Abstract
Concentrated microwave field (CMF) is the technology for preserving liquid food products, where the preservation factor is both a nonthermal effect and a moderate thermal effect. The term “concentrated microwave field” is traditionally used in Poland and is the equivalent to “single-mode-type microwave heating”. The aim of the research was to determine selected physical and rheological properties of liquid egg white, liquid yolk and liquid whole eggs (LWE) after CMF treatment. It was found that both the amount of energy supplied to the system and the intervals between CMF pulses had a statistically significant impact on the analyzed properties of liquid egg raw materials. In industrial practice, it would be recommended to use lower CMF treatment (at the level of 2.2–2.6 kJ) for the yolk, medium (3.2–3.6 kJ) for egg white and higher (4.0–4.4 kJ) for the liquid whole eggs. The “response surface” models presented in the paper may have a practical application in determining the influence of individual technological parameters on the final quality of various liquid food products.
1. Introduction
Preserving food with a microwave field usually has a lesser impact on the deterioration of the nutritional and sensory properties of food compared to other methods of conventional heat treatment or reheating of food [1]. The impact of the microwave field in the food industry is usually reduced to processes such as blanching, cooking, drying, pasteurization, sterilization, thawing, baking, fat rendering or as an element of combined technological processes [2,3]. A relatively common problem encountered when heating food with microwave ovens is an uneven temperature per unit volume of product, especially when the product is nonuniform. In order to minimize this phenomenon, the use of various parameters of microwave treatment is suggested, in relation to the same raw material, earlier preparation of product ingredients of optimal size and shape, use of the pulsed microwave treatment mode (which was used in our research), selection of precise process conditions dedicated to specific products, microwave ovens with a suitable shape of the treatment chamber, using lower power during microwave processing [1,4].
The main preservation factor in the process of thermal treatment, also in the aspect of microwave fields, is the temperature of a certain value applied for a sufficiently long time. In the food industry, microwave pasteurization is often performed when processing juices, and more energetic sterilization is typically used for milk. In addition, the publications describe the effectiveness of the microwave field also in the technology of preserving cider, coconut water or sweet potato puree [5]. In these studies, particular attention is paid to the dielectric properties of the preserved raw materials, as well as to the selection of energy-optimal process parameters in order to achieve the assumed degree of microbial inactivation. The impact of the microwave field can take place both in the case of liquid egg raw materials and eggs in the shell while maintaining a properly selected energy dose [6]. Research on microwave pasteurization in relation to Salmonella Typhimurium in egg yolk proved that the effectiveness of this technology depended on the time and conditions of interaction [7].
The preservation of food products is carried out not only by classical thermal methods, but more and more often using new thermal methods (ohmic heating or using radio frequencies above 70 kHz) or nonthermal methods (high hydrostatic pressure, exposure to ultraviolet light, pulsed electric fields, high-frequency ultrasounds, ionizing radiation, oscillating magnetic field). After using these methods, there are less changes in the color of the food product, its sensory characteristics and the nutritional value. In addition, the amount of energy needed to preserve the product can be reduced by up to 70% compared to classic thermal methods [8,9,10,11,12]. Classic food sterilization is increasingly being replaced not only by microwave sterilization, but also by other technologies, such as, e.g., ozonation, strong UV exposure or cold plasma [13]. Several less intense preservation techniques are also used, previously known as the “hurdle theory”, and now the “hurdle technology”, which lead to the appropriate microbiological purity of the final product with less energy input and less nutritional value loss [14,15].
The mechanism of inactivation of microorganisms in food during exposure to the microwave field is not fully understood. There are several theories on this subject. One of them, called the theory of selective heating, assumes that microorganisms are selectively more heated by the microwave field compared to the surrounding environment, which leads to their inactivation. According to the theory of electroporation, the potential difference on both sides of the bacterial cell membrane causes damage and the formation of the so-called pores through which cellular content passes out, which leads to the death of the bacterial cell [16]. A similar theory of rupture of the cell membrane assumes that the destruction of the cell membrane through ruptures occurs as a result of the generated electrical voltage. A slightly different cause of microbial inactivation is indicated by the coupled magnetic field theory, according to which it is electromagnetic energy, through a coupled influence on important elements for bacteria, i.e., on protein or DNA molecules, that causes its death [3].
In the microwave treatment used in our experiment, the microwave field was densified by means of a specially constructed waveguide, which is why device manufacturer Plazmatronika called this method a “concentrated microwave field” (CMF). The term “concentrated microwave field” may be ambiguous, but it has been traditionally used in Poland among researchers and food technologists for about 20 years. In world literature, other terms related to this technology are popular, such as: “single-mode-type microwave heating” [17], which results from the use of a standardized waveguide, alternatively referred to as “single-mode microwave cavity” [18,19,20]. The authors decided to keep the traditionally used name of this technology as CMF.
