Responses of Medicinal and Aromatic Plants to Engineered Nanoparticles †
Abstract
1. Introduction
2. Effects of Metal-, Metalloid-, and Carbon-Based NPs on Vascular Plants
3. Improved Production of Secondary Metabolites in Presence of NPs
4. Effect of Metal-Based NPs on Medicinal and Aromatic Plants
4.1. Silver-Based NPs
4.1.1. Impact on Plant Growth
4.1.2. Elicitor Effect
4.1.3. Effect on Antioxidant Enzymes
4.1.4. Attenuation of Abiotic Stress
4.1.5. Cytotoxic and Genotoxic Effects of AgNPs
4.2. Copper-Based NPs
4.2.1. Impact on Plant Growth and Photosynthesis
4.2.2. Elicitor Effect
4.2.3. Genotoxicity
4.3. Iron-Based NPs
4.3.1. Impact on Plant Growth and Assimilation Pigments
4.3.2. Elicitor Effect
4.3.3. Attenuation of Abiotic Stress
4.3.4. Genotoxicity
4.4. Zinc-Based NPs
4.4.1. Impact on Germination and Plant Growth
4.4.2. Elicitor Effect
4.4.3. Impact on Antioxidant Enzymes
4.4.4. Cytotoxic and Genotoxic Effects
4.5. TiO2-Based NPs
4.5.1. Impact on Germination, Plant Growth and Assimilation Pigments
4.5.2. Elicitor Effect
4.5.3. Attenuation of Abiotic Stress
4.5.4. Genotoxicity
4.6. NPs of Other Metals
4.6.1. MgO NPs and Mn2O3 NPs
4.6.2. CoNPs
4.6.3. NiNPs and NiO NPs
4.6.4. Cr2O3 NPs
4.6.5. CeO2 NPs
4.6.6. Al2O3 NPs
4.6.7. AuNPs
5. Effect of Metalloid-Based NPs on Medicinal Plants
5.1. Silicon and Silica NPs
5.1.1. Impact on Germination and Plant Growth
5.1.2. Elicitor Effect
5.1.3. Attenuation of Abiotic Stress
5.2. Selenium NPs
5.2.1. Impact on Plant Growth and Elicitor Effect
5.2.2. Elicitor Effect
6. Effects of Carbon-Based NPs on Medicinal and Aromatic Plants
6.1. Carbon Dots
6.2. Graphene Quantum Dots and Graphene Nanosheets
6.3. Graphene Oxide
6.3.1. Impact on Germination and Plant Growth
6.3.2. Attenuation of Abiotic Stress
6.4. SWCNTs
6.5. MWCNTs
6.5.1. Impact on Germination and Plant Growth
6.5.2. Elicitor Effect
6.5.3. Attenuation of Abiotic Stress
6.5.4. Genotoxicity
7. Effects of Organic Material-Derived NPs on Medicinal and Aromatic Plants
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Pimentel, D. Green revolution agriculture and chemical hazards. Sci. Total Environ. 1996, 188, S86–S98. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Sandin-Espana, P.; Sevilla-Moran, B.; Lopez-Goti, C.; Alonso-Prados, J.L. Biopesticides from natural products: Current development, legislative framework, and future trends. BioResources 2016, 11, 5618–5640. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Nanobiopesticides in agriculture: State of the art and future opportunities. In Nano-Biopesticides Today and Future Perspectives; Koul, O., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 397–447. [Google Scholar]
- Salmeron-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Masarovicova, E.; Kralova, K. Medicinal plants—past, nowadays, future. Acta Horticult. 2007, 749, 19–27. [Google Scholar] [CrossRef]
- Masarovicova, E.; Kralova, K.; Vykoukova, I.; Krissakova, Z. Responses of medicinal plants to abiotic stresses. In Handbook of Plant and Crop Stress, 4th ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 713–753. [Google Scholar]
- Bryan, C.P. The Papyrus Ebers; D. Appleton and Co.: New York, NY, USA, 1936. [Google Scholar]
- Duke, J.A. Medicinal Plants of the Bible; Conch Magazine Ltd.: New York, NY, USA, 1983. [Google Scholar]
- Duke, J.A.; Telatnik, M.A.; Duke, P.K. Herbs of the Bible: 2000 Years of Plant. Medicine; Wendell Whitman Co.: Warsaw, IN, USA, 2007. [Google Scholar]
- Gunther, R.T. The Greek Herbal of Dioscorides; Oxford University Press: Oxford, UK, 1934. [Google Scholar]
- Gerard, J. The Herbal or General History of Plants, the 2nd Edition of the Famous 1597 Work; Thomas Johnson, T., Ed.; Dover Publications: Mineola, NY, USA, 1975. [Google Scholar]
- Janick, J. Herbals: The connection between horticulture and medicine. HortTechnology 2003, 13, 229–238. [Google Scholar] [CrossRef]
- Dafni, A.; Petanidou, T.; Vallianatou, I.; Kozhuharova, E.; Blanche, C.; Pacini, E.; Peyman, M.; Stevanovic, Z.D.; Franchi, G.G.; Benitez, G. Myrtle, basil, rosemary, and three-lobed sage as ritual plants in the monotheistic religions: An historical-ethnobotanical comparison. Econ. Bot. 2020, 74, 330–355. [Google Scholar] [CrossRef]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Dolab, J.G.; Lima, B.; Spaczynska, E.; Kos, J.; Cano, N.H.; Feresin, G.; Tapla, A.; Garibotto, F.; Petenatti, E.; Olivella, M.; et al. Antimicrobial activity of Annona emarginata (Schltdl.) H. Rainer and most active isolated compound against clinically important bacteria. Molecules 2018, 23, 1187. [Google Scholar] [CrossRef]
- Zhang, H.J.; Jampilek, J. Anti-infective drug discovery based on diversified plant natural compounds. Curr. Org. Chem. 2017, 21, 1775–1776. [Google Scholar] [CrossRef]
- Jampilek, J. Design of antimalarial agents based on natural products. Curr. Org. Chem. 2017, 21, 1824–1846. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudkacik-Kramarczyk, S.; Rudnicka, K.; Gatkowska, J.; Sobczak-Kupiec, A.; Jampilek, J. In vitro biosafety of pro-ecological chitosan based hydrogels modified with natural substances. J. Biomed. Mater. Res. A 2019, 107, 2501–2511. [Google Scholar] [CrossRef]
- Do Nascimento, L.D.; de Moraes, A.A.B.; da Costa, K.S.; Galucio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.D.; de Faria, L.J.G. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Nieto, G. A review on applications and uses of Thymus in the food industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Amiri, A.; Mottaghipisheh, J.; Jamshidi-Kia, F.; Saeidi, K.; Vitalini, S.; Iriti, M. Antimicorbial potency of major functional foods’ essential oils in liquid and vapor phases: A short review. Appl. Sci. 2020, 10, 8103. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Saleh Al-Aboody, M.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Wang, M. Bioactive substances of plant origin. In Handbook of Food Chemistry; Cheung, P., Mehta, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 967–1008. [Google Scholar]
- Inoue, M.; Hayashi, S.; Craker, L.E. Role of medicinal and aromatic plants: Past, present, and future. In Pharmacognosy–Medicinal Plants; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: Rijeka, Croatia, 2019; Available online: https://www.intechopen.com/books/pharmacognosy-medicinal-plants/role-of-medicinal-and-aromatic-plants-past-present-and-future (accessed on 15 December 2020).
- Porritt, G.M.; Garlic, F. Christopher’s Herbal Legacy. Available online: https://www.herballegacy.com/Porritt_History.html (accessed on 15 December 2020).
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, D.R.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef] [PubMed]
- Sherman, P.W.; Hash, G.A. Why vegetable recipes are not very spicy. Evol. Hum. Behav. 2001, 22, 147–163. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef]
- Steinhoff, B. Laws and regulation on medicinal and aromatic plants in Europe. Acta Hortic. 2005, 678, 13–22. [Google Scholar] [CrossRef]
- Barbieri, C. Medicinal and aromatic plants legislation in the European Union, Italy and several of its regions. Nat. Prod. Res. 2013, 27, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Nanotechnology Initiative. The National Nanotechnology Initiative Strategic Plan. December. Available online: https://www.nano.gov/sites/default/files/pub_resource/nni_strategic_plan_2004.pdf (accessed on 15 December 2020).
- European Commission. 2011. Recommendation of 18 October 2011on the Definition of Nanomaterial (2011/696/EU). Official Journal of European Union L 275/38. Available online: https://ec.europa.eu/research/industrial_technologies/pdf/policy/commission-recommendation-on-the-definition-of-nanomater-18102011_en.pdf (accessed on 15 December 2020).
