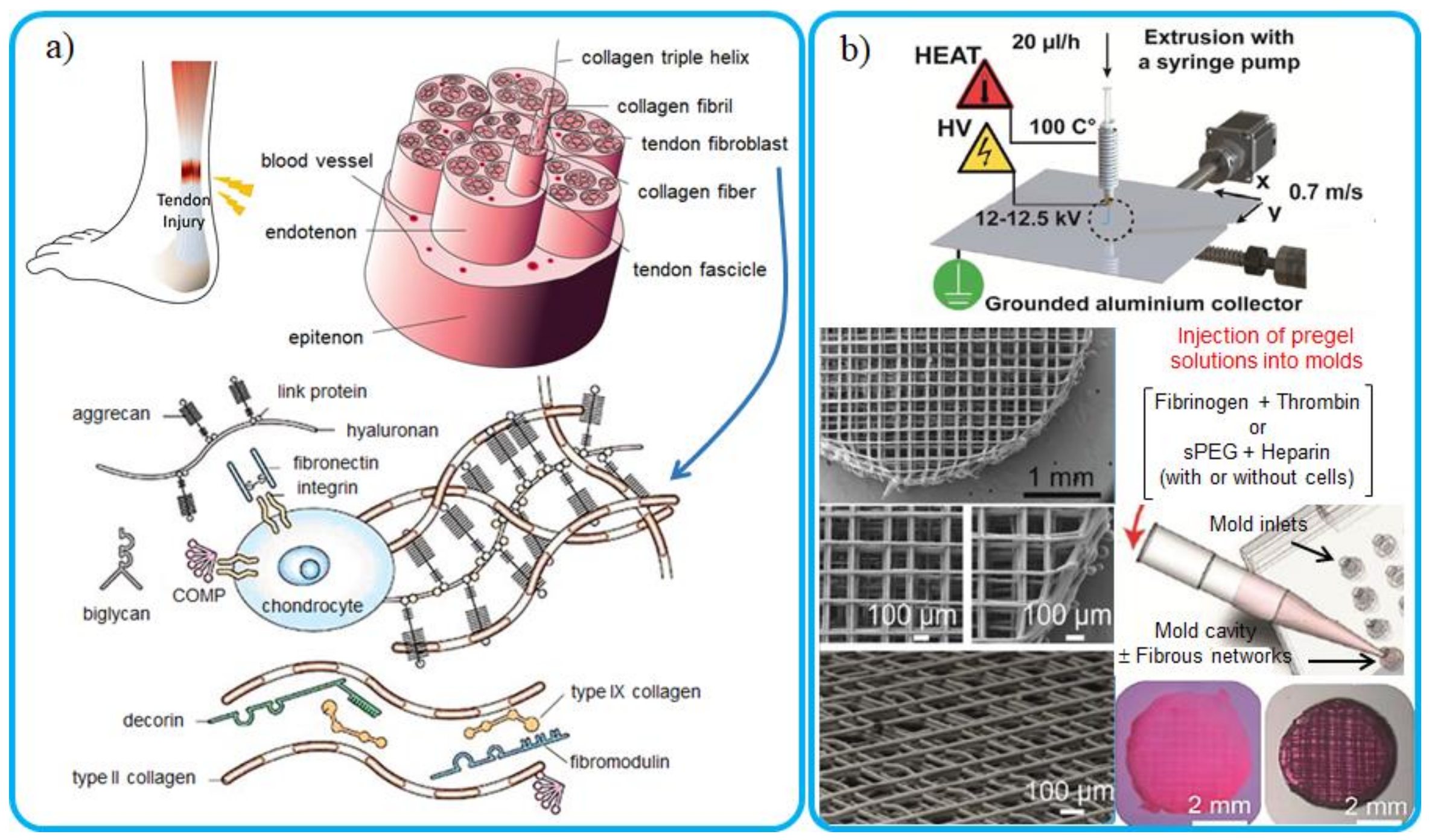
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1D | 1-dimensional |
| 2D | 2-dimensional |
| ATBC | Acetyl Tributyl Citrate |
| Bm-MSC | Bone Marrow-derived Mesenchymal Stromal Cells |
| CAD | Computer-aided Design |
| CaP | Calcium Phosphate |
| CHDH | Chlorhexidine Dihydrochloride |
| CPC | Cardiac Progenitor Cell |
| DOTP | Di-2-ethylhexil terephthalate |
| DPD | Dipyridamole |
| ECM | Extracellular Matrix |
| eMSC | Endometrial Mesenchymal Stem Cells |
| FDA | Food and Drug Administration |
| GCs | Gingival Cells |
| HDPE | High Density Polyethylene |
| hMSC | Human Mesenchymal Stem Cells |
| hOB | Human Osteoblasts |
| Hap | Hidroxyapatite |
| HUVEC | Human Umbilical Vein Endosteal Cells |
| HUVETs | Human Umbilical Vein Endothelial Cells |
| iPP | Isotactic Polypropylene |
| iPSC-CM | Human Induced Pluripotent Stem Cells-derived Cardiomyocytes |
| LF | Lactoferrin |
| MEW | Melt Electrowriting/Melt Electrospinning Writing |
| MFI | Melt Flow Index |
| MFR | Melt Flow Rate |
| MMT | Montmorillonite |
| mOB | Primary Mouse Osteoblasts |
| nHAp | Nanohydroxyapatite |
| NHDF | Normal Human Dermal Fibroblasts |
| p(CL-co-AC) | Copolymer of PCL and acryloyl carbonate |
| PA | Polyamide |
| PA12 | Polyamide 12 |
| PA6 | Polyamide 6 |
| PCL | Poly (ε-caprolactone) |
| PDLCs | Periodontal Ligament Cells |
| PDMS | Poly (dimethyl siloxane) |
| PE | Polyethylene |
| PEG | Poly (ethylene glycol) |
| PEG-b-PCL | Poly (ethylene glycol)-block-poly(ε-caprolactone) |
| PES | Polyester |
| PGA | Poly (glycolic acid) |
| PHB | Polyhydroxy Butyrate |
| pHMGCL | Poly (hydroxymethylglycolide-co-ε-caprolactone) |
| PLA | Polylactic acid |
| PLLA | Poly-L-lactic acid |
| PMMA | Poly (methyl methacrylate) |
| POP | Pelvic Organ Prolapse |
| PP | Polypropylene |
| PPS | Polyphenylene sulphide |
| PEVOH | Poly (ethylene-co-vinyl alcohol) |
| PVB | Polyvinyl butyral |
| PVDF | Poly (vinylidene fluoride) |
| SAN | Styrene-acrylonitrile |
| SD | Sanguis Draconis |
| SF | Silk Fibroin |
| SM | Sodium Myristate |
| SO | Sodium Oleate |
| SS | Sodium Stearate |
| TPU | Polyurethane |
| VEGF | Angiogenic Factor |
| WP | Whey Protein |
References
- Qi, S.; Craig, D. Recent developments in micro- and nanofabrication techniques for the preparation of amorphous pharmaceutical dosage forms. Adv. Drug Deliv. Rev. 2016, 100, 67–84. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Yousefzade, O.; Katsarava, R.; Puiggalí, J. Biomimetic Hybrid Systems for Tissue Engineering. Biomimetics 2020, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wang, M.; Wang, X.; Yu, J.; Sun, G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 13, 16–27. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Tu, D.; Pagliara, S.; Camposeo, A.; Potente, G.; Mele, E.; Cingolani, R.; Pisignano, D. Soft Nanolithography by Polymer Fibers. Adv. Funct. Mater. 2011, 21, 1140–1145. [Google Scholar] [CrossRef]
- Kakroodi, A.R.; Kazemi, Y.; Rodrigue, D.; Park, C.B. Facile production of biodegradable PCL/PLA in situ nanofibrillar composites with unprecedented compatibility between the blend components. Chem. Eng. J. 2018, 351, 976–984. [Google Scholar] [CrossRef]
- Kamps, A.C.; Fryd, M.; Park, S.-J. Hierarchical Self-Assembly of Amphiphilic Semiconducting Polymers into Isolated, Bundled, and Branched Nanofibers. ACS Nano 2012, 6, 2844–2852. [Google Scholar] [CrossRef]
- Mingjun, C.; Youchen, Z.; Haoyi, L.; Xiangnan, L.; Yumei, D.; Bubakir, M.M.; Weimin, Y. An example of industrialization of melt electrospinning: Polymer melt differential electrospinning. Adv. Ind. Eng. Polym. Res. 2019, 2, 110–115. [Google Scholar] [CrossRef]
- Buzgo, M.; Filova, E.; Staffa, A.; Rampichová, M.; Doupnik, M.; Vocetkova, K.; Hedvičáková, V.; Kolcun, R.; Lukas, D.; Necas, A.; et al. Needleless emulsion electrospinning for the regulated delivery of susceptible proteins. J. Tissue Eng. Regen. Med. 2018, 12, 583–597. [Google Scholar] [CrossRef]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Liu, T.Q.; Lv, D.; Zhao, X.L.; Gao, N.; Zhao, N. Study on a Preparation of PS Hollow Submicro-Fiber by Coaxial Electrospinning. Adv. Mater. Res. 2013, 652, 228–233. [Google Scholar] [CrossRef]
- Wu, H.; Bian, F.; Gong, R.H.; Zeng, Y. Effects of Electric Field and Polymer Structure on the Formation of Helical Nanofibers via Coelectrospinning. Ind. Eng. Chem. Res. 2015, 54, 9585–9590. [Google Scholar] [CrossRef]
- Morikawa, K.; Vashisth, A.; Grimme, C.J.; Green, M.J.; Naraghi, M. Wire Melt Electrospinning of Thin Polymeric Fibers via Strong Electrostatic Field Gradients. Macromol. Mater. Eng. 2019, 304. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Duan, X.-P.; Yan, X.; Yu, M.; Ning, X.; Zhao, Y.; Long, Y.-Z. Recent advances in melt electrospinning. RSC Adv. 2016, 6, 53400–53414. [Google Scholar] [CrossRef]
- Góra, A.; Sahay, R.; Thavasi, V.; Ramakrishna, S. Melt-Electrospun Fibers for Advances in Biomedical Engineering, Clean Energy, Filtration, and Separation. Polym. Rev. 2011, 51, 265–287. [Google Scholar] [CrossRef]
- Lyons, J.; Li, C.; Ko, F. Melt-electrospinning part I: Processing parameters and geometric properties. Polymers 2004, 45, 7597–7603. [Google Scholar] [CrossRef]
- Müller, M.; Kulikov, O.; Hornung, K.; Wagner, M.H. The use of thermoplastic elastomers as polymer processing aids in processing of linear low density polyethylene. Polym. Sci. Ser. A 2010, 52, 1163–1170. [Google Scholar] [CrossRef]
- Yousefzade, O.; Jeddi, J.; Franco, L.; Puiggalí, J.; Garmabi, H. Crystallization kinetics of chain extended poly(L-lactide)s having different molecular structures. Mater. Chem. Phys. 2020, 240, 122217. [Google Scholar] [CrossRef]
- Yousefzade, O.; Diarce, G.; Sangroniz, L.; Aguirresarobe, R.H.; Puiggalí, J.; Garmabi, H. Reactive melt processing of poly (L-lactide) in the presence of thermoplastic polyurethane and carboxylated carbon nanotubes. J. Mater. Sci. 2019, 54, 14961–14974. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, P.W.; Wang, X.; Zhang, C.; Pokorski, J.K.; Baer, E. Polyolefin Microfiber Based Antibacterial Fibrous Membrane by Forced Assembly Coextrusion. Macromol. Mater. Eng. 2016, 302. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, H.-C.; Kim, H.-Y. Roles of work of adhesion between carbon blacks and thermoplastic polymers on electrical properties of composites. J. Colloid Interface Sci. 2002, 255, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, F.; Yousefzade, O.; Garmabi, H. Compatibilized low-density polyethylene/linear low-density polyethylene/nanoclay nanocomposites: II. Opposing effects of nanofiller on quiescent and shear-induced crystallization. Adv. Polym. Technol. 2016, 37, 1345–1355. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Wahbi, M.; Kasmi, N.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of additives on the thermal and thermo-oxidative stability of poly(ethylene furanoate) biobased polyester. Thermochim. Acta 2020, 686. [Google Scholar] [CrossRef]