The dielectric properties of the microwave treated food products are the main parameters taken into account in the calculations related to the conversion of microwave energy into heat. The most frequently used concept is the relative complex permittivity ε*, referring to the treated raw material, which has the form of a complex number consisting of the real part ε′ and the imaginary part ε″ according to the following formula [3,21]:
where: ε *—relative complex permittivity,
ε* = ε′ − jε″
ε′—electric permeability,
ε″—dielectric loss factor,
j—imaginary unit of a complex number
The real part ε′ describes the ability of a material to store electrical energy whereas the imaginary part ε″ is related to the loss of electrical energy in terms of heat dissipation [22]. The ratio of the dielectric loss factor ε″ and the dielectric permittivity ε′ is expressed as a tangent delta and is the same as the ratio of the relative dielectric loss coefficient and the relative dielectric permittivity according to the formula [3]:
where: tg δ—tan delta
tg δ = ε″/ε′ = κ″/κ′
ε′—electric permittivity,
ε″—dielectric loss factor,
κ’—relative electric permittivity, also called the dielectric constant,
κ″—relative dielectric loss factor.
The relationships between the values of ε and κ can be described as κ’= ε′/εo and κ″ = ε″/εo where εo is the permittivity of the vacuum (εo = 8.854 × 10−12 F m−1). The dielectric properties of raw materials subjected to the influence of the microwave field also depend on the temperature of the process and the frequency of the microwave waves used [3].
Nevertheless, the aim of the study was not to determine the “relative complex permittivity” or to make other measurements regarding detailed electromagnetic parameters, but to find such ranges of parameters of pulsed CMF treatment, at which relatively small changes in color, pH and viscosity of egg raw material take place, which is important both from the point of view of production technology (e.g., transport with pipes of a liquid product, for which viscosity tests are useful) and consumer quality assessment of the final product (which is influenced, among others, by the color of the product and its acidity).
The total time of the real impact with the pulsed microwave field is shorter than in the continuous mode, which results in a lower energy demand of the process while obtaining similar effectiveness of preserving liquid food products. At the same time, the total time of impacts, due to the specially selected intervals between strictly defined CMF pulse groups, is slightly longer compared to the classic, i.e., continuous operation mode of the device. Earlier own studies demonstrated the effectiveness of the impulse effect of CMF related to the inactivation of microorganisms, especially in the framework of “hurdle technology” [16]. The active acidity (pH) of the egg raw material indicates its degree of freshness also in the sensory property aspect of final egg product, therefore we decided to analyze the pH after the CMF treatments. In the case of the yolk and liquid whole eggs, the color of the final product is particularly important, especially in the context of red and yellow. For this reason, it was decided for all samples to determine the change of individual color components, i.e., L*a*b*, not the total color difference (ΔE). Increasing the apparent viscosity of egg raw materials is disadvantageous in terms of production technology. Increasing the viscosity of the product changes the parameters of its pressing, which may contribute to unfavorable changes during subsequent technological processes as well as may have an impact on the greater energy consumption of the process. Studies showing the change in apparent viscosity of food liquids subjected to new methods of preservation allow a better assessment of their suitability in industrial conditions.
The aim of the study was to analyze selected physical properties, i.e., raw material temperature increase, changes in pH and color (L*a*b*), as well as to evaluate rheological properties expressed as viscosity of liquid yolk, LWE and albumen after low-energy CMF treatment.
2. Material and Method
2.1. Preparing the Egg Raw Material
The raw material for the research was fresh hen eggs obtained from Brown Leghorn laying hens aged 30 to 50 weeks. The raw material, of which the pH of the white part (albumen) was not higher than 9.25, was taken for analysis. The eggs were broken and the yolk was separated from the white part of the egg. Regarding the LWE, the egg white was gently mixed with the yolk (in the proportion 2:1 by weight) and then was homogenized in the “Büchi Mikser B - 400” homogenizer for 2 s. The homogenization of the albumen (for 0.5 s) and the yolk (for 2 s) was carried out separately in the same homogenizer [16] and it was own standard laboratory procedure. Before CMF treatment, the raw material was cooled down to a temperature in the range of 4.4–5.9 °C.
2.2. Device for Microwave Treatment
The device for generating the concentrated microwave field was the Plazmatronika RM2001 microwave reactor with a specially constructed waveguide to concentrate the microwave field in the space where the 10 cm3 test sample was placed in a glass vessel (Figure 1).

Figure 1.
Glass vessel placed in the waveguide which was in the chamber of the device.
The waveguide as a “single mode cavity” was the standard equipment of the device and was made by Plazmatronika according to the standards of the Electronic Industries Alliance (EIA), Radio Components Standardization Committee (RCSC) and the International Electrotechnical Commission (IEC). The waveguide used in the experiment is named WR340 (EIA standard), WG9A (RCSC standard) or R26 (IEC standard). The frequency of 2.45 GHz was used and the inner dimensions of waveguide opening were 86.36 mm × 43.18 mm.
2.3. Microwave Process Parameters
The following constant CMF parameters were used: power 480 W, pulse width 0.1 s, number of pulses in a packet 10, interval between packets 20 s. Variable CMF parameters were intervals between single pulses of 0.5 s, 1.0 s or 1.5 s as well as for each of these intervals 3 different numbers of CMF pulse packets (5, 7 or 9), which resulted in 9 test variants (except the control). The total amount of energy supplied to the system for 5 packets of 10 pulses was 2.40 kJ, for 7 packets (×10 pulses) was 3.36 kJ, and for 9 packets (×10 pulses) was 4.32 kJ. Lower power of CMF treatment (480 W) and pulse mode were used to avoid the possible undesirable high temperature and hot-spot heating of samples which could lead to partial denaturation of egg proteins. The CMF treatment parameters were selected on the basis of our previous studies [15,16].