- Jampilek, J.; Kralova, K. Nano-antimicrobials: Activity, benefits and weaknesses. In Nanostructures in Therapeutic Medicine, Vol. 2—Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, the Netherlands, 2017; pp. 23–54. [Google Scholar]
- Jampilek, J.; Kralova, K. Impact of nanoparticles on toxigenic fungi. In Nanomycotoxicology, Treating Mycotoxins in the Nano Way; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press, Elsevier, 2020; pp. 309–348. [Google Scholar]
- Jampilek, J.; Kralova, K. Nanocomposites: Synergistic nanotools for management of mycotoxigenic fungi. In Nanomycotoxicology, Treating Mycotoxins in the Nano Way; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Jampilek, J.; Kralova, K.; Fedor, P. Bioactivity of nanoformulated synthetic and natural insecticides and their impact on environment. In Nanopesticides; Fraceto, L., de Castro, V.S.S., Grillo, R., Ávila, D., Caixeta Oliveira, H., Lima, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 165–225. [Google Scholar]
- Tarrahi, R.; Mahjouri, S.; Khataee, A. A review on in vivo and in vitro nanotoxicological studies in plants: A headlight for future targets. Environ. Ecotoxicol. Environ. Saf. 2021, 208, 111697. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kos, J.; Kralova, K. Potential of nanomaterial applications in dietary supplements and foods for special medical purposes. Nanomaterials 2019, 9, 296. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Potential of nanonutraceuticals in increasing immunity. Nanomaterials 2020, 10, 2224. [Google Scholar] [CrossRef]
- Placha, D.; Jampilek, J. Graphenic materials for biomedical applications. Nanomaterials 2019, 9, 1758. [Google Scholar] [CrossRef] [PubMed]
- Placha, D.; Jampilek, J. Chronic inflammatory diseases, anti-inflammatory agents and their delivery nanosystems. Pharmaceutics 2021, 13, 64. [Google Scholar] [CrossRef]
- Vaculikova, E.; Grunwaldova, V.; Kral, V.; Dohnal, J.; Jampilek, J. Preparation of candesartan and atorvastatin nanoparticles by solvent evaporation. Molecules 2012, 17, 13221. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.S.H.; Barhoum, A. Fundamentals of Nanoparticles: Classifications, Synthesis Methods, Properties and Characterization; Elsevier: Amsterdam, the Netherlands, 2018. [Google Scholar]
- Luque, R.; Varma, R.S. Sustainable Preparation of Metal. Nanoparticles: Methods and Applications; Royal Society of Chemistry: Cambridge, UK, 2012. [Google Scholar]
- Sharon, M. History of Nanotechnology: From Prehistoric to Modern Times; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Shukla, A.K.; Iravani, S. Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, the Netherlands, 2019. [Google Scholar]
- Jampilek, J.; Kralova, K. Application of nanobioformulations for controlled release and targeted biodistribution of drugs. In Nanobiomaterials: Applications in Drug Delivery; Sharma, A.K., Keservani, R.K., Kesharwani, R.K., Eds.; CRC Press: Warentown, NJ, USA, 2018; pp. 131–208. [Google Scholar]
- Jampilek, J.; Kralova, K. Nanopesticides: Preparation, targeting and controlled release. In New Pesticides and Soil Sensors in Nanotechnology in the Agri-Food Industry; Grumezescu, A.M., Ed.; Elsevier: London, UK, 2017; Volume 10, pp. 81–127. [Google Scholar]
- Jampilek, J.; Kralova, K. Benefits and potential risks of nanotechnology applications in crop protection. In Nanobiotechnology Applications in Plant Protection; Abd-Elsalam, K., Prasad, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 189–246. [Google Scholar]
- Jampilek, J.; Kralova, K. Beneficial effects of metal- and metalloid-based nanoparticles on crop production. In Nanotechnology for Agriculture; Panpatte, D.G., Jhala, Y.K., Eds.; Springer Nature: Singapore, 2019; pp. 161–219. [Google Scholar]
- Jampilek, J.; Kralova, K. Nanoparticles for improving and augmenting plant functions. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., Lima, R., Eds.; Elsevier: Amsterdam, the Netherlands, 2021; pp. 171–227. [Google Scholar]
- Jampilek, J.; Kralova, K. Impact of nanoparticles on photosynthesizing organisms and their use in hybrid structures with some components of the photosynthetic apparatus. In Plant Nanobionics; Prasad, R., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 255–331. [Google Scholar]
- Jampilek, J.; Kralova, K. Application of nanotechnology in agriculture and food industry, its prospects and risks. Ecol. Chem. Eng. S 2015, 22, 321–361. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Nanomaterials for delivery of nutrients and growth-promoting compounds to plants. In Nanotechnology: An Agricultural Paradigm; Prasad, R., Kumar, M., Kumar, V., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2017; pp. 177–226. [Google Scholar]
- Masarovicova, E.; Kralova, K. Metal nanoparticles and plants. Ecol. Chem. Eng. S 2013, 20, 9–22. [Google Scholar]
- Masarovicova, E.; Kralova, K.; Zinjarde, S.S. Metal nanoparticles in plants. Formation and action. In Handbook of Plant and Crop Physiology, 3rd ed.; Pessarakli, M., Ed.; CRC: Boca Raton, FL, USA; Taylor and Francis: Boca Raton, FL, USA, 2014; pp. 683–731. [Google Scholar]
- Kralova, K.; Masarovicova, E.; Jampilek, J. Plant responses to stress induced by toxic metals and their nanoforms. In Handbook of Plant and Crop Stress, 4th ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 479–522. [Google Scholar]
- Oliveira, H.C.; Stolf-Moreira, R.; Martinez, C.B.R.; Grillo, R.; de Jesus, M.B.; Fraceto, L.F. Nanoencapsulation enhances the post-emergence herbicidal activity of atrazine against nustard plants. PLoS ONE 2015, 10, e0132971. [Google Scholar] [CrossRef]
- Bombo, A.B.; Pereira, A.E.S.; Lusa, M.G.; Oliveira, E.D.; de Oliveira, J.L.; Campos, E.V.R.; de Jesus, M.B.; Oliveira, H.C.; Fraceto, L.F.; Mayer, J.L.S. A mechanistic view of interactions of a nanoherbicide with target organism. J. Agric. Food Chem. 2019, 67, 4453–4462. [Google Scholar] [CrossRef]
- Balah, M.A.; Pudake, R.N. Use nanotools for weed control and exploration of weed plants in nanotechnology. In Nanoscience for Sustainable Agriculture; Pudake, R.N., Chauhan, N., Kole, C., Eds.; Springer Nature: Basel, Switzerland, 2019; pp. 207–231. [Google Scholar]
- Patel, K.V.; Nath, M.; Bhatt, M.D.; Dobriyal, A.K.; Bhatt, D. Nanofomulation of zinc oxide and chitosan zinc sustain oxidative stress and alter secondary metabolite profile in tobacco. 3Biotech 2020, 10, 477. [Google Scholar] [CrossRef]
- Ma, Y.J.; Xia, J.; Wang, Y.; Wang, J.W. Stimulation of tanshinone production in Salvia miltiorrhiza hairy roots by beta-cyclodextrin-coated silver nanoparticles. Sustain. Chem. Pharm. 2020, 18, 100271. [Google Scholar] [CrossRef]
- Singh, R.; Singh, D.P.; Gupta, P.; Jain, P.; Sanchita; Mishra, T.; Kumar, A.; Dhawan, S.S.; Shirke, P.A. Nanoparticles alter the withanolide biosynthesis and carbohydrate metabolism in Withania somnifera (Dunal). Ind. Crops Prod. 2019, 127, 94–109. [Google Scholar] [CrossRef]
- Kralova, K.; Masarovicova, E.; Jampilek, J. Risks and benefits of metal-based nanoparticles for vascular plants. In Handbook of Pant and Crop Physiology, 4th ed.; Pessarakli, M., Ed.; Taylor & Francis: Abingdon, UK, 2021; in press. [Google Scholar]
- Moharrami, F.; Hosseini, B.; Sharafi, A.; Farjaminezhad, M. Enhanced production of hyoscyamine and scopolamine from genetically transformed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. In Vitro Cell. Dev. Biol. Plant 2017, 3, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Anjum, I.; Hano, C.; Kousar, S. Advances in nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites: Current status and future outlooks. RSC Adv. 2019, 9, 40404–40423. [Google Scholar] [CrossRef]
- Asl, K.R.; Hosseini, B.; Sharafi, A.; Palazon, J. Influence of nano-zinc oxide on tropane alkaloid production, h6h gene transcription and antioxidant enzyme activity in Hyoscyamus reticulatus L. hairy roots. Eng. Life Sci. 2019, 19, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Linh, T.M.; Mai, N.C.; Hoe, P.T.; Lien, L.Q.; Ban, N.K.; Hien, L.T.T.; Chau, N.H.; Van, N.T. Metal-based nanoparticles enhance drought tolerance in soybean. J. Nanomater. 2020, 2020, 4056563. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; Rehman, M.Z.U.; Hussain, A.; Hussain, K.; Chatha, S.A.S.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Perez-Alvarez, M.; Hernandez-Fuentes, A.D.; de la Fuente, M.C.; Benavides-Mendoza, A.; Valdes-Reyna, J.; Juarez-Maldonado, A. Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Guo, H.Y.; Wang, S.X.; Li, J.L.; Wang, Y.Q.; Xing, B.S. Carbon dots alleviate the toxicity of cadmium ions (Cd2+) toward wheat seedlings. Environ. Sci. Nano 2019, 6, 1493–1506. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant. Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Goswami, P.; Yadav, S.; Mathur, J. Positive and negative effects of nanoparticles on plants and their applications in agriculture. Plant. Sci. Today 2019, 6, 232–234. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Mazarji, M.; Shende, S.; Sushkova, S.; Mandzhieva, S.; Burachevskaya, M.; Chaplygin, V.; Singh, A.; Jatav, H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agric. Sci. 2020, 65, 137–143. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Sushkova, S.; Behal, A.; Maksimov, A.; Blicharska, E.; Ghazaryan, K.; Movsesyan, H.; Barsova, N. ZnO and CuO nanoparticles: A threat to soil organisms, plants, and human health. Environ. Geochem. Health 2020, 42, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Masarovicova, E.; Kralova, K. Essential elements and toxic metals in some crops, medicinal plants, and trees. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; Volume 5, pp. 183–255. [Google Scholar]
- Masarovicova, E.; Kralova, K.; Sersen, F. Plant responses to toxic metal stress. In Handbook of Plant and Crop Stress, 3rd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 595–634. [Google Scholar]
- Masarovicova, E.; Kralova, K. Plant-heavy metal interaction: Phytoremediation, biofortification and nanoparticles. In Advances in Selected Plant. Physiology Aspects; Montanaro, G., Dichio, B., Eds.; InTech: Rijeka, Croatia, 2012; pp. 75–102. [Google Scholar]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Li, Y.D.; Xu, X.K.; Wu, Y.; Zhuang, J.L.; Zhang, X.J.; Zhang, H.R.; Lei, B.F.; Hu, C.F.; Liu, Y.L. A review on the effects of carbon dots in plant systems. Mater. Chem. Front. 2020, 4, 437–448. [Google Scholar]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Pichtel, J.; Hayat, S. Nanoparticles: Biosynthesis, translocation and role in plant metabolism. IET Nanobiotechnol. 2019, 13, 345–352. [Google Scholar] [CrossRef]
- Szollosi, R.; Molnar, A.; Kondak, S.; Kolbert, Z. Dual effect of nanomaterials on germination and seedling growth: Stimulation vs. phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef]
- Masarovicova, E.; Kralova, K.; Kummerova, M. Principles of classification of medicinal plants as hyperaccumulators or excluders. Acta Physiol. Plant. 2010, 32, 823–829. [Google Scholar] [CrossRef]
- Salamon, I.; Kralova, K.; Masarovicova, E. Accumulation of cadmium in chamomile plants cultivated in Eastern Slovakia regions. Acta Hort (ISHS) 2007, 749, 217–222. [Google Scholar] [CrossRef]
- Mehrian, S.K.; De Lima, R. Nanoparticles cyto and genotoxicity in plants: Mechanisms and abnormalities. Environ. Nanotechnol. Monit. Manag. 2016, 6, 184–193. [Google Scholar]
- Koca, F.D.; Duman, F. Genotoxic and cytotoxic activity of green synthesized TiO2 nanoparticles. Appl. Nanosci. 2019, 9, 815–823. [Google Scholar] [CrossRef]
- Liman, R.; Basbug, B.; Ali, M.M.; Acikbas, Y.; Cigerci, I.H. Cytotoxic and genotoxic assessment of tungsten oxide nanoparticles in Allium cepa cells by Allium ana-telophase and comet assays. J. Appl. Genet. 2021, 62, 85–92. [Google Scholar] [CrossRef]
- Youssef, M.S.; Elamawi, R.M. Evaluation of phytotoxicity, cytotoxicity, and genotoxicity of ZnO nanoparticles in Vicia faba. Environ. Sci. Pollut. Res. 2020, 27, 18972–18984. [Google Scholar] [CrossRef]
- Heikal, Y.M.; Sutan, N.A.; Rizwan, M.; Elsayed, A. Green synthesized silver nanoparticles induced cytogenotoxic and genotoxic changes in Allium cepa L. varies with nanoparticles doses and duration of exposure. Chemosphere 2020, 243, 125430. [Google Scholar] [CrossRef]
- Rui, M.M.; Ma, C.X.; Hao, Y.; Guo, J.; Rui, Y.K.; Tang, X.L.; Zhao, Q.; Fan, X.; Zhang, Z.T.; Hou, T.Q.; et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant. Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Alshaal, T.; El-Henawy, A.; Elmahrouk, M.; Bayoumi, Y.; Shalaby, T.; Amer, M.; Shehata, S.; Fari, M.; et al. Plant nano-nutrition: Perspectives and challenges. In Nanotechnology, Food Security and Water Treatment; Book Series: Environmental Chemistry for a Sustainable, World; Gothandam, K.M., Ranjan, S., Dasgupta, N., Ramalingam, C., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 11, pp. 129–161. [Google Scholar]
- Ju, M.; Navarreto-Lugo, M.; Wickramasinghe, S.; Milbrandt, N.B.; McWhorter, A.; Samia, A.C.S. Exploring the chelation-based plant strategy for iron oxide nanoparticle uptake in garden cress (Lepidium sativum) using magnetic particle spectrometry. Nanoscale 2019, 11, 18582–18594. [Google Scholar] [CrossRef] [PubMed]
- Abbasifar, A.; Shahrabadi, F.; ValizadehKaji, B. Effects of green synthesized zinc and copper nano-fertilizers on the morphological and biochemical attributes of basil plant. J. Plant. Nutr. 2020, 43, 1104–1118. [Google Scholar] [CrossRef]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of carbon nanomaterials in the plant system: A perspective view on the pros and cons. Sci. Total Environ. 2017, 667, 485–499. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Dervishi, E.; Chen, J.H.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Tripathi, S.; Kapri, S.; Datta, A.; Bhattacharyya, S. Influence of the morphology of carbon nanostructures on the stimulated growth of gram plant. RSC Adv. 2016, 6, 43864–43873. [Google Scholar] [CrossRef]
- Mathew, S.; Tiwari, D.K.; Tripathi, D. Interaction of carbon nanotubes with plant system: A review. Carbon Lett. 2020, in press. [Google Scholar] [CrossRef]
- Patel, D.K.; Kim, H.B.; Dutta, S.D.; Ganguly, K.; Lim, K.T. Carbon nanotubes-nased manomaterials and their agricultural and biotechnological applications. Materials 2020, 13, 1679. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; Kim, B.S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef]
- Vithanage, M.; Seneviratne, M.; Ahmad, M.; Sarkar, B.; Ok, Y.S. Contrasting effects of engineered carbon nanotubes on plants: A review. Environ. Geochem. Health 2017, 39, 1421–1439. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sun, W.; Zhu, Y.G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant. Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in agriculture: A nutrient or contaminant for crops? Arch. Agron. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Use of Nanofertilizers in Agriculture: Advantages and Safety Concerns. Dextra International. 2020. Available online: http://www.dextrainternational.com/use-of-nanofertilizers-in-agriculture-advantages-and-safety-concerns (accessed on 15 December 2020).