- Dubrocq-Baritaud, C.; Darque-Ceretti, E.; Vergnes, B. Fluoropolymer processing aids in linear-low density polyethylene extrusion: How to improve their efficiency? J. Non-Newton. Fluid Mech. 2014, 208–209, 42–52. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Su, Y.; Kun, E.; Zhang, F. Clay-Polymer Nanocomposites Prepared by Reactive Melt Extrusion for Sustained Drug Release. Pharmaceutics 2020, 12, 51. [Google Scholar] [CrossRef]
- Vlachopoulos, J.; Strutt, D. Polymer processing. Mater. Sci. Technol. 2003, 19, 1161–1169. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, H.W.; Kim, S.H.; Hyun, J.C. Effect of fluid viscoelasticity on the draw resonance dynamics of melt spinning. J. Non-Newton. Fluid Mech. 2001, 99, 159–166. [Google Scholar] [CrossRef]
- Zhou, C.; Kumar, S. Thermal instabilities in melt spinning of viscoelastic fibers. J. Non-Newton. Fluid Mech. 2010, 165, 879–891. [Google Scholar] [CrossRef]
- George, K.E. 2-Non-Newtonian fluid mechanics and polymer rheology. In Advances in Polymer Processing; Thomas, S., Weimin, Y., Eds.; Woodhead Publishing: Sawston/Cambridge, UK, 2009; pp. 13–46. [Google Scholar] [CrossRef]
- Silviya, E.; Varma, S.; Unnikrishnan, G.; Thomas, S. Compounding and mixing of polymers. In Advances in Polymer Processing; Elsevier: Amsterdam, The Netherlands, 2009; pp. 71–105. [Google Scholar]
- Katchy, E.M. Effects of dry blending on morphogy of PVC powder particles. J. Appl. Polym. Sci. 1983, 28, 1847–1869. [Google Scholar] [CrossRef]
- Rawal, A.; Mukhopadhyay, S. Melt spinning of synthetic polymeric filaments. In Advances in Filament Yarn Spinning of Textiles and Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 75–99. [Google Scholar]
- Qu, H.; Skorobogatiy, M. Conductive polymer yarns for electronic textiles. In Electronic Textiles; Elsevier: Amsterdam, The Netherlands, 2015; pp. 21–53. [Google Scholar]
- La Mantia, F.P.; Ceraulo, M.; Mistretta, M.C.; Morreale, M. Effect of Cold Drawing on Mechanical Properties of Biodegradable Fibers. J. Appl. Biomater. Funct. Mater. 2017, 15, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Shimada, N.; Tsutsumi, H.; Nakane, K.; Ogihara, T.; Ogata, N. Poly(ethylene-co-vinyl alcohol) and Nylon 6/12 nanofibers produced by melt electrospinning system equipped with a line-like laser beam melting device. J. Appl. Polym. Sci. 2010, 116, 2998–3004. [Google Scholar] [CrossRef]
- Nayak, R.; Padhye, R.; Arnold, L. Melt-electrospinning of nanofibers. Electrospun Nanofibers 2017, 11–40. [Google Scholar]
- Wang, X.-F.; Zheng, M.-H. Melt-electrospinning of PMMA. Chin. J. Polym. Sci. 2009, 28, 45–53. [Google Scholar] [CrossRef]
- Dalton, P.D.; Grafahrend, D.; Klinkhammer, K.B.; Klee, D.; Möller, M. Electrospinning of polymer melts: Phenomenological observations. Polymer 2007, 48, 6823–6833. [Google Scholar] [CrossRef]
- Buivydiene, D.; Dabasinskaite, L.; Krugly, E.; Kliucininkas, L. Formation of PA12 fibres via melt electrospinning process: Parameter analysis and optimisation. J. Polym. Eng. 2019, 40, 49–56. [Google Scholar] [CrossRef]
- Daenicke, J.; Lämmlein, M.; Steinhübl, F.; Schubert, D.W. Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process. e-Polymers 2019, 19, 330–340. [Google Scholar] [CrossRef]
- Doustgani, A.; Ahmadi, E. Melt electrospinning process optimization of polylactic acid nanofibers. J. Ind. Text. 2014, 45, 626–634. [Google Scholar] [CrossRef]
- Hao, M.F.; Liu, Y.; He, X.T.; Ding, Y.M.; Yang, W.M. Factors Influencing Diameter of Polypropylene Fiber in Melt Electrospinning. Adv. Mater. Res. 2011, 221, 129–134. [Google Scholar] [CrossRef]
- Nayak, R.; Kyratzis, I.L.; Truong, Y.B.; Padhye, R.; Arnold, L. Melt-electrospinning of polypropylene with conductive additives. J. Mater. Sci. 2012, 47, 6387–6396. [Google Scholar] [CrossRef]
- Detta, N.; Brown, T.D.; Edin, F.K.; Albrecht, K.; Chiellini, F.; Chiellini, E.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning of polycaprolactone and its blends with poly(ethylene glycol). Polym. Int. 2010, 59, 1558–1562. [Google Scholar] [CrossRef]
- Mingjun, C.; Zhang, Y.; Chen, X.; Yang, W.; Li, H.; Yousefzadeh, M.; Ramakrishna, S. Polymer melt differential electrospinning from a linear slot spinneret. J. Appl. Polym. Sci. 2020, 137, 48922. [Google Scholar] [CrossRef]
- Zhou, F.; Gong, R.-H.; Porat, I. Three-jet electrospinning using a flat spinneret. J. Mater. Sci. 2009, 44, 5501–5508. [Google Scholar] [CrossRef]
- Mayadeo, N.; Morikawa, K.; Naraghi, M.; Green, M.J. Modeling of downstream heating in melt electrospinning of polymers. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1393–1405. [Google Scholar] [CrossRef]
- Zhmayev, E.; Cho, D.; Joo, Y.L. Modeling of melt electrospinning for semi-crystalline polymers. Polymer 2010, 51, 274–290. [Google Scholar] [CrossRef]
- Shabani, E.; Yancheshme, A.A.; Ronen, A.; Gorga, R.E. Effect of the Spin-Line Temperature Profile on the Translocation of the Solidification Point and Jet Thinning in Unconfined Melt Electrospinning. ACS Appl. Polym. Mater. 2021, 3, 268–278. [Google Scholar] [CrossRef]
- Shabani, E.; Rashid, T.U.; Gorga, R.E.; Krause, W.E. A facile LED backlight in situ imaging technique to investigate sub-micron level processing. Polym. Test. 2020, 92, 106865. [Google Scholar] [CrossRef]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Direct Writing by Way of Melt Electrospinning. Adv. Mater. 2011, 23, 5651–5657. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D. Melt electrowriting with additive manufacturing principles. Curr. Opin. Biomed. Eng. 2017, 2, 49–57. [Google Scholar] [CrossRef]
- Castilho, M.; Feyen, D.; Flandes-Iparraguirre, M.; Hochleitner, G.; Groll, J.; Doevendans, P.A.F.; Vermonden, T.; Ito, K.; Sluijter, J.; Malda, J. Melt Electrospinning Writing of Poly-Hydroxymethylglycolide-co -ε-Caprolactone-Based Scaffolds for Cardiac Tissue Engineering. Adv. Health Mater. 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-M.; Wang, X.-X.; Yu, S.; Zhao, Y.-T.; Yan, X.; Zheng, J.; Yu, M.; Yan, S.-Y.; Long, Y.-Z. Bubble Melt Electrospinning for Production of Polymer Microfibers. Polymer 2018, 10, 1246. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.-H. Bubble Electrospinning for Mass Production of Nanofibers. Int. J. Nonlinear Sci. Numer. Simul. 2007, 8, 393–396. [Google Scholar] [CrossRef]