2.4. Measurements of the Temperature, pH and Color
After completion of the CMF treatment, the temperature of the samples was measured twice, and then they were cooled down to the temperature of 20 °C, where the analysis of physical and rheological properties was carried out. The pH measurement was carried out twice for each sample using a Consort C832 Multi-parameter Analyzer. Product color (L*a*b* scale) was determined using a Minolta CR-400 reflection colorimeter. Each sample (8 cm3) was placed in a Petri dish of 45 mm in diameter. The measurement procedure was performed according to Chen et al. [23] and Necidova et al. [24]. The color space parameters L—lightness, a—redness, and b—yellowness were measured 3 times for each sample using the reflectance method. The instrument was calibrated to white point, and a setting with a D65 light source was used. The light projection tube of Minolta CR-400 was lightly touching the liquid surface of the samples during color measurement.
2.5. Analysis of Apparent Viscosity
The viscosity curve was determined using a Haake RheoStress 6000 rheometer, where a sample with a volume of 1 cm3 was placed in a measuring system consisting of a plate-cone set (C60/10° TiL). The shear rate ranged from 0 to 1000 s−1, and the apparent viscosity value for the shear rate 1000 s-1 expressed in millipascal-seconds (mPa·s) was used for the analysis. The apparent viscosity was determined in triplicate.
2.6. Statistical Analysis
The results obtained during the research were statistically processed using the Statistica 13.0 computer program. A one-way analysis of variance was performed where the means in the post hoc test were compared using Duncan’s test (statistically significant differences were those at the level of p ≤ 0.05). In the case of temperature increase and active acidity (pH), the mean was determined from two repetitions, and in the remaining cases, from three. The “Response Surface Methodology” charts were plotted and the significance of individual Pareto coefficients for all analyzed variants was also presented collectively in tabular form, separately for each raw material. “Response Surface Methodology” is used in much research into technological parameters, especially in the context of their optimization. The correlations between the studied discriminants within the individual egg raw materials subjected to CMF influence were also calculated.
3. Results
Tables 1, 3 and 5 show the obtained results, which were compared with each other (in columns) using analysis of variance and Duncan’s test to determine the significance of the differences. The average increase in the temperature of the raw material after the CMF effects was presented in relation to 9 research variants. In the case of the pH value, color (L*a*b*) and apparent viscosity the mean values for the control sample were also given. Additionally, the analysis of the “response surface” showed which discriminants (i.e., the energy emitted to the system and the distance between CMF pulses) had the strongest influence on the final values of the analyzed parameters. Thanks to the applied methodology, it was possible to estimate the tested values and the significance of variables both in the linear (L), quadratic (Q) aspect and linear−linear interactions of the main factors (L·L). In Figure 2, Figure 3 and Figure 4, separately for individual egg raw materials, only 4 selected discriminants that were subject to the greatest influence of CMF are presented in aggregate. The Pareto coefficients are summarized in Tables 2, 4 and 6, where significant variables are marked in bold and with an asterisk. The results are presented separately depending on the raw material, i.e., the albumen, yolk and LWE.
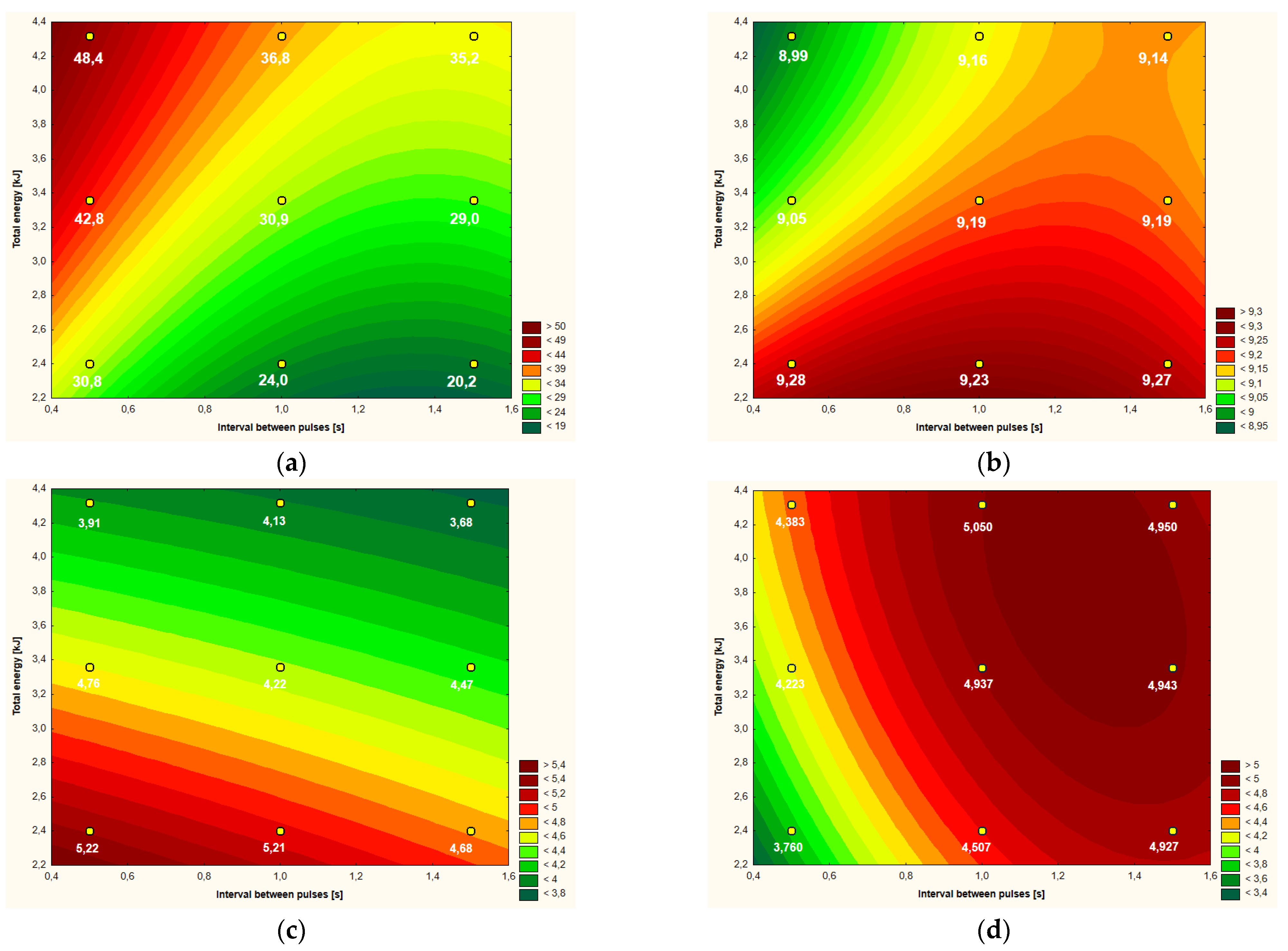
Figure 2.