- Kopittke, P.M.; Lombi, E.; Wang, P.; Schjoerring, J.K.; Husted, S. Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. Nano 2019, 6, 3513–3524. [Google Scholar] [CrossRef]
- Santiago, E.F.; Pontes, M.S.; Arruda, G.J.; Caires, A.R.L.; Colbeck, I.; Maldonado-Rodriguez, R.; Grillo, R. Understanding the interaction of nanopesticides with plants. In Nanopesticides; Fraceto, L.F., de Castro, V.L., Grillo, R., Avila, D., Caixeta Oliveira, H., Lima, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 69–109. [Google Scholar]
- Bundschuh, M.; Filser, J.; Luderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [PubMed]
- Paramo, L.A.; Feregrino-Perez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in agroindustry: Applications, toxicity, challenges, and trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Jasim, B.; Thomas, R.; Mathew, J.; Radhakrishnan, E.K. Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm. J. 2017, 25, 443–447. [Google Scholar] [CrossRef]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A., Shanker, C., Eds.; IntechOpen: Rijeka, Croatia, 2016; pp. 247–278. [Google Scholar]
- Javed, R.; Yucesan, B.; Zia, M.; Gurel, E. Elicitation of secondary metabolites in callus cultures of Stevia rebaudiana Bertoni grown under ZnO and CuO nanoparticles stress. Sugar Tech. 2018, 20, 194–201. [Google Scholar] [CrossRef]
- Kavianifar, S.; Ghodrati, K.; Naghdi Badi, H.; Etminan, A. Effects of nano elicitors on callus induction and mucilage production in tissue culture of Linum usitatissimum L. J. Med. Plant. 2018, 17, 45–54. [Google Scholar]
- Abraham, J.; Thomas, T.D. Hairy root culture for the production of useful secondary metabolites. In Biotechnology and Production of Anti-Cancer Compounds; Malik, S., Ed.; Springer: Cham, Switzerland, 2017; pp. 201–230. [Google Scholar]
- Dhiman, N.; Patial, V.; Bhattacharya, A. The current status and future applications of hairy root cultures. In Biotechnological Approaches for Medicinal and Aromatic Plants; Kumar, N., Ed.; Springer: Singapore, 2018; pp. 87–155. [Google Scholar]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Elicitation of silver nanoparticles enhanced the secondary metabolites and pharmacological activities in cell suspension cultures of bitter gourd. 3 Biotech 2019, 8, 412. [Google Scholar] [CrossRef]
- Ramezannezhad, R.; Aghdasi, M.; Fatemi, M. Enhanced production of cichoric acid in cell suspension culture of Echinacea purpurea by silver nanoparticle elicitation. Plant. Cell Tissue Organ. Cult. 2019, 139, 261–273. [Google Scholar] [CrossRef]
- Karakas, O. Effect of silver nanoparticles on production of indole alkaloids in Isatis constricta. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 621–627. [Google Scholar] [CrossRef]
- Ali, A.; Mohammad, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z.U.R. Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma tuberculata. Artif. Cells Nanomed. Biotechnol. 2019, 47, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.; Nadeem, M.; Ahmad, W.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Chemogenic silver nanoparticles enhance lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Plant. Cell Tissue Organ. Cult. 2019, 136, 589–596. [Google Scholar] [CrossRef]
- Golkar, P.; Moradi, M.; Garousi, G.A. Elicitation of Stevia glycosides using salicylic acid and silver nanoparticles under callus culture. Sugar Tech. 2019, 21, 569–577. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Perez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant. Cell Tissue Organ. Cult. 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Farida, S.H.M.; Karamian, R.; Albrectsen, B.R. Silver nanoparticle pollutants activate oxidative stress responses and rosmarinic acid accumulation in sage. Physiol. Plant 2020, in press. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M.; Ali, S.S.; Khan, A.; Wei, D.Q. Sustainable production of biomass and industrially important secondary metabolites in cell cultures of selfheal (Prunella vulgaris L.) elicited by silver and gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, A.; Jadczak, P.; Kulpa, D.; Przewodowski, W. Gas chromatography-mass spectrometry (GC-MS) analysis of essential oils from AgNPs and AuNPs elicited Lavandula angustifolia in vitro cultures. Molecules 2019, 24, 606. [Google Scholar] [CrossRef]
- Hussain, M.; Raja, N.I.; Mashwani, Z.U.R.; Iqbal, M.; Sabir, S.; Yasmeen, F. In vitro seed germination and biochemical profiling of Artemisia absinthium exposed to various metallic nanoparticles. 3 Biotech 2017, 7, 101. [Google Scholar] [CrossRef]
- Sharifi-Rad, R.; Esmaeilzadeh, B.S.; Samzadeh-Kermani, A.; Gholami, M. The effect of non-biological elicitors on physiological and biochemical properties of medicinal plant Momordica charantia L. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 1315–1326. [Google Scholar] [CrossRef]
- Lafmejani, Z.N.; Jafari, A.A.; Moradi, P.; Moghadam, A.L. Impact of foliar application of iron-chelate and iron nano particles on some morpho-physiological traits and essential oil composition of peppermint (Mentha piperita L.). J. Essent. Oil-Bear. Plants 2018, 21, 1374–1384. [Google Scholar] [CrossRef]
- Ghasemi, B.; Hosseini, R.; Dehghan Nayeri, F. Effects of cobalt nanoparticles on artemisinin production and gene expression in Artemisia annua. Turk. J. Bot. 2015, 39, 769–777. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Ao, Q.; Yang, Y.S. Engineered ZnO and CuO nanoparticles ameliorate morphological and biochemical response in tissue culture regenerants of candyleaf (Stevia rebaudiana). Molecules 2020, 25, 1356. [Google Scholar] [CrossRef]
- Chung, I.M.; Rajakumar, G.; Subramanian, U.; Venkidasamy, B.; Thiruvengadam, M. Impact of copper oxide nanoparticles on enhancement of bioactive compounds using cell suspension cultures of Gymnema sylvestre (Retz.) R. Br. Appl. Sci. 2019, 9, 2165. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Yucesan, B.; Zia, M.; Gurel, E. Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant. Physiol. Biochem. 2017, 110, 94–99. [Google Scholar] [CrossRef]
- Zaeem, A.; Drouet, S.; Anjum, S.; Khursihd, R.; Younas, M.; Blondeau, J.P.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Effects of biogenic zinc oxide nanoparticles on growth and oxidative stress response in flax seedlings vs. in vitro cultures: A comparative analysis. Biomolecules 2020, 10, 918. [Google Scholar] [CrossRef]
- Velazquez-Gamboa, M.C.; Rodriguez, H.L.; Abud-Archila, M.; Gutierrez-Miceli, F.A.; Gonzalez-Mendoza, D.; Valdez-Salas, B.; Gonzalez-Terreros, E.; Lujan-Hidalgo, M.C. Agronomic biofortification of Stevia rebaudiana with zinc oxide (ZnO) phytonanoparticles and antioxidant compounds. Sugar Tech. 2020, in press. [Google Scholar] [CrossRef]
- Karimzadeh, F.; Haddad, R.; Garoosi, G.; Khademian, R. Effects of nanoparticles on activity of lignan biosynthesis enzymes in cell suspension culture of Linum usitatissimum L. Russ. J. Plant. Physiol. 2019, 66, 756–762. [Google Scholar] [CrossRef]
- Sharafi, E.; Fotokian, M.H.; Davoodi, D.; Hasanloo, H.H. Improvement of hypericin and hyperforin production using zinc and iron nano-oxides as elicitors in cell suspension culture of St John’s wort (Hypericum perforatum L.). JMPB 2013, 2, 177–184. [Google Scholar]
- Mohasseli, V.; Farbood, F.; Moradi, A. Antioxidant defense and metabolic responses of lemon balm (Melissa officinalis L.) to Fe-nano-particles under reduced irrigation regimes. Ind. Crops Prod. 2020, 149, 112338. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Nasibi, F.; Kalantari, K.M.; Ghanati, F. Evaluation of secondary metabolites and antioxidant activity in Dracocephalum polychaetum Bornm. cell suspension culture under magnetite nanoparticles and static magnetic field elicitation. Plant. Cell Tissue Organ. Cult. 2019, 136, 489–498. [Google Scholar] [CrossRef]
- Ahmad, B.; Shabbir, A.; Jaleel, H.; Masroor, M.; Khan, A.; Sadiq, Y. Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr. Plant. Biol. 2018, 13, 6–15. [Google Scholar] [CrossRef]
- Shabbir, A.; Khan, M.M.A.; Ahmad, B.; Sadiq, Y.; Jaleel, H.; Uddin, M. Efficacy of TiO2 nanoparticles in enhancing the photosynthesis, essential oil and khusimol biosynthesis in Vetiveria zizanioides L. Nash. Photosynthetica 2019, 57, 599–606. [Google Scholar] [CrossRef]
- Ghorbanpour, M. Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Indian J. Plant. Physiol. 2015, 20, 249–256. [Google Scholar] [CrossRef]
- Rezaizad, M.; Hashemi-Moghaddam, H.; Abbaspour, H.; Gerami, M.; Mueller, A. Photocatalytic effect of TiO2 nanoparticles on morphological and photochemical properties of stevia plant (Stevia rebaudiana Bertoni). Sugar Tech. 2019, 21, 1024–1030. [Google Scholar] [CrossRef]
- Modarresi, M.; Chahardoli, A.; Karimi, N.; Chahardoli, S. Variations of glaucine, quercetin and kaempferol contents in Nigella arvensis against Al2O3, NiO, and TiO2 nanoparticles. Heliyon 2020, 6, e04265. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Kamalizadeh, M.; Bihamta, M.; Zarei, A. Drought stress and TiO2 nanoparticles affect the composition of different active compounds in the Moldavian dragonhead plant. Acta Physiol. Plant. 2019, 41, 21. [Google Scholar] [CrossRef]
- Tian, H.; Ghorbanpour, M.; Kariman, K. Manganese oxide nanoparticle-induced changes in growth, redox reactions and elicitation of antioxidant metabolites in deadly nightshade (Atropa belladonna L.). Ind. Crops Prod. 2018, 126, 403–414. [Google Scholar] [CrossRef]
- Ahmad, B.; Khan, M.M.A.; Jaleel, H.; Shabbir, A.; Sadiq, Y.; Uddin, M. Silicon nanoparticles mediated increase in glandular trichomes and regulation of photosynthetic and quality attributes in Mentha piperita L. J. Plant. Growth Regul. 2020, 39, 346–357. [Google Scholar] [CrossRef]
- Torabzadeh, D.; Hassabpour, H.; Asgarpanah, J.; Rezayian, M. Nanoparticles induced antioxidative compounds in Matricaria chamomilla. Iran. J. Plant. Physiol. 2019, 9, 2955–2961. [Google Scholar]
- Ebadollahi, R.; Jafarirad, S.; Kosari-Nasab, M.; Mahjouri, S. Effect of explant source, perlite nanoparticles and TiO2/perlite nanocomposites on phytochemical composition of metabolites in callus cultures of Hypericum perforatum. Sci. Rep. 2019, 9, 12998. [Google Scholar] [CrossRef]