- Zhmayev, E.; Cho, D.; Joo, Y.L. Nanofibers from gas-assisted polymer melt electrospinning. Polymer 2010, 51, 4140–4144. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Wu, W.; Chen, H.; Ding, Y.; Yang, W. Effect of electric field on gas-assisted melt differential electrospinning with hollow disc electrode. J. Polym. Eng. 2015, 35, 61–70. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wang, J.; Li, C. Preparation and characterization of PLLA/nHA nonwoven mats via laser melt electrospinning. Mater. Lett. 2012, 73, 103–106. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wang, J.; Li, C. Preparation and characterization of poly(ɛ-caprolactone) nonwoven mats via melt electrospinning. Polymer 2012, 53, 248–253. [Google Scholar] [CrossRef]
- Zakaria, M.; Nakane, K. Fabrication of Polypropylene Nanofibers from Polypropylene/Polyvinyl Butyral Blend Films Using Laser-Assisted Melt-Electrospinning. Polym. Eng. Sci. 2019, 60, 362–370. [Google Scholar] [CrossRef]
- Ogata, N.; Yamaguchi, S.; Shimada, N.; Lu, G.; Iwata, T.; Nakane, K.; Ogihara, T. Poly(lactide) nanofibers produced by a melt-electrospinning system with a laser melting device. J. Appl. Polym. Sci. 2007, 104, 1640–1645. [Google Scholar] [CrossRef]
- Ogata, N.; Lu, G.; Iwata, T.; Yamaguchi, S.; Nakane, K.; Ogihara, T. Effects of ethylene content of poly(ethylene-co-vinyl alcohol) on diameter of fibers produced by melt-electrospinning. J. Appl. Polym. Sci. 2007, 104, 1368–1375. [Google Scholar] [CrossRef]
- Richaud, E.; Verdu, J.; Fayolle, B. Tensile properties of polypropylene fibres. In Handbook of Tensile Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2009; pp. 315–331. [Google Scholar]
- Larrondo, L.; Manley, R.S.J. Electrostatic fiber spinning from polymer melts. I. Experimental observations on fiber formation and properties. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 909–920. [Google Scholar] [CrossRef]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Effect of viscosity and electrical conductivity on the morphology and fiber diameter in melt electrospinning of polypropylene. Text. Res. J. 2012, 83, 606–617. [Google Scholar] [CrossRef]
- Malakhov, S.N.; Belousov, S.I.; Orekhov, A.S.; Chvalun, S.N. Electrospinning of Nonwoven Fabrics from Polypropylene Melt with Additions of Stearates of Divalent Metals. Fibre Chem. 2018, 50, 27–32. [Google Scholar] [CrossRef]
- Grob, M.C.; Minder, E. Permanent antistatic additives: New developments. Plast. Addit. Compd. 1999, 1, 20–26. [Google Scholar] [CrossRef]
- Cao, L.; Su, D.-F.; Su, Z.; Chen, X. Morphology, crystallization behavior and tensile properties of β-nucleated isotactic polypropylene fibrous membranes prepared by melt electrospinning. Chin. J. Polym. Sci. 2014, 32, 1167–1175. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, T.Q.; Liu, R.X. Study of Preparation Metallocene Based LLDPE Extra-Fibers by Melt Electrospinning Process. Adv. Mater. Res. 2012, 512, 2424–2427. [Google Scholar] [CrossRef]
- Shabani, E.; Li, C.; Komer, R.; Clarke, L.; Bochinski, J.; Gorga, R.; Boland, B.; Sheoran, N. Strategies to improve the unconfined melt electrospinning process via incorporation of ionically conductive particles. In Proceedings of the APS March Meeting 2019, Boston, MA, USA, 4–8 March 2019; Volume 64, p. B50.004. [Google Scholar]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Isitman, N.A.; Gunduz, H.O.; Kaynak, C. Nanoclay synergy in flame retarded/glass fibre reinforced polyamide 6. Polym. Degrad. Stab. 2009, 94, 2241–2250. [Google Scholar] [CrossRef]
- Wu, H.; Krifa, M.; Koo, J.H. Flame retardant polyamide 6/nanoclay/intumescent nanocomposite fibers through electrospinning. Text. Res. J. 2014, 84, 1106–1118. [Google Scholar] [CrossRef]
- Isitman, N.A.; Aykol, M.; Kaynak, C. Nanoclay assisted strengthening of the fiber/matrix interface in functionally filled polyamide 6 composites. Compos. Struct. 2010, 92, 2181–2186. [Google Scholar] [CrossRef]
- Malakhov, S.N.; Belousov, S.I.; Shcherbina, M.A.; Meshchankina, M.Y.; Chvalun, S.N.; Shepelev, A.D. Effect of low molecular additives on the electrospinning of nonwoven materials from a polyamide-6 melt. Polym. Sci. Ser. A 2016, 58, 236–245. [Google Scholar] [CrossRef]
- Luo, C.J.; Stride, E.; Edirisinghe, M. Mapping the Influence of Solubility and Dielectric Constant on Electrospinning Polycaprolactone Solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Wright, L.; Andric, T.; Freeman, J. Utilizing NaCl to increase the porosity of electrospun materials. Mater. Sci. Eng. C 2011, 31, 30–36. [Google Scholar] [CrossRef]
- Piyasin, P.; Yensano, R.; Pinitsoontorn, S. Size-Controllable Melt-Electrospun Polycaprolactone (PCL) Fibers with a Sodium Chloride Additive. Polymer 2019, 11, 1768. [Google Scholar] [CrossRef]
- Davachi, S.M.; Heidari, B.S.; Hejazi, I.; Seyfi, J.; Oliaei, E.; Farzaneh, A.; Rashedi, H. Interface modified polylactic acid/starch/poly ε-caprolactone antibacterial nanocomposite blends for medical applications. Carbohydr. Polym. 2017, 155, 336–344. [Google Scholar] [CrossRef]
- Kylie, K.; Hermanns, S.; Ellerkmann, J.; Saralidze, K.; Langensiepen, F.; Seide, G. The effect of additives and process parameters on the pilot-scale manufacturing of polylactic acid sub-microfibers by melt electrospinning. Text. Res. J. 2020, 90, 1948–1961. [Google Scholar] [CrossRef]
- Kylie, K.; Balakrishnan, N.; Hermanns, S.; Langensiepen, F.; Seide, G. Biobased Dyes as Conductive Additives to Reduce the Diameter of Polylactic Acid Fibers during Melt Electrospinning. Materials 2020, 13, 1055. [Google Scholar] [CrossRef]
- Reddy, C.S.; Sharma, A.; Rao, V.N. Effect of plasticizer on electrical conductivity and cell parameters of PVP+PVA+KClO3 blend polymer electrolyte system. J. Power Sources 2002, 111, 357–360. [Google Scholar] [CrossRef]
- Qin, Y.; Cheng, L.; Zhang, Y.; Chen, X.; Wang, X.; He, X.; Yan, H.; An, Y.; Li, H. Efficient preparation of poly(lactic acid) nanofibers by melt differential electrospinning with addition of acetyl tributyl citrate. J. Appl. Polym. Sci. 2018, 135, 46554. [Google Scholar] [CrossRef]
- Takeshita, A.; Igarashi-Migitaka, J.; Nishiyama, K.; Takahashi, H.; Takeuchi, Y.; Koibuchi, N. Acetyl Tributyl Citrate, the Most Widely Used Phthalate Substitute Plasticizer, Induces Cytochrome P450 3A through Steroid and Xenobiotic Receptor. Toxicol. Sci. 2011, 123, 460–470. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Balogh, A.; Drávavölgyi, G.; Ferguson, J.; Pataki, H.; Vajna, B.; Marosi, G. Solvent-Free Melt Electrospinning for Preparation of Fast Dissolving Drug Delivery System and Comparison with Solvent-Based Electrospun and Melt Extruded Systems. J. Pharm. Sci. 2013, 102, 508–517. [Google Scholar] [CrossRef]