Temperature increment (a), active acidity (pH) (b), color parameter b* (c,d) apparent viscosity (at shear rate 1000 s−1) of egg albumen after CMF treatment (9 values) depending on intervals between pulses and total energy supplied to system.
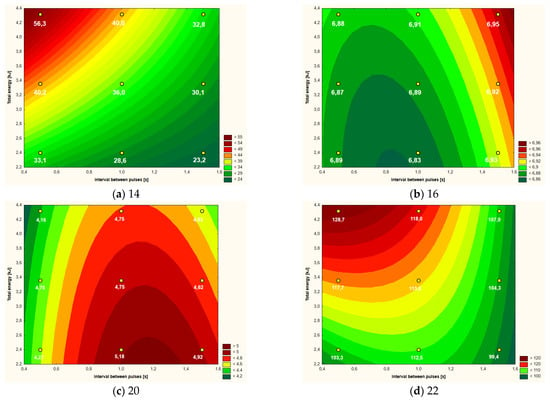
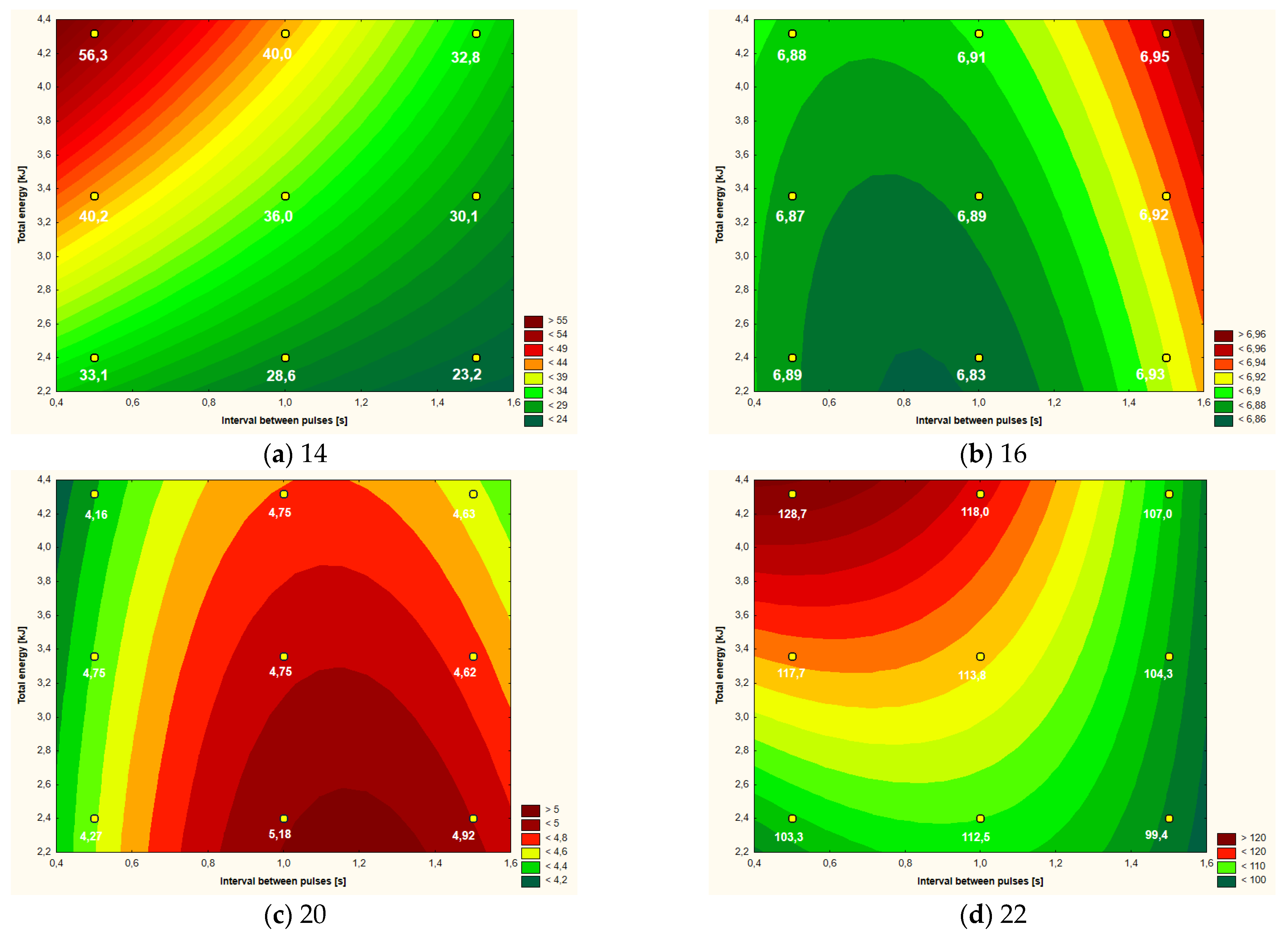
Figure 3.