- Rahmani, N.; Radjabian, T.; Soltani, B.M. Impacts of foliar exposure to multi-walled carbon nanotubes on physiological and molecular traits of Salvia verticillata L., as a medicinal plant. Plant. Physiol. Biochem. 2020, 150, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, M.; Iranbakhsh, A.; Ebadi, M.; Ardebili, Z.O. Multi-walled carbon nanotubes improved growth, anatomy, physiology, secondary metabolism, and callus performance in Catharanthus roseus: An in vitro study. 3 Biotech 2019, 9, 404. [Google Scholar] [CrossRef]
- Samadi, S.; Saharkhiz, M.J.; Azizi, M.; Samiei, L.; Ghorbanpour, M. Multi-walled carbon nanotubes stimulate growth, redox reactions and biosynthesis of antioxidant metabolites in Thymus daenensis celak. in vitro. Chemosphere 2020, 249, 126069. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J. Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 2015, 94, 749–759. [Google Scholar] [CrossRef]
- Heydari, H.R.; Chamani, E.; Esmaielpour, B. Cell line selection through gamma irradiation combined with multi-walled carbon nanotubes elicitation enhanced phenolic compounds accumulation in Salvia nemorosa cell culture. Plant. Cell Tissue Organ. Cult. 2020, 142, 353–367. [Google Scholar] [CrossRef]
- Ahmadi, S.Z.; Ghorbanpour, M.; Aghaee, A.; Hadian, J. Deciphering morpho-physiological and phytochemical attributes of Tanacetum parthenium L. plants exposed to C60 fullerene and salicylic acid. Chemosphere 2020, 259, 127406. [Google Scholar] [CrossRef]
- Kole, C.; Kole, P.; Randunu, K.M.; Choudhary, P.; Podila, R.; Ke, P.C.; Rao, A.M.; Marcus, R.K. Nanobiotechnology can boost crop production and quality: First evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol. 2013, 13, 37. [Google Scholar] [CrossRef]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Mandoulakani, B.A. Iron oxide nanoparticles: A novel elicitor to enhance anticancer flavonoid production and gene expression in Dracocephalum kotschyi hairy-root cultures. J. Sci. Food Agric. 2019, 99, 6418–6430. [Google Scholar] [CrossRef] [PubMed]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Mandoulakani, B.A. Pharmaceutical important phenolic compounds overproduction and gene expression analysis in Dracocephalum kotschyi hairy roots elicited by SiO2 nanoparticles. Ind. Crops Prod. 2019, 133, 435–446. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.P.; Li, W.Y.; Wang, J.W. Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr. Nanosci. 2013, 9, 363–370. [Google Scholar] [CrossRef]
- Shakeran, Z.; Keyhanfar, M.; Asghari, G.; Ghanadian, M. Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel. Turk. J. Biol. 2015, 39, 111–118. [Google Scholar] [CrossRef]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of bioactive compounds and gene expression alterations in hairy root cultures of chinese cabbage elicited by copper oxide nanoparticles. Plant. Cell Tissue Organ. Cult. 2018, 134, 95–106. [Google Scholar] [CrossRef]
- Juarez-Maldonado, A.; Ortega-Ortiz, H.; Morales-Diaz, A.B.; Gonzalez-Morales, S.; Morelos-Moreno, A.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theoret. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Delgoda, R.; Murray, J.E. Evolutionary Perspectives on the Role of Plant Secondary Metabolites. In Pharmacognosy: Fundamentals, Applications and Strategies; Badal, S., Delgoda, R., Eds.; Academic Press & Elsevier: London, UK, 2017; pp. 93–100. [Google Scholar]
- Mottaki, Z.; Rezayian, M.; Niknam, V.; Ebrahimzadeh, H.; Mirmasoumi, M. Using hairy roots for production of secondary metabolites in Artemisia. Plant. Biotechnol. Rep. 2019, 13, 263–271. [Google Scholar] [CrossRef]
- Mosavat, N.; Golkar, P.; Yousefifard, M.; Javed, R. Modulation of callus growth and secondary metabolites in different Thymus species and Zataria multiflora micropropagated under ZnO nanoparticles stress. Biotechnol. Appl. Biochem. 2019, 66, 316–322. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Pistelli, L.; Giovannini, A.; Ruffoni, B.; Bertoli, A.; Pistelli, L. Hairy root cultures for secondary metabolites production. Adv. Exp. Med. Biol. 2010, 698, 167–184. [Google Scholar]
- Sharma, P.; Radh, H.; Shrivastava, N. Hairy root cultures: A suitable biological system for studying secondary metabolic pathways in plants. Eng. Life Sci. 2013, 13, 62–75. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Moyano, E.; Cusido, R.M.; Oksman-Caldentey, K.M. Exploring the Metabolic stability of engineered hairy roots after 16 years maintenance. Front. Plant. Sci. 2016, 7, 1486. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, A.; Hosseini, B.; Palazon, J.; Maleki, R. Improved tropane alkaloid production and changes in gene expression in hairy root cultures of two Hyoscyamus species elicited by silicon dioxide nanoparticles. Plant. Physiol. Biochem. 2020, 155, 416–428. [Google Scholar] [CrossRef]
- Tkalec, M.; Stefanic, P.P.; Balen, B. Phytotoxicity of silver nanoparticles and defence mechanisms. Compr. Anal. Chem. 2019, 84, 145–198. [Google Scholar]
- Khalaki, M.A.; Ghorbani, A.; Moameri, M. Effects of silica and silver nanoparticles on seed germination traits of Thymus kotschyanus in laboratory conditions. J. Rangel. Sci. 2016, 6, 221–231. [Google Scholar]
- Darvishzadeh, F.; Nejatzadeh, F.; Iranbakhsh, A.R. Effects of silver nanoparticles on salinity tolerance in basil plant (Ocimum basilicum L.) during germination in vitro. New Cell. Nilecular Biotechnol. J. 2015, 5, 63–70. [Google Scholar]
- Jadczak, P.; Kulpa, D.; Bihun, M.; Przewodowski, W. Positive effect of AgNPs and AuNPs in in vitro cultures of Lavandula angustifolia Mill. Plant. Cell Tissue Organ. Cult. 2019, 139, 191–197. [Google Scholar] [CrossRef]
- Sahandi, S.M.; Sorooshzadeh, A.; Rezazadeh, H.S.; Naghdibadi, H.A. Effect of nano silver and silver nitrate on seed yield of borage. J. Med. Plant. Res. 2011, 5, 706–710. [Google Scholar]
- Seifsahandi, M.; Sorooshzadeh, A. Comparison between the influences of silver nanoparticles and silver nitrate on the growth and phytochemical properties of borage (Borago officinalis L). Curr. Nanosci. 2013, 9, 241–247. [Google Scholar] [CrossRef]
- Pastelin-Solano, M.C.; Ramirez-Mosqueda, M.A.; Bogdanchikova, N.; Castro-Conzalez, C.G.; Bello-Bello, J.J. Silver nanoparticles affect the micropropagation of vanilla (Vanilla planifolia Jacks. ex Andrews). Agrociencia 2020, 54, 1–13. [Google Scholar]
- Timoteo, C.D.; Paiva, R.; dos Reis, M.V.; Claro, P.I.C.; da Silva, D.P.C.; Marconcini, J.M.; de Oliveira, J.E. Silver nanoparticles in the micropropagation of Campomanesia rufa (O. Berg) Nied. Plant. Cell Tissue Organ. Cult. 2019, 137, 359–368. [Google Scholar] [CrossRef]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Influence of silver nanoparticles on the enhancement and transcriptional changes of glucosinolates and phenolic compounds in genetically transformed root cultures of Brassica rapa ssp rapa. Bioprocess. Biosyst. Eng. 2018, 41, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull. Natl. Res. Cent. 2019, 43, 38. [Google Scholar] [CrossRef]
- Soltanabad, M.H.; Bagherieh-Najjar, M.B.; Mianabadi, M. Carnosic acid content increased by silver nanoparticle treatment in rosemary (Rosmarinus officinalis L.). Appl. Biochem. Biotechnol. 2020, 191, 482–495. [Google Scholar] [CrossRef]
- Anum, F.; Raja, N.I.; Hussain, M.; Iqbal, M.; Chaudhari, S.K.; Ehsan, M.; Javaid, U.; Zafar, N.U. Effect of green synthesised silver nanoparticles on morphogenic and biochemical variations in callus cultures of kinnow mandarin (Citrus reticulata L.). IET Nanobiotechnol. 2019, 13, 541–545. [Google Scholar] [CrossRef]
- Saha, N.; Gupta, S.D. Promotion of shoot regeneration of Swertia chirata by biosynthesized silver nanoparticles and their involvement in ethylene interceptions and activation of antioxidant activity. Plant. Cell Tissue Organ. Cult. 2018, 134, 289–300. [Google Scholar] [CrossRef]
- Jalilzadeh, E.; Jamei, E.; Sarghein, S.H. Magnetic field and silver nanoparticles induced changes on phenolic compound and oxidative status of marigold seedlings. J. Plant. Physiol. Breed. 2018, 8, 75–88. [Google Scholar]
- Ghanati, F.; Bakhtiarian, S. Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula officinalis L (Asteraceae). Trop. J. Pharm. Res. 2014, 13, 1783–1789. [Google Scholar] [CrossRef]
- Lawal, A.T.; Azeez, L.; Sulaiman, W.K. Silver nanoparticles (AgNPs) alleviate naphthalene-triggered oxidative stress and physiological deficiencies in Moringa oleifera. Chem. Ecol. 2020, in press. [Google Scholar] [CrossRef]
- Cvjetko, P.; Milosic, A.; Domijan, A.M.; Vrcek, I.V.; Tolic, S.; Stefanic, P.P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotox. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef]
- Ghavam, M. Effect of silver nanoparticles on tolerance to drought stress in Thymus daenensis Celak and Thymus vulgaris L. in germination and early growth stages. Environ. Stress. Crop. Sci. 2019, 12, 555–566. [Google Scholar]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Hafez, R.M.; Fouad, A.S. Mitigation of genotoxic and cytotoxic effects of silver nanoparticles on onion root tips using some antioxidant scavengers. Egypt. J. Bot. 2020, 60, 133–145. [Google Scholar] [CrossRef]
- Durairaj, K.; Roy, B.; Chandrasekaran, N.; Krishnan, S.P.; Mukherjee, A. Silver nanorods induced oxidative stress and chromosomal aberrations in the Allium cepa model. IET Nanobiotechnol. 2020, 14, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.R.; Silva, L.R.; Fernandes, L.S.P.; Salgado, L.D.; Silva, H.C.D.; Firak, D.S.; Bach, L.; Santos-Filho, R.; Voigt, C.L.; Barros, A.C. Visible-light reduced silver nanoparticles’ toxicity in Allium cepa test system. Environ. Pollut. 2020, 257, 113551. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Spinoso-Castillo, J.L.; Arano-Avalos, S.; Martinez-Estrada, E.; Arellano-Garcia, M.E.; Pestryakov, A.; Toledano-Magana, Y.; Garcia-Ramos, J.C.; Bogdanchikova, N. Cytotoxic, genotoxic, and polymorphism effects on Vanilla planifolia Jacks ex Andrews after long-term exposure to Argovit® silver nanoparticles. Nanomaterials 2018, 8, 754. [Google Scholar] [CrossRef]
- Rawat, S.; Adisa, I.O.; Wang, Y.; Sun, Y.P.; Fadil, A.S.; Niu, G.H.; Sharma, N.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Differential physiological and biochemical impacts of nano vs micron Cu at two phenological growth stages in bell pepper (Capsicum annuum) plant. Nanoimpact 2019, 14, 100161. [Google Scholar] [CrossRef]
- Ghidan, A.Y.; Al-Antary, T.M.; Awwad, A.M.; Ayad, J.Y. Physiological effect of some nanomaterials on pepper (Capsicum annuum L.) plants. Fresen. Environ. Bull. 2018, 27, 7872–7878. [Google Scholar]