- Ye, M.; Kim, S.; Park, K. Issues in long-term protein delivery using biodegradable microparticles. J. Control. Release 2010, 146, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Bastiancich, C.; Danhier, F.; Préat, V. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J. Control. Release 2016, 243, 29–42. [Google Scholar] [CrossRef]
- Lian, H.; Meng, Z. Melt electrospinning vs. solution electrospinning: A comparative study of drug-loaded poly (ε-caprolactone) fibres. Mater. Sci. Eng. C 2017, 74, 117–123. [Google Scholar] [CrossRef]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.-K. Electrospinning Nanofibers for Therapeutics Delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Mazalevska, O.; Struszczyk, M.; Krucińska, I. Design of vascular prostheses by melt electrospinning-structural characterizations. J. Appl. Polym. Sci. 2012, 129, 779–792. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef]
- Zhao, F.W.; Liu, Y.; Ding, Y.M.; Yan, H.; Xie, P.C.; Yang, W.M. Effect of Plasticizer and Load on Melt Electrospinning of PLA. Key Eng. Mater. 2012, 501, 32–36. [Google Scholar] [CrossRef]
- Balogh, A.; Drávavölgyi, G.; Faragó, K.; Farkas, A.; Vigh, T.; Sóti, P.L.; Wagner, I.; Madarász, J.; Pataki, H.; Marosi, G.; et al. Plasticized Drug-Loaded Melt Electrospun Polymer Mats: Characterization, Thermal Degradation, and Release Kinetics. J. Pharm. Sci. 2014, 103, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Immergut, E.H.; Mark, H.F. Principles of Plasticization. In Solid Surfaces; American Chemical Society (ACS): Washington, DC, USA, 1965; Volume 48, pp. 1–26. [Google Scholar]
- Ruffolo, J.R.R.; Feuerstein, G.Z. Pharmacology of Carvedilol: Rationale for Use in Hypertension, Coronary Artery Disease, and Congestive Heart Failure. Cardiovasc. Drugs Ther. 1997, 11, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Semjonov, K.; Lust, A.; Kogermann, K.; Laidmäe, I.; Maunu, S.; Hirvonen, S.-P.; Yliruusi, J.; Nurk, G.; Lust, E.; Heinämäki, J. Melt-electrospinning as a method to improve the dissolution and physical stability of a poorly water-soluble drug. Eur. J. Pharm. Sci. 2018, 121, 260–268. [Google Scholar] [CrossRef]
- Buschle-Diller, G.; Cooper, J.; Xie, Z.; Wu, Y.; Waldrup, J.; Ren, X. Release of antibiotics from electrospun bicomponent fibers. Cellulose 2007, 14, 553–562. [Google Scholar] [CrossRef]
- Huang, X.; Li, W.; Wang, M.; Tan, X.; Wang, Q.; Zhang, M.; Wang, C.; Zhang, H. Synthesis of multiple-shelled organosilica hollow nanospheres via a dual-template method by using compressed CO 2. Microporous Mesoporous Mater. 2017, 247, 66–74. [Google Scholar] [CrossRef]
- Xie, Z.; Buschle-Diller, G. Electrospun poly(D,L-lactide) fibers for drug delivery: The influence of cosolvent and the mechanism of drug release. J. Appl. Polym. Sci. 2010, 115, 1–8. [Google Scholar] [CrossRef]
- Okuda, T.; Tominaga, K.; Kidoaki, S. Time-programmed dual release formulation by multilayered drug-loaded nanofiber meshes. J. Control. Release 2010, 143, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Huang, X.B.; Cai, X.; Lu, J.; Yuan, J.; Shen, J. The influence of fiber diameter of electrospun poly(lactic acid) on drug delivery. Fibers Polym. 2012, 13, 1120–1125. [Google Scholar] [CrossRef]
- Natu, M.V.; De Sousa, H.C.; Gil, M.H. Effects of drug solubility, state and loading on controlled release in bicomponent electrospun fibers. Int. J. Pharm. 2010, 397, 50–58. [Google Scholar] [CrossRef]
- Holländer, J.; Genina, N.; Jukarainen, H.; Khajeheian, M.; Rosling, A.; Mäkilä, E.; Sandler, N. Three-Dimensional Printed PCL-Based Implantable Prototypes of Medical Devices for Controlled Drug Delivery. J. Pharm. Sci. 2016, 105, 2665–2676. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, J.; Wang, H.; Xia, Q.; Xu, S.; Han, C.C. Controlled Antibiotics Release System through Simple Blended Electrospun Fibers for Sustained Antibacterial Effects. ACS Appl. Mater. Interfaces 2015, 7, 26400–26404. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Zhu, K. Encapsulation and controlled release of lysozyme from electrospun poly(ε-caprolactone)/poly(ethylene glycol) non-woven membranes by formation of lysozyme–oleate complexes. J. Mater. Sci. Mater. Med. 2008, 19, 827–832. [Google Scholar] [CrossRef]
- Mittal, V.; Akhtar, T.; Matsko, N. Mechanical, Thermal, Rheological and Morphological Properties of Binary and Ternary Blends of PLA, TPS and PCL. Macromol. Mater. Eng. 2015, 300, 423–435. [Google Scholar] [CrossRef]
- Thomas, V.; Jagani, S.; Johnson, K.; Jose, M.V.; Dean, D.R.; Vohra, Y.K.; Nyairo, E. Electrospun Bioactive Nanocomposite Scaffolds of Polycaprolactone and Nanohydroxyapatite for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2006, 6, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Y.; Wang, J.; Zhang, C.; Yan, H.; Zhu, M.; Wang, K.; Li, C.; Xu, Q.; Kong, D. Effect of Resveratrol on Modulation of Endothelial Cells and Macrophages for Rapid Vascular Regeneration from Electrospun Poly(ε-caprolactone) Scaffolds. ACS Appl. Mater. Interfaces 2017, 9, 19541–19551. [Google Scholar] [CrossRef] [PubMed]
- Salles, T.H.C.; Sendyk, D.I.; De Oliveira, N.K.; Machado, D.; Lancellotti, M.; Deboni, M.C.Z.; Kiang, C.T.; D’Ávila, M.A. In vitro and in vivo evaluation of electrospun membranes of poly (ε-caprolactone) and poly (rotaxane). Mater. Sci. Eng. C 2017, 77, 912–919. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Padalhin, A.R.; Seo, H.S.; Lee, B.-T. A hybrid electrospun PU/PCL scaffold satisfied the requirements of blood vessel prosthesis in terms of mechanical properties, pore size, and biocompatibility. J. Biomater. Sci. Polym. Ed. 2013, 24, 1692–1706. [Google Scholar] [CrossRef]
- Chignell, C.F.; Bilskj, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and Photochemical Properties of Curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef]
- Jafari, Y.; Sabahi, H.; Rahaie, M. Stability and loading properties of curcumin encapsulated in Chlorella vulgaris. Food Chem. 2016, 211, 700–706. [Google Scholar] [CrossRef]
- Lian, H.; Meng, Z. Melt electrospinning of daunorubicin hydrochloride-loaded poly (ε-caprolactone) fibrous membrane for tumor therapy. Bioact. Mater. 2017, 2, 96–100. [Google Scholar] [CrossRef]
- He, F.-L.; Deng, X.; Zhou, Y.-Q.; Zhang, T.-D.; Liu, Y.-L.; Ye, Y.-J.; Yin, D.-C. Controlled release of antibiotics from poly-ε-caprolactone/polyethylene glycol wound dressing fabricated by direct-writing melt electrospinning. Polym. Adv. Technol. 2019, 30, 425–434. [Google Scholar] [CrossRef]