Temperature increment (a), active acidity (pH) (b), color parameter a* (c,d) apparent viscosity (at shear rate 1000 s−1) of yolk after CMF treatment (9 values) depending on intervals between pulses and total energy supplied to system.
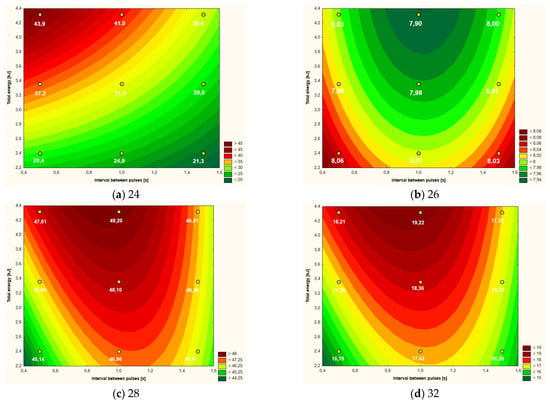
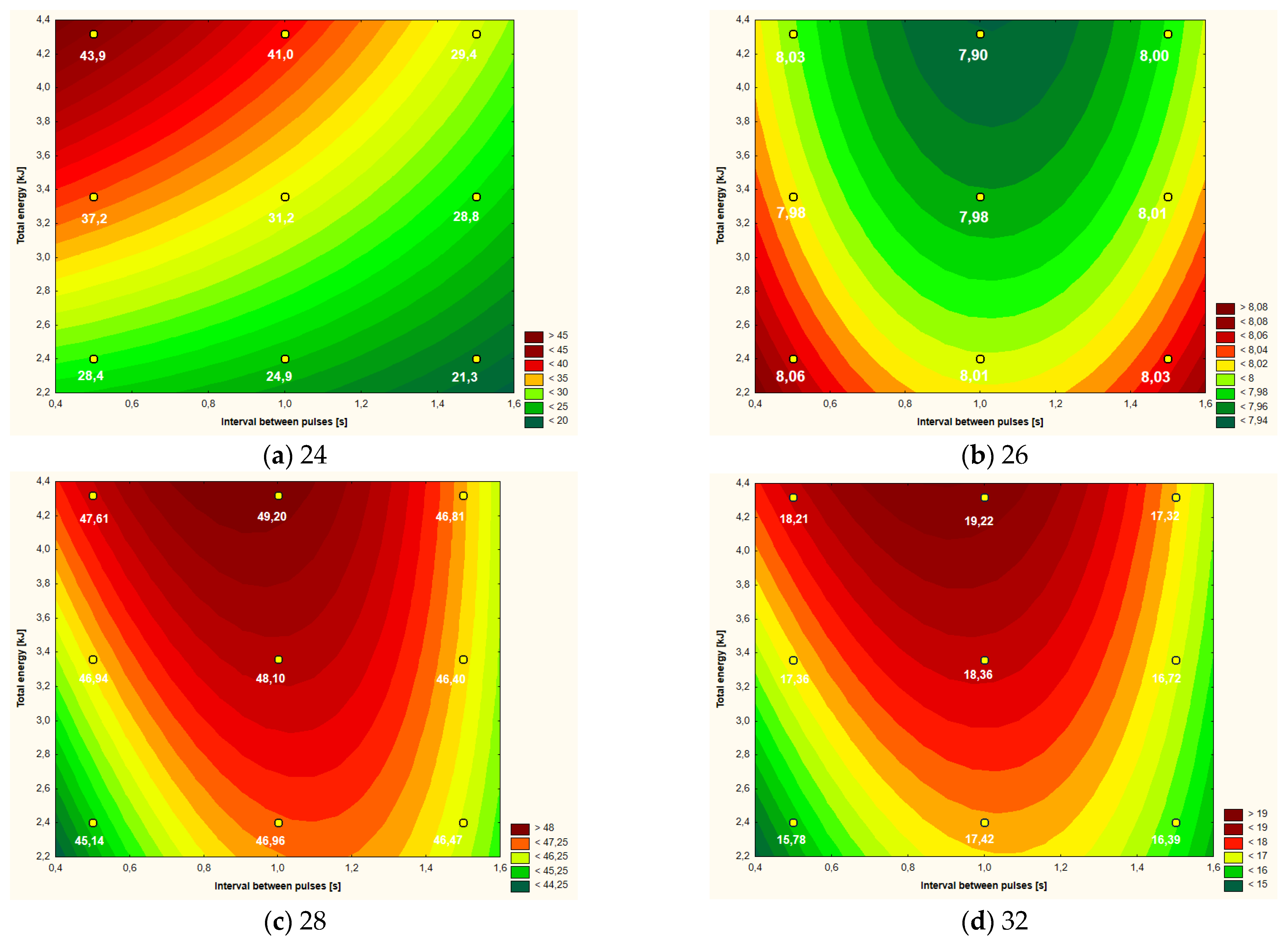
Figure 4.