- Rawat, S.; Pullagurala, V.L.R.; Hernandez-Molina, M.; Sun, Y.P.; Niu, G.H.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Impacts of copper oxide nanoparticles on bell pepper (Capsicum annum L.) plants: A full life cycle study. Environ. Sci. Nano 2018, 5, 83–95. [Google Scholar] [CrossRef]
- Talankova-Sereda, T.E.; Liapina, K.V.; Shkopinskij, E.A.; Ustinov, A.I.; Kovalyova, A.V.; Dulnev, P.G.; Kucenko, N.I. The influence of Cu and Co nanoparticles on growth characteristics and biochemical structure of Mentha longifolia in vitro. Nanosci. Nanoeng. 2016, 4, 31–39. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Fahmy, A.H.; Ahmed, S.S. Copper nanoparticles elevate regeneration capacity of (Ocimum basilicum L.) plant via somatic embryogenesis. Plant. Cell Tissue Organ. Cult. 2019, 136, 41–50. [Google Scholar] [CrossRef]
- Du, W.C.; Tan, W.J.; Yin, Y.; Ji, R.; Peralta-Videa, J.R.; Guo, H.Y.; Gardea-Torresdey, J.L. Differential effects of copper nanoparticles/microparticles in agronomic and physiological parameters of oregano (Origanum vulgare). Sci. Total Environ. 2018, 618, 306–312. [Google Scholar] [CrossRef] [PubMed]
- AlQuraidi, A.O.; Mosa, K.A.; Ramamoorthy, K. Phytotoxic and genotoxic effects of copper nanoparticles in coriander (Coriandrum sativum-Apiaceae). Plants 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, C.Y.; Cota-Ruiz, K.; Peralta-Videa, J.R.; Sun, Y.P.; Rawat, S.; Tan, W.J.; Reyes, A.; Hernandez-Viezcas, J.A.; Niu, G.H.; et al. Improvement of nutrient elements and allicin content in green onion (Allium fistulosum) plants exposed to CuO nanoparticles. Sci. Total Environ. 2020, 725, 138387. [Google Scholar] [CrossRef]
- Javed, R.; Mohamed, A.; Yucesan, B.; Gurel, E.; Kausar, R.; Zia, M. CuO nanoparticles significantly influence in vitro culture, steviol glycosides, and antioxidant activities of Stevia rebaudiana Bertoni. Plant. Cell Tissue Organ. Cult. 2017, 131, 611–620. [Google Scholar] [CrossRef]
- Ghazal, B.; Saif, S.; Farid, K.; Khan, A.; Rehman, S.; Reshma, A.; Fazal, H.; Ali, M.; Ahmad, A.; Rahman, L.; et al. Stimulation of secondary metabolites by copper and gold nanoparticles in submerge adventitious root cultures of Stevia rebaudiana (Bert.). IET Nanobiotechnol. 2018, 12, 569–573. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Yucesan, B.; Gurel, E. Abiotic stress of ZnO-PEG, ZnO-PVP, CuO-PEG and CuO-PVP nanoparticles enhance growth, sweetener compounds and antioxidant activities in shoots of Stevia rebaudiana Bertoni. IET Nanobiotechnol. 2017, 11, 898–902. [Google Scholar] [CrossRef]
- Lala, S. Enhancement of secondary metabolites in Bacopa monnieri (L.) Pennell plants treated with copper-based nanoparticles in vivo. IET Nanobiotechnol. 2020, 14, 78–85. [Google Scholar] [CrossRef]
- Pramanik, A.; Datta, A.K.; Das, D.; Kumbhakar, D.V.; Ghosh, B.; Mandal, A.; Gupta, S.; Saha, A.; Sengupta, S. Assessment of nanotoxicity (cadmium sulphide and copper oxide) using cytogenetical parameters in Coriandrum sativum L. (Apiaceae). Cytol. Genet. 2018, 52, 299–308. [Google Scholar] [CrossRef]
- Bahrami, M.K.; Movafeghi, A.; Mahdavinia, G.R.; Hassanpouraghdam, M.B.; Gohari, G. Effects of bare and chitosan-coated Fe3O4 magnetic nanoparticles on seed germination and seedling growth of Capsicum annuum L. Biointerface Res. Appl. Chem. 2018, 8, 3552–3559. [Google Scholar]
- Jangir, H.; Das, C.K.; Kumar, J.; Mahapatra, S.S.; Srivastava, G.; Bhardwaj, A.; Das, M. Nano pyrite (FeS2) root priming enhances chilli and marigold production in nutrients-deficient soil: A nano strategy for fertiliser tuning. Appl. Nanosci. 2019, 9, 327–340. [Google Scholar] [CrossRef]
- Nechitailo, G.S.; Bogoslovskaya, O.A.; Ol’khovskaya, I.P.; Glushchenko, N.N. Influence of iron, zinc, and copper nanoparticles on some growth indices of pepper plants. Nanotechnol. Russ. 2018, 13, 161–167. [Google Scholar] [CrossRef]
- Siva, G.V.; Benita, L.F.J. Iron oxide nanoparticles promotes agronomic traits of ginger (Zingiber officinale Rosc.). Int. J. Adv. Res. Biol. Sci. 2016, 3, 230–237. [Google Scholar]
- Sabet, H.; Mortazaeinezhad, F. Yield, growth and Fe uptake of cumin (Cuminum cyminum L.) affected by Fe-nano, Fe-chelated and Fe-siderophore fertilization in the calcareous soils. J. Trace Elem. Med. Biol. 2018, 50, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Mohebodini, M.; Fathi, R.; Mehri, N. Optimization of hairy root induction in chicory (Cichorium intybus L.) and effects of nanoparticles on secondary metabolites accumulation. Iran. J. Genet. Plant. Breed. 2017, 6, 60–68. [Google Scholar]
- Khan, M.A.; Ali, A.; Mohammad, S.; Ali, H.; Khan, T.; Mashwani, Z.U.R.; Jan, A.; Ahmad, P. Iron nano modulated growth and biosynthesis of steviol glycosides in Stevia rebaudiana. Plant. Cell Tissue Organ. Cult. 2020, 143, 121–130. [Google Scholar] [CrossRef]
- Kiapour, H.; Moaveni, P.; Sani, B.; Rajabzadeh, F.; Mozafari, H. Investigating the effect of magnesium and iron oxide nanoparticles on the levels of enzymatic and non-enzymatic antioxidants in roselle. JMPB 2020, 9, 19–31. [Google Scholar]
- Tavallali, V.; Kiani, M.; Hojati, S. Iron nano-complexes and iron chelate improve biological activities of sweet basil (Ocimum basilicum L.). Plant. Physiol. Biochem. 2019, 144, 445–454. [Google Scholar] [CrossRef]
- Tavallali, V.; Rowshan, V.; Gholami, H.; Hojati, S. Iron-urea nano-complex improves bioactive compounds in essential oils of Ocimum basilicum L. Sci. Hortic. 2020, 265, 109222. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Fahad; Balouch, A.; Agheem, M.H.; Memon, S.A.; Baloch, A.R.; Tunio, A.; Abdullah; Pato, A.H.; Jagirani, M.S.; Panah, P.; et al. Efficient mitigation of cadmium and lead toxicity in coriander plant utilizing magnetite (Fe3O4) nanofertilizer as growth regulator and antimicrobial agent. Int. J. Environ. Anal. Chem. 2020, in press. [Google Scholar] [CrossRef]
- Askary, M.; Talebi, S.M.; Amini, F.; Bangan, A.D.B. Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologia 2017, 63, 65–75. [Google Scholar] [CrossRef]
- Ghosh, I.; Mukherjee, A.; Mukherjee, A. In planta genotoxicity of nZVI: Influence of colloidal stability on uptake, DNA damage, oxidative stress and cell death. Mutagenesis 2017, 32, 371–387. [Google Scholar] [CrossRef]
- Younes, N.A.; Hassan, H.S.; Elkady, M.F.; Hamed, A.M.; Dawood, M.F.A. Impact of synthesized metal oxide nanomaterials on seedlings production of three Solanaceae crops. Heliyon 2020, 6, e03188. [Google Scholar] [CrossRef]
- Garcia-Lopez, J.I.; Zavala-Garcia, F.; Olivares-Saenz, E.; Lira-Saldivar, R.H.; Diaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vazquez-Alvarado, R.; Nino-Medina, G. Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Garcia-Lopez, J.I.; Nino-Medina, G.; Olivares-Saenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vazquez-Alvarado, R.; Rodriguez-Salinas, P.A.; Zavala-Garcia, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Gokak, I.B.; Taranath, T.C. Morphological and biochemical responses of Abelmoschus esculantus (L.) Moench to zinc nanoparticles. Adv. Nat. Sci. Nanosci. 2015, 6, 025017. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Kim, S.; Lee, I. Assessment of phytotoxicity of ZnO NPs on a medicinal plant, Fagopyrum esculentum. Environ. Sci. Pollut. Res. 2013, 20, 848–854. [Google Scholar] [CrossRef]
- Karimi, N.; Behbahani, M.; Dini, G.; Razmjou, A. Enhancing the secondary metabolite and anticancer activity of Echinacea purpurea callus extracts by treatment with biosynthesized ZnO nanoparticles. Adv. Nat. Sci. Nanosci. 2018, 9, 045009. [Google Scholar] [CrossRef]
- Shahhoseini, R.; Azizi, M.; Asili, J.; Moshtaghi, N.; Samiei, L. Effects of zinc oxide nanoelicitors on yield, secondary metabolites, zinc and iron absorption of Feverfew (Tanacetum parthenium (L.) Schultz Bip.). Acta Physiol. Plant. 2020, 42, 52. [Google Scholar] [CrossRef]
- Linh, N.T.N.; Tung, H.T.; Hien, V.T.; Luan, V.Q.; Huy, N.P.; Loc, N.H.; Nhut, D.T. Effect of metal nanoparticles on the growth of Ngoc Linh ginseng (Panax vietnamensis Ha et Grushv.) lateral roots cultured in vitro. HU JOS 2017, 126, 47–56. [Google Scholar]
- Radi, A.A.; Farghaly, F.A.; Al-Kahtany, F.A.; Hamada, A.M. Zinc oxide nanoparticles-mediated changes in ultrastructure and macromolecules of pomegranate callus cells. Plant. Cell Tissue Organ. Cult. 2018, 135, 247–261. [Google Scholar] [CrossRef]
- Desai, C.V.; Desai, H.B.; Suthar, K.P.; Singh, D.; Patel, R.M.; Taslim, A. Phytotoxicity of zinc-nanoparticles and its influence on stevioside production in Stevia rebaudiana Bertoni. Appl. Biol. Res. 2015, 17, 1–7. [Google Scholar] [CrossRef]
- Oloumi, H.; Soltaninejad, R.; Baghizadeh, A. The comparative effects of nano and bulk size particles of CuO and ZnO on glycyrrhizin and phenolic compounds contents in Glycyrrhiza glabra L. seedlings. Indian J. Plant. Physiol. 2015, 20, 157–161. [Google Scholar] [CrossRef]
- Nadernejad, N. Investigation of toxicity of zinc oxide nanoparticles synthesized by olive extract on growth and pigments in Borago officinalis. J. Plant. Process. Funct. 2019, 8, 303–315. [Google Scholar]
- Sun, Z.Q.; Xiong, T.T.; Zhang, T.; Wang, N.F.; Chen, D.; Li, S.S. Influences of zinc oxide nanoparticles on Allium cepa root cells and the primary cause of phytotoxicity. Ecotoxicology 2019, 28, 175–188. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A.; Mukherjee, A. Effects of ZnO nanoparticles in plants: Cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 807, 25–32. [Google Scholar] [CrossRef]
- Ahmed, B.; Dwivedi, S.; Abdin, M.Z.; Azam, A.; Al-Shaeri, M.; Khan, M.S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Mitochondrial and chromosomal damage induced by oxidative stress in Zn2+ ions, ZnO-bulk and ZnO NPs treated Allium cepa roots. Sci. Rep. 2017, 7, 40685. [Google Scholar] [CrossRef]
- Kumari, M.; Khan, S.S.; Pakrashi, S.; Mukherjee, A.; Chandrasekaran, N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J. Hazard. Mater. 2011, 190, 613–621. [Google Scholar] [CrossRef]
- Debnath, P.; Mondal, A.; Sen, K.; Mishra, D.; Mondal, N.K. Genotoxicity study of nano Al2O3, TiO2 and ZnO along with UV-B exposure: An Allium cepa root tip assay. Sci. Total Environ. 2020, 713, 136592. [Google Scholar] [CrossRef] [PubMed]