- Montaseri, H.; Forbes, P.B. A review of monitoring methods for triclosan and its occurrence in aquatic environments. TrAC Trends Anal. Chem. 2016, 85, 221–231. [Google Scholar] [CrossRef]
- Bhargava, H.; Leonard, P.A. Triclosan: Applications and safety. Am. J. Infect. Control. 1996, 24, 209–218. [Google Scholar] [CrossRef]
- Davachi, S.M.; Kaffashi, B. Polylactic Acid in Medicine. Polym. Technol. Eng. 2015, 54, 944–967. [Google Scholar] [CrossRef]
- Davachi, S.M.; Kaffashi, B. Preparation and Characterization of Poly L-Lactide/Triclosan Nanoparticles for Specific Antibacterial and Medical Applications. Int. J. Polym. Mater. 2015, 64, 497–508. [Google Scholar] [CrossRef]
- Davoodi, S.; Oliaei, E.; Davachi, S.M.; Heidari, B.S.; Hejazi, I.; Seyfi, J.; Ebrahimi, H. Preparation and characterization of interface-modified PLA/starch/PCL ternary blends using PLLA/triclosan antibacterial nanoparticles for medical applications. RSC Adv. 2016, 6, 39870–39882. [Google Scholar] [CrossRef]
- Mohammadi-Rovshandeh, J.; Davachi, S.M.; Kaffashi, B.; Hassani, A.; Bahmeyi, A.; Pouresmaeel-Selakjani, P. Effect of lignin removal on mechanical, thermal, and morphological properties of polylactide/starch/rice husk blend used in food packaging. J. Appl. Polym. Sci. 2014, 131, 41095. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Allo, B.A.; Costa, D.O.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactive and Biodegradable Nanocomposites and Hybrid Biomaterials for Bone Regeneration. J. Funct. Biomater. 2012, 3, 432–463. [Google Scholar] [CrossRef]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Osteoblast adhesion on nanophase ceramics. Biomaterials 1999, 20, 1221–1227. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Kaffashi, B.; Davoodi, S.; Oliaei, E. Poly(ε-caprolactone)/triclosan loaded polylactic acid nanoparticles composite: A long-term antibacterial bionanocomposite with sustained release. Int. J. Pharm. 2016, 508, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Yandi, W.; Mieszkin, S.; Martin-Tanchereau, P.; Callow, M.E.; Callow, J.A.; Tyson, L.; Liedberg, B.; Ederth, T. Hydration and Chain Entanglement Determines the Optimum Thickness of Poly(HEMA-co-PEG10MA) Brushes for Effective Resistance to Settlement and Adhesion of Marine Fouling Organisms. ACS Appl. Mater. Interfaces 2014, 6, 11448–11458. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Zhang, C.; Advincula, A.A.; Baer, E.; Pokorski, J.K. Protein and Bacterial Antifouling Behavior of Melt-Coextruded Nanofiber Mats. ACS Appl. Mater. Interfaces 2016, 8, 8928–8938. [Google Scholar] [CrossRef]
- Huang, J.; Murata, H.; Koepsel, R.R.; Russell, A.J.; Matyjaszewski, K. Antibacterial Polypropylene via Surface-Initiated Atom Transfer Radical Polymerization. Biomacromolecules 2007, 8, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, T.D. Some antibacterial properties of chlorhexidine. J. Periodontal Res. 1973, 8, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D.; Day, M.J. Antibacterial activity of chlorhexidine. J. Hosp. Infect. 1993, 25, 229–238. [Google Scholar] [CrossRef]
- Cao, K.; Liu, Y.; Olkhov, A.; Siracusa, V.; Iordanskii, A.L. PLLA-PHB fiber membranes obtained by solvent-free electrospinning for short-time drug delivery. Drug Deliv. Transl. Res. 2018, 8, 291–302. [Google Scholar] [CrossRef]
- Ali, A.Q.; Kannan, T.P.; Ahmad, A.; Samsudin, A.R. In vitro genotoxicity tests for polyhydroxybutyrate—A synthetic biomaterial. Toxicol. Vitr. 2008, 22, 57–67. [Google Scholar] [CrossRef]
- Xu, Y.; Li, K.; Liu, Y.; An, Y.; Xing, C. Black plaster composite fiber prepared by upward electrospinning. J. Appl. Polym. Sci. 2019, 136, 47662. [Google Scholar] [CrossRef]
- Wang, W.; Olson, D.; Cheng, B.; Guo, X.; Wang, K. Sanguis Draconis resin stimulates osteoblast alkaline phosphatase activity and mineralization in MC3T3-E1 cells. J. Ethnopharmacol. 2012, 142, 168–174. [Google Scholar] [CrossRef]
- Yamashita, K.; Nakate, T.; Okimoto, K.; Ohike, A.; Tokunaga, Y.; Ibuki, R.; Higaki, K.; Kimura, T. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int. J. Pharm. 2003, 267, 79–91. [Google Scholar] [CrossRef]
- Chan, K.L.A.; Kazarian, S.G. FTIR Spectroscopic Imaging of Dissolution of a Solid Dispersion of Nifedipine in Poly(ethylene glycol). Mol. Pharm. 2004, 1, 331–335. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Nanocomposite Electrospun Nanofiber Membranes for Environmental Remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Kaur, S.; Ramakrishna, S. Electrospun polymer nanocomposite fibers: Fabrication and physical properties. In Physical Properties and Applications of Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2010; pp. 616–637. [Google Scholar]
- Haroosh, H.J.; Dong, Y. 4-Electrospun poly(lactic acid) (PLA): Poly(ε-caprolactone) (PCL)/halloysite nanotube (HNT) composite fibers: Synthesis and characterization. In Fillers and Reinforcements for Advanced Nanocomposites; Dong, Y., Umer, R., Lau, A.K.-T., Eds.; Woodhead Publishing: Sawston/Cambridge, UK, 2015; pp. 59–80. [Google Scholar] [CrossRef]
- Shang, M.; Li, N.; Zhang, S.; Zhao, T.; Zhang, C.; Liu, C.; Li, H.; Wang, Z. Full-Spectrum Solar-to-Heat Conversion Membrane with Interfacial Plasmonic Heating Ability for High-Efficiency Desalination of Seawater. ACS Appl. Energy Mater. 2017, 1, 56–61. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An Effective 2D Light-to-Heat Conversion Material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Q.; Liang, X.; Xu, J.; Chang, C.; Song, C.; Shang, W.; Wu, T.D.A.J.; Tao, P.; Deng, T. Paper-based membranes on silicone floaters for efficient and fast solar-driven interfacial evaporation under one sun. J. Mater. Chem. A 2017, 5, 16359–16368. [Google Scholar] [CrossRef]
- Chala, T.F.; Wu, C.-M.; Chou, M.-H.; Guo, Z.-L. Melt Electrospun Reduced Tungsten Oxide /Polylactic Acid Fiber Membranes as a Photothermal Material for Light-Driven Interfacial Water Evaporation. ACS Appl. Mater. Interfaces 2018, 10, 28955–28962. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Kwon, B.; Byun, H. Preparation of PVdF nanofiber membranes by electrospinning and their use as secondary battery separators. J. Membr. Sci. 2011, 378, 111–116. [Google Scholar] [CrossRef]
- Pan, J.-L.; Zhang, Z.; Zhang, H.; Zhu, P.-P.; Wei, J.-C.; Cai, J.-X.; Yu, J.; Koratkar, N.; Yang, Z. Ultrathin and Strong Electrospun Porous Fiber Separator. ACS Appl. Energy Mater. 2018, 1, 4794–4803. [Google Scholar] [CrossRef]