Temperature increment (a), active acidity (pH) (b), color parameter L* (c) and color parameter b* (d) of liquid whole eggs after CMF treatment (9 values) depending on intervals between pulses and total energy supplied to system.
3.1. Egg White (Albumen)
In all research variants relating to the albumen, statistical differentiation of the analyzed parameters was found (Table 1). The highest increase of the test temperature (by 48.4 °C) took place in the variant in which the highest amount of energy (4.32 kJ) was introduced into the system with the shortest pause between pulses (0.5 s). The means of all variants differed in a statistically significant manner. The lowest temperature increase (by 20.2 °C) was found in the case of CMF treatment with the lowest amount of energy (2.40 kJ) and the largest intervals between pulses.

Table 1.
Physical and rheological parameters of the egg albumen after the CMF treatment (n=2 or n=3).
Table 2 presents the Pareto coefficients understood as the quotients of the “effect” and “standard error” calculated within the “assessment of ANOVA effects” for the analysis of the experiment with the master plan composition of the “response surface”. For all analyzed discriminants, a significant influence of the variables (or their linear interaction) on the obtained “response area” was found. Most often, the amount of energy supplied to the system in the linear aspect had the greatest impact on the variability of the discriminants in the “response surface” model (marked in bold).

Table 2.
Values of linear (L) and quadratic (Q) Pareto coefficients and linear−linear interactions of the main factors (L·L) of the analyzed variables in the “response surface” model for egg white after CMF treatment.
The temperature increase of hen egg white after the CMF treatment in the range from 0.5 to 1.5 s in the case of the variable “CMF pulse interval” and from 2.40 to 4.32 kJ in relation to the second variable, i.e., “total amount of energy supplied to the system” is shown in Figure 2a. The estimation determined with the help of “response curves” can be considered only in the given ranges of variability, which applies to all graphs of this type presented in the paper. In the case of an increase of the temperature of egg white, the greatest changes in this value took place when higher doses of energy were applied to the system, i.e., greater than approximately 4 kJ, with simultaneous shorter intervals between pulses, i.e., not greater than 1 s. In the given range of variables, the greater impact on the final temperature increase had the distance between the pulses, and the smaller amount of energy supplied to the system, which is represented in Figure 2a by isothermal lines running in the vast majority (in the given range of variability) at an angle greater than 45° in relation to the OX axis. In the case of the variability range above approximately 1 s (in relation to the intervals between pulses) and below approximately 3.5 kJ (in terms of the total amount of energy supplied to the system) we have the opposite situation. In this area of variability, the increase in temperature of hen egg white was mainly influenced by one variation factor, i.e., the amount of energy supplied to the system, which is visible in the system of isothermal lines running at an angle much smaller than 45° with respect to the OX axis. The absolute temperature increase of egg white in this area was much smaller (to approximately 31 °C) than in the first discussed range, where this value was over 48 °C.
Taking into account the entire range of variability presented in Figure 2a and analyzing the Pareto coefficients in Table 2, it can be concluded that the total amount of energy (in the linear aspect) supplied to the system had a slightly greater impact on the increase in the temperature of the protein than the intervals between the pulses (also in terms of linear). The quadratic aspects of both variables also had an effect on the endpoint temperature of the protein—except that in this case the effect of the intervals between the pulses was greater. If we add the absolute values of the linear and quadratic Pareto coefficients of these variables, which statistically significantly influenced the final temperature, the obtained values would be similar: 28.25 for the total energy and 29.20 for the intervals between the pulses. The interaction of the linear values of the main factors was not statistically significant for the value of the egg white temperature increase (Table 2).
The main factor influencing the shape of the “response surface” model for the active acidity (pH) of the egg white (Figure 2b) was the amount of energy supplied to the system, albeit only in a linear aspect (Table 2). A general observation was made that with the increase in the total energy supplied to the system, the pH value decreased from the level of about 9.23–9.27 to the value 8.99–9.16 (Figure 2b). Apart from the square aspect of the amount of energy supplied to the system, all other factors had a statistically significant influence on the shape of the curves obtained in Figure 2b.
The L* color parameter of hen’s egg white, expressing its brightness, had the highest values of over 57, and the lowest being 53–54. Pareto analysis showed that the only factor influencing the response area model was the linear interaction of the main factors (Table 2). The highest brightness was characteristic of the egg white subjected to the effects of CMF with a high amount of energy (with small intervals between pulses) and with lower total energy, but with the simultaneous use of the largest intervals between CMF pulses.
The a* color parameter of hen’s egg white ranged from −2.12 to −2.68 and corresponded to the gray color with a slight hint of green (negative values of the a* parameter). The only parameter that statistically significantly influenced the shape of the “response surface” model curves was the total amount of energy (in a linear aspect) supplied to the system (Table 2).