- Tighe-Neira, R.; Carmora, E.; Recio, G.; Nunes-Nesi, A.; Reyes-Diaz, M.; Alberdi, M.; Rengel, Z.; Inostroza-Blancheteau, C. Metallic nanoparticles influence the structure and function of the photosynthetic apparatus in plants. Plant. Physiol. Biochem. 2018, 130, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Ghorbanpour, M.; Salehiarjomand, H. Nano-anatase TiO2 modulates the germination behavior and seedling vigority of some commercially important medicinal and aromatic plants. J. Biol. Environ. Sci. 2014, 8, 5359. [Google Scholar]
- Dehkourdi, E.H.; Mosavi, M. Effect of anatase nanoparticles (TiO2) on parsley seed germination (Petroselinum crispum) in vitro. Biol. Trace Elem. Res. 2013, 155, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Feizi, H.; Kamali, M.; Jafari, L.; Moghaddam, P.R. Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill). Chemosphere 2013, 91, 506–511. [Google Scholar] [CrossRef]
- Khater, M.S.; Osman, Y.A.H. Influence of TiO2 nanoparticles on growth, chemical constituents and toxicity of fennel plant. AJNSA 2015, 48, 178–186. [Google Scholar]
- Motyka, O.; Strbova, K.; Zinicovscaia, I. Chlorophyll content in two medicinal plant species following nano-TiO2 exposure. Bull. Environ. Contam. Toxicol. 2020, 104, 373–379. [Google Scholar] [CrossRef]
- Morteza, E.; Moaveni, P.; Morteza, T.; Saemi, H.; Joorabloo, A. Effects of TiO2 (nano and bulk) foliar application on physiological traits and grain yield of Safflower (Carthamus tinctorius L.). Biol. Forum 2015, 7, 1725–1731. [Google Scholar]
- Ogunkunle, C.O.; Adegboye, E.F.; Okoro, H.K.; Vishwakarma, V.; Alagarsamy, K.; Fatoba, P.O. Effect of nanosized anatase TiO2 on germination, stress defense enzymes, and fruit nutritional quality of Abelmoschus esculentus (L.) Moench (okra). Arab. J. Geosci. 2020, 13, 120. [Google Scholar] [CrossRef]
- Hu, J.; Wu, X.Y.; Wu, F.; Chen, W.X.; White, J.C.; Yang, Y.; Wang, B.; Xing, B.S.; Tao, S.; Wang, X.L. Potential application of titanium dioxide nanoparticles to improve the nutritional quality of coriander (Coriandrum sativum L.). J. Hazard. Mater. 2020, 389, 121837. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.J.; Du, W.C.; Darrouzet-Nardi, A.J.; Hernandez-Viezcas, J.A.; Ye, Y.Q.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of the exposure of TiO 2 nanoparticles on basil (Ocimum basilicum) for two generations. Sci. Total Environ. 2018, 636, 240–248. [Google Scholar] [CrossRef]
- Morteza, E.; Morteza, T.; Saemi, H.; Joorabloo, A. Effect of nano titanium dioxide spraying on essential oil yield and traits of cumin (Cuminum cyminum L.). Biol. Environ. Sci. 2016, 8, 101–108. [Google Scholar]
- Kahila, M.M.H.; Najy, A.M.; Rahaie, M.; Mir-Derikvand, M. Effect of nanoparticle treatment on expression of a key gene involved in thymoquinone biosynthetic pathway in Nigella sativa L. Nat. Prod. Res. 2018, 32, 1858–1862. [Google Scholar] [CrossRef]
- Golami, A.; Abbaspour, H.; Hashemi-Moghaddam, H.; Gerami, M. Photocatalytic effect of TiO2 nanoparticles on essential oil of Rosmarinus officinalis. J. Biochem. Technol. 2018, 9, 50–56. [Google Scholar]
- Khajavi, M.; Rahaie, M.; Ebrahimi, A. The effect of TiO2 and SiO2 nanoparticles and salinity stress on expression of genes involved in parthenolide biosynthesis in Feverfew (Tanacetum parthenium L.). Caryologia 2019, 72, 3–14. [Google Scholar]
- Karamian, R.; Ghasemlou, F.; Amiri, H. Physiological evaluation of drought stress tolerance and recovery in Verbascum sinuatum plants treated with methyl jasmonate, salicylic acid and titanium dioxide nanoparticles. Plant. Biosyst. 2020, 154, 277–287. [Google Scholar] [CrossRef]
- Mohammadi, H.; Esmailpour, M.; Gheranpaye, A. Effects of TiO2 nanoparticles and water-deficit stress on morpho-physiological characteristics of dragonhead (Dracocephalum moldavica L.) plants. Acta Agric. Slov. 2016, 107, 385–396. [Google Scholar] [CrossRef]
- Fazeli-Nasab, B.; Sirousmehr, A.R.; Azad, H. Effect of titanium dioxide nanoparticles on essential oil quantity and quality in Thymus vulgaris under water deficit. JMPB 2018, 7, 125–133. [Google Scholar]
- Ghabel, V.K.; Karamian, R. Effects of TiO2 nanoparticles and spermine on antioxidant responses of Glycyrrhiza glabra L. to cold stress. Acta Bot. Croat. 2020, 79, 137–147. [Google Scholar] [CrossRef]
- Mohammadi, R.; Maali-Amiri, R.; Abbasi, A. Effect of TiO2 nanoparticles on chickpea response to cold stress. Biol. Trace Elem. Res. 2013, 152, 403–410. [Google Scholar] [CrossRef]
- Rikabad, M.M.; Pourakbar, L.; Moghaddam, S.S.; Popovic-Djordjevic, J. Agrobiological, chemical and antioxidant properties of saffron (Crocus sativus L.) exposed to TiO2 nanoparticles and ultraviolet-B stress. Ind. Crops Prod. 2019, 137, 137–143. [Google Scholar] [CrossRef]
- Aghdam, M.T.B.; Mohammadi, H.; Ghorbanpour, M. Effects of nanoparticulate anatase titanium dioxide on physiological and biochemical performance of Linum usitatissimum (Linaceae) under well-watered and drought stress conditions. Braz. J. Bot. 2016, 39, 139–146. [Google Scholar] [CrossRef]
- Pakrashi, S.; Jain, N.; Dalai, S.; Jayakumar, J.; Chandrasekaran, P.T.; Raichur, A.M.; Chandrasekaran, N.; Mukherjee, A. In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations. PLoS ONE 2014, 9, e87789. [Google Scholar] [CrossRef]
- Ahmed, B.; Shahid, M.; Khan, M.S.; Musarrat, J. Chromosomal aberrations, cell suppression and oxidative stress generation induced by metal oxide nanoparticles in onion (Allium cepa) bulb. Metallomics 2018, 10, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Kaya, N.; Kaya, B. Genotoxic effects of zinc oxide and titanium dioxide nanoparticles on root meristem cells of Allium cepa by comet assay. Turk. J. Biol. 2014, 38, 31–39. [Google Scholar] [CrossRef]
- Rezaei, Z.; Jafarirad, S.; Kosari-Nasab, M. Modulation of secondary metabolite profiles by biologically synthesized MgO/perlite nanocomposites in Melissa officinalis plant organ cultures. J. Hazard. Mater. 2019, 380, 120878. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.Q.; Medina-Velo, I.A.; Cota-Ruiz, K.; Moreno-Olivas, F.; Gardea-Torresdey, J.L. Can abiotic stresses in plants be alleviated by manganese nanoparticles or compounds? Ecotoxicol. Environ. Saf. 2019, 184, 109671. [Google Scholar] [CrossRef]
- Mangalampalli, B.; Dumala, N.; Grover, P. Allium cepa root tip assay in assessment of toxicity o magnesium oxide nanoparticles and microparticles. J. Environ. Sci. 2018, 66, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.H.; Shakib, E.S.; Ebrahimi, O.; Sharifi-Rad, J. Impacts of nickel nanoparticles on grow characteristics, photosynthetic pigment content and antioxidant activity of Coriandrum sativum L. Orient. J. Chem. 2017, 33, 1297–1303. [Google Scholar] [CrossRef]
- Baskar, V.; Safia, N.; Preethy, K.S.; Dhivya, S.; Thiruvengadam, M.; Sathishkumar, R. A comparative study of phytotoxic effects of metal oxide (CuO, ZnO and NiO) nanoparticles on in-vitro grown Abelmoschus esculentus. Plant. Biosyst. 2020, in press. [Google Scholar] [CrossRef]
- Manna, I.; Bandyopadhyay, M. Engineered nickel oxide nanoparticle causes substantial physicochemical perturbation in plants. Front. Chem. 2017, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Rajeshwari, A.; Jadon, P.S.; Chaudhuri, G.; Mukherjee, A.; Chandrasekaran, N.; Mukherjee, A. Cytogenetic studies of chromium (III) oxide nanoparticles on Allium cepa root tip cells. J. Environ. Sci. 2015, 38, 150–157. [Google Scholar] [CrossRef]
- Liman, R. Genotoxic effects of Bismuth (III) oxide nanoparticles by Allium and Comet assay. Chemosphere 2013, 93, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.I.; Rico, C.M.; Hernandez-Viezcas, J.A.; Nunez, J.C.; Barrios, A.C.; Tafoy, A.; Flores-Marges, J.P.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J. Agric. Food Chem. 2013, 61, 6224–6230. [Google Scholar] [CrossRef]
- Maqbool, Q.; Sidorowicz, A.; Nazar, M.; Jabeen, N.; Anwaar, S.; Ahmad, I. Organometallic cerium oxide nanostructures acted as nanofertilizer during callogenesis and organogenesis of recalcitrant plant Berberis lycium Royle. Mater. Today Commun. 2019, 20, 100544. [Google Scholar] [CrossRef]
- Liman, R.; Acikbas, Y.; Cigerci, I.H. Cytotoxicity and genotoxicity of cerium oxide micro and nanoparticles by Allium and Comet tests. Ecotoxicol. Environ. Saf. 2019, 168, 408–414. [Google Scholar] [CrossRef]
- De, A.; Chakrabarti, M.; Ghosh, I.; Mukherjee, A. Evaluation of genotoxicity and oxidative stress of aluminium oxide nanoparticles and its bulk form in Allium cepa. Nucleus 2016, 59, 219–225. [Google Scholar] [CrossRef]
- Kang, H.; Hwang, Y.G.; Lee, T.G.; Jin, C.R.; Cho, C.H.; Jeong, H.Y.; Kim, D.O. Use of gold nanoparticle fertilizer enhances the ginsenoside contents and anti-inflammatory effects of red ginseng. J. Microbiol. Biotechnol. 2016, 26, 26–1668. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant. Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sharifi-Rad, M.; da Silva, J.A.T. Morphological, physiological and biochemical responses of crops (Zea mays L., Phaseolus vulgaris L.), medicinal plants (Hyssopus officinalis L., Nigella sativa L.), and weeds (Amaranthus retroflexus L., Taraxacum officinale F. H. Wigg) exposed to SiO2 nanoparticles. J. Agric. Sci. Technol. 2016, 18, 1027–1040. [Google Scholar]
- Ashkavand, P.; Tabari, M.; Zarafshar, M.; Tomaskova, I.; Struve, D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. For. Res. Pap. 2015, 76, 350–359. [Google Scholar] [CrossRef]
- Rahimi, S.; Hatami, M.; Ghorbanpour, M. Effect of seed priming with nanosilicon on morpho-physiological characteristics, quercetin content and antioxidant capacity in Calendula officinalis L. under drought stress conditions. J. Med. Plants 2020, 18, 186–203. [Google Scholar]
- Khan, M.A.; Wallace, W.T.; Sambi, J.; Rogers, D.T.; Littleton, J.M.; Rankin, S.E.; Knutson, B.L. Nanoharvesting of bioactive materials from living plant cultures using engineered silica nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110190. [Google Scholar] [CrossRef]
- Kalteh, M.; Alipour, Z.T.; Ashraf, S.; Aliabadi, M.M.; Nosratabadi, A.F. Effect of silica nanoparticles on basil (Ocimum basilicum) under salinity stress. J. Chem. Health Risks 2014, 4, 49–55. [Google Scholar]
- Koce, J.D.; Drobne, D.; Klancnik, K.; Makovec, D.; Novak, S.; Hocevar, M. Oxidative potential of ultraviolet-A irradiated or nonirradiated suspensions of titanium dioxide or silicon dioxide nanoparticles on Allium cepa roots. Environ. Toxicol. Chem. 2014, 33, 858–867. [Google Scholar] [CrossRef]
- Brasted, R.C. Selenium. Available online: https://www.britannica.com/science/selenium (accessed on 14 January 2021).