- Wu, S.; Ning, J.; Jiang, F.; Shi, J.; Huang, F. Ceramic Nanoparticle-Decorated Melt-Electrospun PVDF Nanofiber Membrane with Enhanced Performance as a Lithium-Ion Battery Separator. ACS Omega 2019, 4, 16309–16317. [Google Scholar] [CrossRef]
- Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Recent Trends in Nanofibrous Membranes and Their Suitability for Air and Water Filtrations. Membranes 2011, 1, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Bunyakan, C.; Wiyaratn, W.; Chungsiriporn, J. Photocatalytic decolorization of basic dye by TiO 2 nanoparticle in photoreactor. Songklanakarin J. Sci. Technol. 2012, 34, 203–210. [Google Scholar]
- Karahaliloğlu, Z.; Hacker, C.; Demirbilek, M.; Seide, G.; Denkbaş, E.B.; Gries, T. Photocatalytic performance of melt-electrospun polypropylene fabric decorated with TiO2 nanoparticles. J. Nanoparticle Res. 2014, 16, 2615. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.; Zheng, J.; Yao, X.; Liu, W.; Cui, P.; Li, Y. Relationship between Microstructure and Tensile Properties of PET/Silica Nanocomposite Fibers. J. Macromol. Sci. Part B 2008, 47, 368–377. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wang, J.; Li, C. Preparation and properties of PET/SiO2composite micro/nanofibers by a laser melt-electrospinning system. J. Appl. Polym. Sci. 2012, 125, 2050–2055. [Google Scholar] [CrossRef]
- Hong, Z.; Zhang, P.; He, C.; Qiu, X.; Liu, A.; Chen, L.; Zhu, X.; Jing, X. Nano-composite of poly(-lactide) and surface grafted hydroxyapatite: Mechanical properties and biocompatibility. Biomaterials 2005, 26, 6296–6304. [Google Scholar] [CrossRef] [PubMed]
- Malakhov, S.N.; Bakirov, A.V.; Dmitryakov, P.V.; Chvalun, S.N. Nanocomposite nonwoven materials based on polyamide-6 and montmorillonite, prepared by electrospinning of the polymer melt. Russ. J. Appl. Chem. 2016, 89, 165–172. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, C.; Han, J.; Han, N.; Xi, J.; Fan, L.; Guo, R. Controllable Synthesis of Gold Nanorod/Conducting Polymer Core/Shell Hybrids Toward in Vitro and in Vivo near-Infrared Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 12323–12330. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.; Orza, A.; Lu, Q.; Guo, P.; Wang, L.; Yang, L.; Mao, H. Magnetic Nanoparticle Facilitated Drug Delivery for Cancer Therapy with Targeted and Image-Guided Approaches. Adv. Funct. Mater. 2016, 26, 3818–3836. [Google Scholar] [CrossRef]
- Song, C.; Wang, X.; Zhang, J.; Nie, G.-D.; Luo, W.-L.; Fu, J.; Ramakrishna, S.; Long, Y.-Z. Electric Field-Assisted In Situ Precise Deposition of Electrospun γ-Fe2O3/Polyurethane Nanofibers for Magnetic Hyperthermia. Nanoscale Res. Lett. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Hu, P.-Y.; Zhao, Y.-T.; Zhang, J.; Yu, S.-X.; Yan, J.-S.; Wang, X.-X.; Hu, M.-Z.; Xiang, H.-F.; Long, Y. In situ melt electrospun polycaprolactone/Fe3O4 nanofibers for magnetic hyperthermia. Mater. Sci. Eng. C 2020, 110, 110708. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D.L. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Silva, M.; Cyster, L.; Barry, J.; Yang, X.; Oreffo, R.; Grant, D.; Scotchford, C.; Howdle, S.; Shakesheff, K.; Rose, F. The effect of anisotropic architecture on cell and tissue infiltration into tissue engineering scaffolds. Biomaterials 2006, 27, 5909–5917. [Google Scholar] [CrossRef] [PubMed]
- Abdal-Hay, A.; Abbasi, N.; Gwiazda, M.; Hamlet, S.; Ivanovski, S. Novel polycaprolactone/hydroxyapatite nanocomposite fibrous scaffolds by direct melt-electrospinning writing. Eur. Polym. J. 2018, 105, 257–264. [Google Scholar] [CrossRef]
- Pukánszky, B. Interfaces and interphases in multicomponent materials: Past, present, future. Eur. Polym. J. 2005, 41, 645–662. [Google Scholar] [CrossRef]
- Keridou, I.; Cailloux, J.; Martínez, J.C.; Santana, O.; Maspoch, M.L.; Puiggalí, J.; Franco, L. Biphasic polylactide/polyamide 6,10 blends: Influence of composition on polyamide structure and polyester crystallization. Polymer 2020, 202, 122676. [Google Scholar] [CrossRef]
- Valenti, S.; Yousefzade, O.; Puiggalí, J.; Macovez, R. Phase-selective conductivity enhancement and cooperativity length in PLLA/TPU nanocomposite blends with carboxylated carbon nanotubes. Polymer 2020, 191, 122279. [Google Scholar] [CrossRef]
- Van Puyvelde, P.; Vananroye, A.; Cardinaels, R.; Moldenaers, P. Review on morphology development of immiscible blends in confined shear flow. Polymer 2008, 49, 5363–5372. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Kim, G.-M.; Le, K.H.T.; Giannitelli, S.M.; Lee, Y.J.; Rainer, A.; Trombetta, M. Electrospinning of PCL/PVP blends for tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1425–1442. [Google Scholar] [CrossRef]
- Nazari, T.; Garmabi, H. Thermo-rheological and interfacial properties of polylactic acid/polyethylene glycol blends toward the melt electrospinning ability. J. Appl. Polym. Sci. 2016, 133, 44120. [Google Scholar] [CrossRef]
- Nazari, T.; Garmabi, H. The effects of processing parameters on the morphology of PLA/PEG melt electrospun fibers. Polym. Int. 2018, 67, 178–188. [Google Scholar] [CrossRef]
- Nazari, T.; Garmabi, H. Polylactic acid/polyethylene glycol blend fibres prepared via melt electrospinning: Effect of polyethylene glycol content. Micro Nano Lett. 2014, 9, 686–690. [Google Scholar] [CrossRef]
- Cao, L.; Dong, M.; Zhang, A.; Liu, Y.; Yang, W.; Su, Z.; Chen, X. Morphologies and crystal structures of styrene-acrylonitrile/isotactic polypropylene ultrafine fibers fabricated by melt electrospinning. Polym. Eng. Sci. 2013, 53, 2674–2682. [Google Scholar] [CrossRef]
- Fujii, T.; Mizutani, Y.; Nakane, K. Melt-electrospun fibers obtained from polypropylene/poly(ethylene-co -vinyl alcohol)/polypropylene three-layer films. J. Appl. Polym. Sci. 2018, 135, 46393. [Google Scholar] [CrossRef]
- Sahay, R.; Thavasi, V.; Ramakrishna, S. Design Modifications in Electrospinning Setup for Advanced Applications. J. Nanomater. 2011, 2011, 1–17. [Google Scholar] [CrossRef]
- Braeken, L.; Van Der Bruggen, B.; Vandecasteele, C. Regeneration of brewery waste water using nanofiltration. Water Res. 2004, 38, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Wang, Z. Oilfield produced water treatment with surface-modified fiber ball media filtration. Water Sci. Technol. 2002, 46, 165–170. [Google Scholar] [CrossRef]
- Watanabe, K.; Kim, B.-S.; Kim, I.-S. Development of Polypropylene Nanofiber Production System. Polym. Rev. 2011, 51, 288–308. [Google Scholar] [CrossRef]
- Buivydiene, D.; Krugly, E.; Ciuzas, D.; Tichonovas, M.; Kliucininkas, L.; Martuzevicius, D. Formation and characterisation of air filter material printed by melt electrospinning. J. Aerosol Sci. 2019, 131, 48–63. [Google Scholar] [CrossRef]