The amount of energy supplied to the system in a linear aspect had the main impact on the b* color parameter of hen egg white (Table 2). The second statistically significant factor, albeit with a smaller influence on the “response surface” model, was the intervals between CMF pulses—also in a linear aspect. Positive values of the b* parameter indicated a slight yellow shade of the raw material. With the increase in the amount of energy, a decrease in the value of the b* parameter was observed from 4.68–5.22 to 3.68–4.13. According to the “response surface” model, the hen’s egg white lost its yellow shade mainly with increasing energy input and to a slightly lesser extent with the simultaneous application of the intervals between CMF pulses (Figure 2c).
The value of hen egg white viscosity expressed in mPa·s (determined at the shear rate of 1000 s−1) according to the “response surface” model (Figure 2d) had the highest values (approximately 5 mPa·s) in the case of using longer intervals between CMF pulses while using rather higher doses of energy supplied to the system. The intervals between the CMF pulses (both in the linear and quadratic aspect) had a greater impact on the shape of the curves than the amount of energy supplied to the system (albeit only in the linear aspect, as the quadratic aspect did not have a significant impact on the model), which is shown in Table 2. Less sticky egg white (up to the value not higher than about 4.5 mPa·s) was obtained during the CMF treatment with a lower value of energy supplied to the system and shorter intervals between pulses.
Taking into account the analyzed variants of egg white, shown in Figure 2, it can be concluded that the most favorable changes took place at medium energy-intensive CMF treatments, i.e., at the level of about 3.2–3.6 kJ. The intervals between the CMF pulses were also significant, but appear to have a smaller effect on the overall physical and rheological characteristics of the egg white.
3.2. Yolk
With regard to the yolk samples, Table 3 shows the mean values of the analyzed parameters depending on the research variant used. Except for the temperature increment, in all other cases the parameters of the control sample were also determined. Statistical differences were found for all analyzed parameters (within the columns). The greatest number of homogeneous groups, and thus the greatest differentiation between the variants, was found in the case of the temperature increment and the active acidity of the yolk after the CMF treatment.

Table 3.
Physical and rheological parameters of the egg albumen after the CMF treatment (n = 2 or n = 3).
In the case of the significance of the influence of the variables on the model of the “response surface”, it can be concluded that in the case of the yolk, the greatest significant impact on the analyzed parameters was the distance between the CMF pulses (Table 4), mainly in the linear aspect (5 out of 6 examined parameters), but in two cases (pH, parameter a*) also in the square aspect. The amount of energy supplied to the system (in a linear aspect) influenced the model of the “response surface” in the case of three parameters, i.e., temperature increment, active acidity and apparent viscosity. The only differentiating factor in the case of yolk, which was not statistically significantly influenced by the analyzed variables, was the b* color parameter.

Table 4.
Values of linear (L) and quadratic (Q) Pareto coefficients and linear−linear interactions of the main factors (L·L) of the analyzed variables in the “response surface” model for egg yolk after CMF treatment.
The amount of energy supplied to the system and the intervals between the CMF pulses (Table 4) had a similar impact on the yolk temperature increase. The interaction of linear factors also had a statistically significant influence on the “response surface” curves. The lowest temperature increase of the yolk at a level slightly higher than 23 °C occurred when the lowest energy values and the longest intervals between pulses were used (Figure 3a). The highest temperature increase (over 56 °C) was recorded with the use of the highest doses of energy and the shortest intervals between pulses.
The pH values ranged in a relatively narrow range from 6.83 to 6.95, although the determined “response area” (Figure 3b) was statistically significantly dependent mainly on the intervals between CMF pulses—both in the linear and square (Table 4). Relatively small, but still statistically significant, was the influence of the amount of energy supplied to the system on the course of the curves.
The brightness of the yolk, expressed as the L* color parameter depended on the intervals between CMF pulses—in a linear aspect. When using shorter intervals between CMF pulses, the brightness of the yolk expressed as the L* parameter was approximately 46–49. With the lengthening of the intervals between the pulses, the yolk darkened slightly and obtained L* values at the level of 44–45.
In the case of the color parameter a* (in positive values showing the red color) only the intervals between the CMF pulses (both in the square and linear aspect) had a significant impact on the model of the “response surface” (Figure 3c). On the basis of the plot of the “response surface” it can be concluded that slightly longer intervals between CMF pulses (over 1 s) result in obtaining a yolk with a slightly stronger red color, i.e., parameter b* of approximately 5.0 (Figure 3c).
The yolk viscosity expressed in mPa·s (at the shear rate of 1000 s−1) was statistically significantly dependent to a similar extent on the intervals between the CMF pulses and the total amount of energy used—in both cases in a linear aspect (Figure 3d). On the basis of the plot of the “response surface”, it was found that the yolk was characterized by the lowest viscosity at the level of about 100 mPa·s, which was subjected to low-energy CMF effects and longer intervals between pulses (Table 4). The highest yolk viscosity (close to 130 mPa·s) was found in the samples subjected to the high-energy CMF impact with short intervals between pulses. When using CMF pulse intervals shorter than 1 s, the amount of energy supplied to the system had a major influence on the increase in viscosity. Above 1 s intervals between the CMF pulses, the influence of the amount of energy on the yolk viscosity clearly decreases, and the effect of the interval time increases (Figure 3d).