- Kurokawa, S.; Berry, M.J. Selenium. Role of the essential metalloid in health. Met. Ions Life Sci. 2013, 13, 499–534. [Google Scholar]
- Abedi, S.; Iranbakhsh, A.; Ardebili, Z.O.; Ebadi, M. Nitric oxide and selenium nanoparticles confer changes in growth, metabolism, antioxidant machinery, gene expression, and flowering in chicory (Cichorium intybus L.): Potential benefits and risk assessment. Environ. Sci. Pollut. Res. 2020, in press. [Google Scholar] [CrossRef]
- Sotoodehnia-Korani, S.; Iranbakhsh, A.; Ebadi, M.; Majd, A.; Ardebili, Z.O. Selenium nanoparticles induced variations in growth, morphology, anatomy, biochemistry, gene expression, and epigenetic DNA methylation in Capsicum annuum; an in vitro study. Environ. Pollut. 2020, 265, 114727. [Google Scholar] [CrossRef]
- Alamo-Nole, L.; Su, Y.F. Translocation of cadmium in Ocimum basilicum at low concentration of CdSSe nanoparticles. Appl. Mater. Today 2017, 9, 314–318. [Google Scholar] [CrossRef]
- Behbahani, S.R.; Iranbakhsh, A.; Ebadi, M.; Majd, A.; Ardebili, Z.O. Red elemental selenium nanoparticles mediated substantial variations in growth, tissue differentiation, metabolism, gene transcription, epigenetic cytosine DNA methylation, and callogenesis in bittermelon (Momordica charantia); an in vitro experiment. PLoS ONE 2020, 15, e0235556. [Google Scholar]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon nanomaterials in agriculture: A critical review. Front. Plant. Sci. 2016, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Osheen, S.; Gupta, A.; Kumar, R. Carbon nanomaterials in agriculture. In Nanoscience for Sustainable Agriculture; Pudake, R., Chauhan, N., Kole, C., Eds.; Springer: Cham, Switzerland, 2019; pp. 153–170. [Google Scholar]
- Crista, M.A.; da Silva, J.C.G.E.; da Silva, L.P. Evaluation of different bottom-up routes for the fabrication of carbon dots. Nanomaterials 2020, 10, 1316. [Google Scholar] [CrossRef]
- Zarzycki, P.K. Pure and Functionalized Carbon Based Nanomaterials: Analytical, Biomedical, Civil. and Environmental Engineering Applications; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Chandra, S.; Pradhan, S.; Mitra, S.; Patra, P.; Bhattacharya, A.; Pramanik, P.; Goswami, A. High throughput electron transfer from carbon dots to chloroplast: A rationale of enhanced photosynthesis. Nanoscale 2014, 6, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- Li, D.N.; Li, W.; Zhang, H.R.; Zhang, X.J.; Zhuang, J.L.; Liu, Y.L.; Hu, C.F.; Lei, B.F. Far-red carbon dots as efficient light-harvesting agents for enhanced photosynthesis. ACS Appl. Mater. Interfaces 2020, 12, 21009–21019. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Erande, M.B.; Late, D.J. Graphene quantum dots as enhanced plant growth regulators: Effects on coriander and garlic plants. J. Sci. Food Agric. 2015, 95, 2772–2778. [Google Scholar] [CrossRef]
- Younes, N.A.; Dawood, M.F.A.; Wardany, A.A. Biosafety assessment of graphene nanosheets on leaf ultrastructure, physiological and yield traits of Capsicum annuum L. and Solanum melongena L. Chemosphere 2019, 228, 318–327. [Google Scholar] [CrossRef]
- Hatami, M.; Hosseini, S.M.; Ghorbanpour, M.; Kariman, K. Physiological and antioxidative responses to GO/PANI nanocomposite in intact and demucilaged seeds and young seedlings of Salvia mirzayanii. Chemosphere 2019, 233, 920–935. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Farahani, A.H.K.; Hadian, J. Potential toxicity of nano-graphene oxide on callus cell of Plantago major L. under polyethylene glycol-induced dehydration. Ecotoxicol. Environ. Saf. 2018, 148, 910–922. [Google Scholar] [CrossRef]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Mehrjardi, H.A.; Jonoubi, P.; Majd, A.; Hosseini, R.H.; Abarghouei, H.B.; Hosseinizadeh, S.A. Study of MWCNTs effects on growth rate, germination and morphological characteristics of two Salvia species seedlings, Salvia sclarea and Salvia macrosiphon. J. Curr. Res. Sci. 2015, 3, 110–118. [Google Scholar]
- Fatemi, F.; Abdollahi, M.R.; Mirzaie-Asl, A.; Dastan, D.; Papadopoulou, K. Phytochemical, antioxidant, enzyme activity and antifungal properties of Satureja khuzistanica in vitro and in vivo explants stimulated by some chemical elicitors. Pharm. Biol. 2020, 58, 286–296. [Google Scholar] [CrossRef]
- McGehee, D.L.; Alimohammadi, M.; Khodakovskaya, M.V. Carbon-based nanomaterials as stimulators of production of pharmaceutically active alkaloids in cell culture of Catharanthus roseus. Nanotechnology 2019, 30, 275102. [Google Scholar] [CrossRef] [PubMed]
- Gohari, G.; Safai, F.; Panahirad, S.; Akbari, A.; Rasouli, F.; Dadpour, M.R.; Fotopoulos, V. Modified multiwall carbon nanotubes display either phytotoxic or growth promoting and stress protecting activity in Ocimum basilicum L. in a concentration-dependent manner. Chemosphere 2020, 249, 126171. [Google Scholar] [CrossRef]
- Ghosh, M.; Bhadra, S.; Adegoke, A.; Bandyopadhyay, M.; Mukherjee, A. MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper-methylation. Mutat. Res. Fund. Mol. Mech. Mut. 2015, 774, 49–58. [Google Scholar] [CrossRef]
- Asgari-Targhi, G.; Iranbakhsh, A.; Ardebili, Z.O. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant. Physiol. Biochem. 2018, 127, 393–402. [Google Scholar] [CrossRef]
- Samarah, N.H.; Wang, H.; Welbaum, G.E. Pepper (Capsicum annuum) seed germination and vigour following nanochitin, chitosan or hydropriming treatments. Seed Sci. Technol. 2016, 44, 609–623. [Google Scholar] [CrossRef]
- Giorgetti, L.; Spano, C.; Muccifora, S.; Bottega, S.; Barbieri, F.; Bellani, L.; Castiglione, M.R. Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination: Internalization in root cells, induction of toxicity and oxidative stress. Plant. Physiol. Biochem. 2020, 149, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Chatterjee, A.; Guchhait, R.; De, S.; Pramanick, K. Cytogenotoxic potential of a hazardous material, polystyrene microparticles on Allium cepa L. J. Hazard. Mater. 2020, 385, 121560. [Google Scholar] [CrossRef] [PubMed]
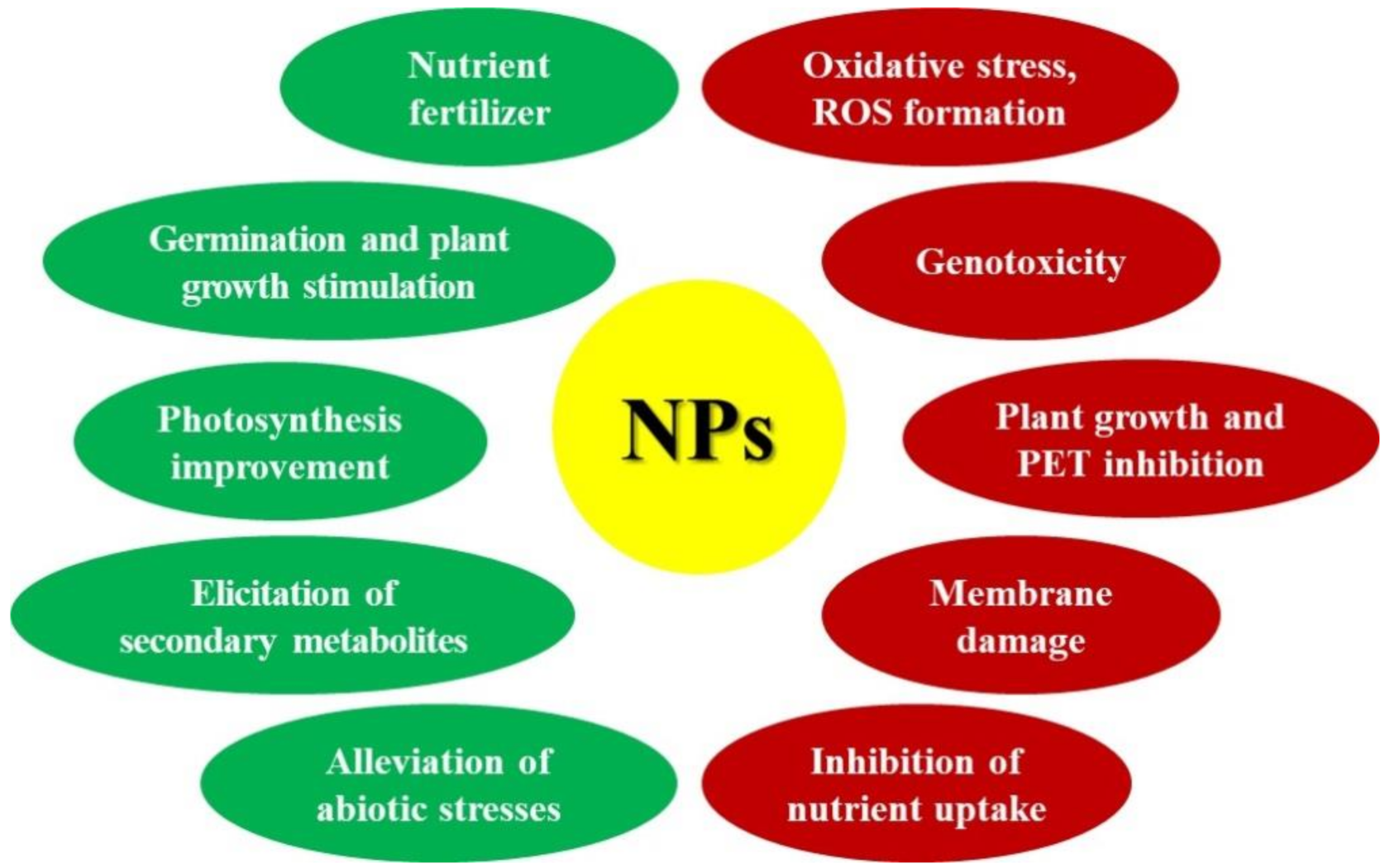
| NPs of Elicitor | Plant | Cultivation Mode | Elicitor Dose | SM | Multiple of SM Content in Control | Refs. |
|---|---|---|---|---|---|---|
| Ag | Momordica charantia L. | CSC | 5 mg/L | TPC TFC flavonoids hydroxybenzoic acid hydroxycinnamic acid | 1.40 1.56 1.52 1.23 1.15 | [121] |
| Ag | Echinacea purpurea L. | CSC, root derived callus (48 h) CSC, leaf derived callus (72 h) | 2 mg/L | cichoric acid cichoric acid | ≈2.22 ≈1.80 | [122] |
| Ag | Isatis constricta L. | leaves of shoots regenerated in MS (5 days) | 2 mg/L | tryptanthrin | 1.71 | [123] |
| Ag | Caralluma tuberculata L. | callus culture | 90 µg/L | TPC TFC | ≈3.75 ≈3.60 | [124] |
| Ag | Trigonella foenum-graecum L | 2% agar (5 days) and then cultivation in sterile soil | 200 μg/plant | diosgenin | 1.30 | [115] |
| Ag | Linum usitatissimum L | cell suspension culture, 20 days | 30 μg/L | secoisolariciresinol diglucoside lariciresinol diglucoside dehydrodiconiferyl alcohol glucoside guaiacylglycerol-coniferyl alcohol ether glucoside | 10.0 2.8 5.0 1.75 | [125] |
| Ag | Stevia rebaudiana L. | callus culture | 45 mg/L, after 2 d 30 mg/L, 45 mg/L, after 6 d | stevioside stevioside rebaudioside A | ≈4.32 ≈3.26 ≈1.70 | [126] |
| Ag | Vanilla planifolia L. | in vitro cultivation in MS | 25 mg/L 50 mg/L | TPC | ≈1.83 ≈1.83 | [127] |
| Ag | Salvia officinalis L. | foliar spaying | 100 mg/L | rosmarinic acid | ≈11.0 | [128] |
| AgAu (1:3) | Prunella vulgaris L | cell culture (+ 2 mg/L NAA), 14 days | 30 μg/L | TPC TFC | 1.54 1.39 | [129] |
| Au | Lavandula angustifolia L. | in vitro culture | 50 mg/L 10 mg/L | (E,E)-farnesol bisabolol oxide A cadale necaryophyllene oxide | 1.91 1.15 1.76 1.23 | [130] |