- An, Y.; Yu, S.; Li, S.; Wang, X.; Yang, W.; Yousefzadeh, M.; Bubakir, M.M.; Li, H. Melt-electrospinning of Polyphenylene Sulfide. Fibers Polym. 2018, 19, 2507–2513. [Google Scholar] [CrossRef]
- Arkoun, M.; Ardila, N.; Heuzey, M.-C.; Ajji, A. Chitosan-Based Structures/Coatings with Antibacterial Properties. In Handbook of Antimicrobial Coatings; Elsevier: Amsterdam, The Netherlands, 2018; pp. 357–389. [Google Scholar]
- Randazzo, W.; Fabra, M.J.; Falcó, I.; López-Rubio, A.; Sánchez, G. Polymers and Biopolymers with Antiviral Activity: Potential Applications for Improving Food Safety. Compr. Rev. Food Sci. Food Saf. 2018, 17, 754–768. [Google Scholar] [CrossRef]
- Topuza, F.; Uyarb, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.K.; Kaya, B.; Jun, M.B.-G. Development of Bioactive Packaging Structure Using Melt Electrospinning. J. Polym. Environ. 2015, 23, 416–423. [Google Scholar] [CrossRef]
- Hollister, S. Scaffold Design and Manufacturing: From Concept to Clinic. Adv. Mater. 2009, 21, 3330–3342. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S. Scaffold engineering: A bridge to where? Biofabrication 2009, 1, 012001. [Google Scholar] [CrossRef]
- Hollister, S.; Murphy, W.L. Scaffold Translation: Barriers Between Concept and Clinic. Tissue Eng. Part B Rev. 2011, 17, 459–474. [Google Scholar] [CrossRef]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [PubMed]
- Gazzarri, M.; Bartoli, C.; Mota, C.; Puppi, D.; Dinucci, D.; Volpi, S.; Chiellini, F. Fibrous star poly(ε-caprolactone) melt-electrospun scaffolds for wound healing applications. J. Bioact. Compat. Polym. 2013, 28, 492–507. [Google Scholar] [CrossRef]
- Ahangar, P.; Cooke, M.E.; Weber, M.H.; Rosenzweig, D.H. Current Biomedical Applications of 3D Printing and Additive Manufacturing. Appl. Sci. 2019, 9, 1713. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef]
- Giannitelli, S.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Muerza-Cascante, M.L.; Haylock, D.; Hutmacher, D.W.; Dalton, P.D. Melt Electrospinning and Its Technologization in Tissue Engineering. Tissue Eng. Part B Rev. 2015, 21, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Wang, H.; Lin, Y.; Zhou, Y.; Wang, Z.; Chen, X.; Cai, N.; Yang, F.; Wu, P.; He, Y.; et al. Near-field melt electrospinning of poly(ε-caprolactone) (PCL) micro-line array for cell alignment study. Mater. Res. Express 2018, 6, 015401. [Google Scholar] [CrossRef]
- Xie, C.; Gao, Q.; Wang, P.; Shao, L.; Yuan, H.; Fu, J.; Chen, W.; He, Y. Structure-induced cell growth by 3D printing of heterogeneous scaffolds with ultrafine fibers. Mater. Des. 2019, 181. [Google Scholar] [CrossRef]
- Visser, J.; Melchels, F.P.; Jeon, J.E.; Van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef]
- Kearney, E.M.; Prendergast, P.J.; Campbell, V.A. Mechanisms of Strain-Mediated Mesenchymal Stem Cell Apoptosis. J. Biomech. Eng. 2008, 130, 061004. [Google Scholar] [CrossRef]
- Huesa, C.; Helfrich, M.H.; Aspden, R.M. Parallel-plate fluid flow systems for bone cell stimulation. J. Biomech. 2010, 43, 1182–1189. [Google Scholar] [CrossRef]
- Song, K.H.; Highley, C.B.; Rouff, A.; Burdick, J.A. Complex 3D-Printed Microchannels within Cell-Degradable Hydrogels. Adv. Funct. Mater. 2018, 28, 1801331. [Google Scholar] [CrossRef]
- Kim, B.N.; Ko, Y.-G.; Yeo, T.; Kim, E.J.; Kwon, O.K. Guided Regeneration of Rabbit Calvarial Defects Using Silk Fibroin Nanofiber–Poly(glycolic acid) Hybrid Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 5266–5272. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.I.; Reis, R.L. Vascularization in Bone Tissue Engineering: Physiology, Current Strategies, Major Hurdles and Future Challenges. Macromol. Biosci. 2009, 10, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Lokmic, Z.; Mitchell, G.M. Engineering the Microcirculation. Tissue Eng. Part B Rev. 2008, 14, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Grainger, S.J.; Putnam, A.J. Assessing the Permeability of Engineered Capillary Networks in a 3D Culture. PLoS ONE 2011, 6, e22086. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.L.; Radisic, M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials 2010, 31, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Kety, S.S. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol. Rev. 1951, 3, 1–41. [Google Scholar]
- Krogh, A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J. Physiol. 1919, 52, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; Van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Janssen, F.W.; Oostra, J.; Van Oorschot, A.; Van Blitterswijk, C.A. A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: In vivo bone formation showing proof of concept. Biomaterials 2006, 27, 315–323. [Google Scholar] [CrossRef]
- Clark, E.R.; Clark, E.L. Microscopic observations on the growth of blood capillaries in the living mammal. Am. J. Anat. 1939, 64, 251–301. [Google Scholar] [CrossRef]
- Malda, J.; Rouwkema, J.; Martens, D.E.; Le Comte, E.P.; Kooy, F.K.; Tramper, J.; Van Blitterswijk, C.A.; Riesle, J. Oxygen gradients in tissue-engineered Pegt/Pbt cartilaginous constructs: Measurement and modeling. Biotechnol. Bioeng. 2004, 86, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bertlein, S.; Hikimoto, D.; Hochleitner, G.; Hümmer, J.; Jungst, T.; Matsusaki, M.; Akashi, M.; Groll, J. Development of Endothelial Cell Networks in 3D Tissues by Combination of Melt Electrospinning Writing with Cell-Accumulation Technology. Small 2018, 14, 1701521. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, S.; Brown, T.D.; Reichert, J.C.; Berner, A. Poly(ε-caprolactone) Scaffolds Fabricated by Melt Electrospinning for Bone Tissue Engineering. Materials 2016, 9, 232. [Google Scholar] [CrossRef]
- Youssef, A.; Hollister, S.J.; Dalton, P.D. Additive manufacturing of polymer melts for implantable medical devices and scaffolds. Biofabrication 2017, 9, 012002. [Google Scholar] [CrossRef]
- Hrynevich, A.; Elçi, B.Ş.; Haigh, J.N.; McMaster, R.; Youssef, A.; Blum, C.; Blunk, T.; Hochleitner, G.; Groll, J.; Dalton, P.D. Dimension-Based Design of Melt Electrowritten Scaffolds. Small 2018, 14, e1800232. [Google Scholar] [CrossRef]
- Brown, T.D.; Slotosch, A.; Thibaudeau, L.; Taubenberger, A.V.; Loessner, D.; Vaquette, C.; Dalton, P.D.; Hutmacher, D.W. Design and Fabrication of Tubular Scaffolds via Direct Writing in a Melt Electrospinning Mode. Biointerphases 2012, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, A.U.; Mikos, A.G. Mikos, A.G. Electrospun Poly(ε-caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Soliman, S.; Pagliari, S.; Rinaldi, A.; Forte, G.; Fiaccavento, R.; Pagliari, F.; Franzese, O.; Minieri, M.; Di Nardo, P.; Licoccia, S. Multiscale three-dimensional scaffolds for soft tissue engineering via multimodal electrospinning. Acta Biomater. 2010, 6, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Ahn, S.; Choi, H.; Cho, D.; Kim, G. Fabrication, characterization, and in vitro biological activities of melt-electrospun PLA micro/nanofibers for bone tissue regeneration. J. Mater. Chem. B 2013, 1, 3670–3677. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Schakenraad, J.M.; Nieuwenhuis, P.; Molenaar, I.; Helder, J.; Dijkstra, P.J.; Feijen, J. In vivo andin vitro degradation of glycine/DL-lactic acid copolymers. J. Biomed. Mater. Res. 1989, 23, 1271–1288. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Friedman, R.J.; Powers, D.L.; Draughn, R.A.; Latour, R.A. Fixation of Osteotomies Using Bioabsorbable Screws in the Canine Femur. Clin. Orthop. Relat. Res. 1998, 355, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.J.; Midha, S.; Ghosh, S.; Ito, K.K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2013, 41, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Saifzadeh, S.; Farag, A.; Hutmacher, D.; Ivanovski, S. Periodontal Tissue Engineering with a Multiphasic Construct and Cell Sheets. J. Dent. Res. 2019, 98, 673–681. [Google Scholar] [CrossRef]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.; Wagner, F.; Martine, L.; Holzapfel, B.; Theodoropoulos, C.; Bas, O.; Savi, F.; Werner, C.; De-Juan-Pardo, E.; Hutmacher, D. Periosteum tissue engineering in an orthotopic in vivo platform. Biomaterials 2017, 121, 193–204. [Google Scholar] [CrossRef]
- Fuchs, A.; Youssef, A.; Seher, A.; Hartmann, S.; Brands, R.C.; Müller-Richter, U.D.; Kübler, A.C.; Linz, C. A new multilayered membrane for tissue engineering of oral hard- and soft tissue by means of melt electrospinning writing and film casting—An in vitro study. J. Cranio-Maxillofac. Surg. 2019, 47, 695–703. [Google Scholar] [CrossRef]
- Muerza-Cascante, M.L.; Shokoohmand, A.; Khosrotehrani, K.; Haylock, D.; Dalton, P.D.; Hutmacher, D.W.; Loessner, D. Endosteal-like extracellular matrix expression on melt electrospun written scaffolds. Acta Biomater. 2017, 52, 145–158. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health A Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Bas, O.; De-Juan-Pardo, E.M.; Meinert, C.; D’Angella, D.; Baldwin, J.G.; Bray, L.J.; Wellard, R.M.; Kollmannsberger, S.; Rank, E.; Werner, C.; et al. Biofabricated soft network composites for cartilage tissue engineering. Biofabrication 2017, 9, 025014. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 203–222. [Google Scholar] [CrossRef]
- Tozer, S.; Duprez, D. Tendon and ligament: Development, repair and disease. Birth Defects Res. Part C Embryo Today Rev. 2005, 75, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hochleitner, G.; Chen, F.; Blum, C.; Dalton, P.D.; Amsden, B.; Groll, J. Melt electrowriting below the critical translation speed to fabricate crimped elastomer scaffolds with non-linear extension behaviour mimicking that of ligaments and tendons. Acta Biomater. 2018, 72, 110–120. [Google Scholar] [CrossRef]
- Gwiazda, M.; Kumar, S.; Świeszkowski, W.; Ivanovski, S.; Vaquette, C. The effect of melt electrospun writing fiber orientation onto cellular organization and mechanical properties for application in Anterior Cruciate Ligament tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103631. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Murry, C.E. Heart regeneration. Nat. Cell Biol. 2011, 473, 326–335. [Google Scholar] [CrossRef]
- Castilho, M.; Van Mil, A.; Maher, M.; Metz, C.H.G.; Hochleitner, G.; Groll, J.; Doevendans, P.A.; Ito, K.; Sluijter, J.; Malda, J. Melt Electrowriting Allows Tailored Microstructural and Mechanical Design of Scaffolds to Advance Functional Human Myocardial Tissue Formation. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Liao, S.; Theodoropoulos, C.; Blackwood, K.A.; Woodruff, M.A.; Gregory, S.D. Melt Electrospun Bilayered Scaffolds for Tissue Integration of a Suture-Less Inflow Cannula for Rotary Blood Pumps. Artif. Organs 2018, 42, E43–E54. [Google Scholar] [CrossRef] [PubMed]
- Saidy, N.T.; Wolf, F.; Bas, O.; Keijdener, H.; Hutmacher, D.W.; Mela, P.; De-Juan-Pardo, E.M. Biologically Inspired Scaffolds for Heart Valve Tissue Engineering via Melt Electrowriting. Small 2019, 15, e1900873. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Garcia, A.; Oliver-De-La-Cruz, J.; Mirabet, V.; Gandía, C.; Villagrasa, A.; Sodupe, E.; Escobedo-Lucea, C. Heart valve tissue engineering: How far is the bedside from the bench? Expert Rev. Mol. Med. 2015, 17, e16. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e467–e492. [Google Scholar] [CrossRef]
- Hewitt, E.; Mros, S.; McConnell, M.; Cabral, J.D.; Ali, A. Melt-electrowriting with novel milk protein/PCL biomaterials for skin regeneration. Biomed. Mater. 2019, 14, 055013. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Vandrovcova, M.; Kročilová, N.; Keppler, J.K.; Zarubova, J.; Skirtach, A.G.; Bačáková, L. Application of whey protein isolate in bone regeneration: Effects on growth and osteogenic differentiation of bone-forming cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Ali, M.A.; Selvanesan, L.; Dias, G.J. Structure–function characteristics of the biomaterials based on milk-derived proteins. Int. J. Biol. Macromol. 2010, 46, 404–411. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin Acts as an Alarmin to Promote the Recruitment and Activation of APCs and Antigen-Specific Immune Responses. J. Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachs-Herrera, A.; Yousefzade, O.; del Valle, L.J.; Puiggali, J. Melt Electrospinning of Polymers: Blends, Nanocomposites, Additives and Applications. Appl. Sci. 2021, 11, 1808. https://doi.org/10.3390/app11041808
Bachs-Herrera A, Yousefzade O, del Valle LJ, Puiggali J. Melt Electrospinning of Polymers: Blends, Nanocomposites, Additives and Applications. Applied Sciences. 2021; 11(4):1808. https://doi.org/10.3390/app11041808
Chicago/Turabian StyleBachs-Herrera, Anna, Omid Yousefzade, Luis J. del Valle, and Jordi Puiggali. 2021. "Melt Electrospinning of Polymers: Blends, Nanocomposites, Additives and Applications" Applied Sciences 11, no. 4: 1808. https://doi.org/10.3390/app11041808
APA StyleBachs-Herrera, A., Yousefzade, O., del Valle, L. J., & Puiggali, J. (2021). Melt Electrospinning of Polymers: Blends, Nanocomposites, Additives and Applications. Applied Sciences, 11(4), 1808. https://doi.org/10.3390/app11041808