| Cu | Artemisia absinthium L. | seeds inoculated on MS medium | 30 µg/mL | TPC TFC | ≈1.66 ≈1.07 | [131] |
| Zn | Momordica charantia L. | foliar spraying | 20 ppm | carotenoids anthocyanins TPC TFC | 2.55 1.11 1.19 1.45 | [132] |
| Zn-Ag (0.95:0.05) (0.75:0.25) | Withania somnifera L. Dunal | in vitro culture (MS medium; 1 month) | 20 mg/L | withanolide | ≈14.08 ≈5.60 | [63] |
| Ni | Withania somnifera L. Dunal | in vitro culture (MS medium; 1 month) | 20 mg/L | withanolide | ≈7.90 | [63] |
| Fe | Mentha piperita L. | foliar application, 3 times | 0.5 g/L 0.5 g/L 1.5 g/L | menthone menthol menthofuran | 1.65 1.30 2.50 | [133] |
| Co | Artemisia annua L. | cell suspension culture (24 h) | 5 mg/L | artemisinin | 2.25 | [134] |
| CdSe QDs | Withania somnifera L. Dunal | in vitro culture (MS medium; 1 month) | 20 mg/L | withanolide | ≈3.75 | [63] |
| CuO | Stevia rebaudiana | leaf regenerants tissue culture | 20 mg/L | rebaudioside A stevioside | 1.50 1.94 | [135] |
| CuO | Gymnema sylvestre (Retz.) R. Br | cell suspension culture | 3 mg/L | gymnemic acid II TPC TFC | 9.0 ≈2.56 ≈1.79 | [136] |
| ZnO | Stevia rebaudiana L. Bertoni | tissue culture grown shoots | 0.1 mg/L 1.0 mg/L | rebaudioside A stevioside TPC stevioside TPC | 1.35 1.53 1.42 1.60 1.63 | [137] |
| ZnO | Stevia rebaudiana L. | leaf regenerants tissue culture | 2 mg/L | rebaudioside A stevioside | 1.49 1.27 | [135] |
| ZnO | Linum usitatissimum L. | in vitro culture (MS medium) | 500 mg/L | secoisolariciresinol diglucoside lariciresinol diglucoside dehydrodiconiferyl alcohol glucoside guaiacylglycerol-β-coniferyl alcohol ether glucoside | 1.28 1.35 1.60 1.54 | [138] |
| ZnO | Stevia rebaudiana L. | hydroponic cultivation | 75 mg/L | TPC TFC | 1.61 1.88 | [139] |
| ZnO | Linum usitatissimum L. | cell suspension culture | 60 mg/L | total lignan | ≈1.59 | [140] |
| ZnO | Hypericum perforatum L. | cell suspension culture | 100 ppb | hypericin hyperforin | 3.8013.36 | [141] |
| Fe2O3 | Melissa officinalis L. | plant irrigation at 60% FC | 30 µM | EO per plant | ≈1.60 | [142] |
| Fe2O3 | Hypericum perforatum L. | cell suspension culture | 100 ppb | hypericin hyperforin | 5.40 12.02 | [141] |
| Fe3O4 | Dracocephalum polychaetum Bornm. | cell suspension culture | 100 ppm | naringin rutin quercetin apigenin rosmarinic acidt hymol carvacrol | 2.02 3.15 6.29 4.81 3.18 5.07 3.22 | [143] |
| TiO2 | Linum usitatissimum L. | cell suspension culture | 150 mg/L | total lignan | 1.50 | [140] |
| TiO2 | Mentha piperita L. | foliar spraying | 150 mg/L | menthol menthone menthyl acetate | 1.09 1.32 1.11 | [144] |
| TiO2 | Vetiveria zizanioides L | foliar spraying | 90 mg/L measurement 300 DAT) | khusimol | 1.24 | [145] |
| TiO2 | Salvia officinalis L. | spraying of the 4-month old plants | 200 mg/L | TPC TFC p-cymene 1,8-cineol cis-thujonecamphor | 1.63 1.72 1.61 2.23 1.88 1.31 | [146] |
| TiO2 | Stevia rebaudiana L. Bertoni | cultivation in soil, spraying 3-fold in 3 weeks | 60 mg/L 200 mg/L | stevioside | ≈1.67 ≈1.77 | [147] |
| TiO2 | Nigella arvensis L. | hydroponic cultivation, 21 d | 2500 mg/L 50 mg/L 50 mg/L 2500 mg/L 1000 mg/L | glaucine (shoots) glaucine (roots) quercetin (shoots) quercetin (roots) TPC | 2.4 1.7 1.5 1.3 2.2 | [148] |
| TiO2 | Dracocephalum moldavica L. | irrigation of hydroponically grown plants | 100 mg/L | geraniol geranial (E-citral)Z-citral | 1.41 1.05 1.07 | [149] |
| TiO2 | Dracocephalum moldavica L. | spraying of plants grown in pots | 30 ppm 30 ppm 100 ppm | rosmarinic acid ellagitan ninchlorogenic acid caffeic acid | 1.23 1.40 1.22 1.41 | [150] |
| NiO | Nigella arvensis L. | hydroponic cultivation, 21 d | 1000 mg/L 2500 mg/L 50 mg/L 50 mg/L 100 mg/L | glaucine (shoots) glaucine (roots) quercetin (shoots) quercetin (roots) TFC | 3.20 2.60 2.2 1.2 2.5 | [148] |
| Al2O3 | Nigella arvensis L. | hydroponic cultivation, 21 d | 2500 mg/L 2500 mg/L 2500 mg/L 1000 mg/L | glaucine (shoots) glaucine (roots) quercetin(shoots) quercetin(roots) | 1.37 1.60 1.22 1.43 | [148] |
| Mn2O3 | Atropa belladonna L | in vitro culture on MS medium | 100 mg/L 25 mg/L | TPC TFC | 3.05 4.49 2.92 | [151] |
| Si | Mentha piperita L. | foliar spraying | 100 mg/L | menthol/per plant menthone/per plant menthyl-acetate/per plant | 1.811.751.83 | [152] |
| SiO2 | Matricaria recutita L. | seed treatment (1 h), then in vitro culture on MS | 4 g/L 6 g/L 6 g/L | TPC TPC TFC | 2.50 * 4.40 * 10.58 * | [153] |
| perlite | Hypericum perforatum L. | callus cultures from in vitro grown plants field grown plants | 25 mg/L 50 mg/L 50 mg/L | alkaloids | 14.24 12.69 1.75 | [154] |
| perlite-TiO2 | Hypericum perforatum L. | callus cultures from in vitro grown plants field grown plants | 25 mg/L 50 mg/L 25 mg/L | alkaloids | 12.35 11.80 1.34 | [154] |
| MWCNTs | Salvia verticillata L | spraying of plants | 50 mg/L 50 mg/L 1000 mg/L 250 mg/L | TPC rosmarinic acid rosmarinic acid caffeic acid | 1.20 3.40 3.99 2.88 | [155] |
| MWCNTs | Catharanthus roseus L. | in vitro cultivation (MS medium) | 50 mg/L 100 mg/L 150 mg/L 50 mg/L | alkaloids TPC | ≈1.86 ≈1.63 ≈1.86 ≈1.36 | [156] |
| MWCNTs | Thymus daenensis Celak. | in vitro culture (MS medium) | 250 mg/L | TPC TFC | 2.10 1.09 | [157] |
| MWCNTs | Satureja khuzistanica | Leaf segments cultured in B5 basal medium | 100 µg/mL | TPC TFC rosmarinic acid caffeic acid | 1.96 2.61 2.60 1.76 | [158] |
| MWCNT- COOH | Salvia nemorosa L. | cell suspension culture + 70 Gy γ-irradiation | 100 mg/L | rosmarinic acid salvianolic acid B ferulic acid cinnamic acid | 13.0 * 14.2 * 20.0 * 3.0 * | [159] |
| C60 fullerene | Tanacetum parthenium L., Pharmasaat genotype | foliar spraying (harvest at full flowering stage) | 250 mg/L | parthenolide | ≈8.2 | [160] |
| fullerenol [C60(OH)20] | Momordica charantia | seed treatment | 10.88 nM | cucurbitacin-B lycopene charantin insulin | 1.74 1.09 1.05 1.91 | [161] |
| NPs | Medicinal Plant | Dose (Length of Treatment) | SM | Multiple of Control SM Content | Refs. |
|---|---|---|---|---|---|
| ZnO | Hyoscyamus reticulatus L. | 100 mg/L (48 h) 100 mg/L (72 h) | hyoscyamine scopolamine | 4.61 3.20 | [67] |
| Fe2O3 | Hyoscyamus reticulatus L. | 900 mg/L (24 h) 450 mg/L (48 h) | hyoscyamine scopolamine | ≈5.0 ≈5.0 | [65] |
| Fe2O3 | Dracocephalum kotschyi Boiss | 75 mg/L (24 h) | rosmarinic acid xanthomicrol cirsimaritin isokaempferide | 9.7 11.87 3.85 2.27 | [162] |
| SiO2 | Dracocephalum kotschyi | 100 mg/L | rosmarinic acid xanthomicrol cirsimaritin isokaempferide | 8.26 13.00 13.42 10.00 | [163] |
| Ag-SiO2 | Artemisia annua L. | 900 mg/L (3 days) | artemisinin | 3.90 | [164] |
| Ag | Datura metel L. | 20 ppm (12 h) (24 h) (48 h) | atropine | 1.147 1.117 2.420 | [165] |
| CuO | Brassica rapa L. spp. pekinensis | 50, 100, 250 mg/L for 24, 48, 72 h | gluconapin glucobrassicanapin 4-methoxyglucobrassicin neoglucobrassicin 4-hydroxyglucobrassicin | ≈1.38 ≈1.34 ≈1.51 ≈1.54 ≈1.82 | [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kralova, K.; Jampilek, J. Responses of Medicinal and Aromatic Plants to Engineered Nanoparticles. Appl. Sci. 2021, 11, 1813. https://doi.org/10.3390/app11041813
Kralova K, Jampilek J. Responses of Medicinal and Aromatic Plants to Engineered Nanoparticles. Applied Sciences. 2021; 11(4):1813. https://doi.org/10.3390/app11041813
Chicago/Turabian StyleKralova, Katarina, and Josef Jampilek. 2021. "Responses of Medicinal and Aromatic Plants to Engineered Nanoparticles" Applied Sciences 11, no. 4: 1813. https://doi.org/10.3390/app11041813
APA StyleKralova, K., & Jampilek, J. (2021). Responses of Medicinal and Aromatic Plants to Engineered Nanoparticles. Applied Sciences, 11(4), 1813. https://doi.org/10.3390/app11041813