In the case of yolk, taking the overall characteristics of the RSM curves (Figure 3) into account, it seems that the lower the energy of CMF interactions was used, the better the physical and rheological parameters were obtained, so a treatment of 2.2–2.6 kJ can be recommended. The role of the CMF intervals between the pulses is important, but less than the amount of energy introduced into the system.
3.3. Liquid Whole Eggs
Table 5 presents the obtained values for the six analyzed parameters in relation to the liquid whole eggs. The smallest impact of the CMF treatment was found in the case of viscosity, where only two variants differed from each other in a statistically significant manner, i.e., the variant in which energy was supplied to the system at the level of 2.40 kJ at 0.5 s intervals between CMF pulses and variant with 3.6 kJ at 1.5 s intervals. The temperature increase of the samples was highly diversified and the highest values were found for variants with short intervals between CMF pulses and higher doses of emitted energy. With regard to the color parameters and the pH value, statistically significant differences were found.

Table 5.
Physical and rheological parameters of the liquid whole eggs after the CMF treatment (n = 2 or n = 3).
Based on the data analysis in Table 6, it can be concluded that for the majority of the analyzed parameters (apart from viscosity), a significant influence of variables or their linear interaction was found. Most often, the amount of energy supplied to the system in the linear aspect (five out of six examined parameters) had the greatest impact on the variability of the parameters in the “response surface” model. None of the analyzed variables influenced the viscosity of the egg mass.

Table 6.
Values of linear (L) and quadratic (Q) Pareto coefficients and linear−linear interactions of the main factors (L·L) of the analyzed variables in the “response surface” model for liquid whole eggs after CMF treatment.
The course of the curves in the plot of “response surface” estimating the temperature rise of the liquid whole eggs (Figure 4a) was similar to the plot for egg white (Figure 2a) and especially for the yolk (Figure 3a). The relationship in all three cases was generally similar, i.e., the greater the energy dose delivered to the system and the shorter the time between CMF pulses, the greater the temperature rise. In the case of LWE, the maximum values of increment were at the level of nearly 44 °C, while the lowest ones were slightly above 21 °C. As in the case of the yolk, the shape of the “response surface” regarding LWE was also dependent on the linear aspects of the amount of energy and the intervals between the pulses as well as their interactions (Table 6).
The active acidity of LWE was the lowest (about 7.90) in the case of high-energy CMF treatment, especially at the average intervals between pulses, i.e., in the range of about 0.8–1.2 s (Figure 4b). Higher pH values (around 8.05) were found with low-energy CMF treatment with simultaneously applied short pulse intervals (0.5–0.6 s) or long (1.4–1.5). The amount of energy supplied to the system (in the linear aspect) and the intervals between the CMF pulses (in the square aspect) had a similar, statistically significant influence on the shape of the “response surface” curves (Table 6).
The main influence on the shape of the curves shown in the graph of the “response surface” of the brightness of the liquid whole eggs (expressed as the color parameter L*) was exerted by the intervals between the pulses (in the square aspect) and the amount of energy—in the linear aspect (Table 6). The darkest variants of LWE (i.e., with the lowest values of the L* parameter of approximately 45–46) were found after the use of low-energy treatment, especially with the simultaneous use of short (0.5–0.7 s) or long (approximately 1.5 s) of the CMF pulse intervals (Figure 4c). The brightest samples of LWE with the L* color parameter at the level of 48–49 were found using medium- and high-energy CMF treatments (at the level of approximately 3.0–4.2 kJ) and average intervals between CMF pulses, i.e., within the range of approximately 0.8–1.3 s (Figure 4c).
The a* color parameter ranged from 1.83 to 3.10, which corresponds essentially to a gray color with a slight shade of red. The main influence on the shape of the “response surface” curves was the amount of energy supplied to the system, both in the linear and quadratic aspect (Table 6). The linear−linear interactions of the main factors also significantly influenced the “response surface”. The linear aspect of the treatment of the energy supplied to the system is particularly visible in the range of values above approximately 3.4 kJ, where the value of the a* parameter is at the level of 2.60–3.10, i.e., with a slightly stronger shade of red, than in the other less energetic ranges of CMF treatment. The liquid whole eggs with the lowest values of the parameter a* (approximately 1.8–2.0) was obtained with low-energy CMF treatment and short intervals between pulses.
In the case of the b* color parameter, i.e., the intensity of the yellow color of LWE, we can see the similarity of the plot of the “response curves” (Figure 4d) to the graph showing the brightness (parameter L*) of this raw material (Figure 4c). The highest values of the b* parameter (approximately 18.0–19.0) were found for high-energy treatment and average values of the interval between CMF pulses, i.e., in the range of approximately 0.8–1.2. The lowest intensity of the yellow color (parameter b* amounting to approximately 16.0) was characteristic for the samples after low-energy CMF treatment and simultaneously short (0.5–0.6 s) or long (1.4–1.5) intervals between pulses (Figure 4d). The total energy supplied to the system (in the linear aspect) and the intervals between the CMF pulses (in the square aspect) had a similar, statistically significant influence on the shape of the obtained “response surface” curves (Table 6).
As in the case of egg white and yolk, the amount of energy introduced into the system was the main factor influencing the physical and rheological characteristics of the liquid whole eggs. Based on the analysis of the characteristics of the RSM curves (Figure 4), it can be recommended in the case of LWE to use higher energy CMF treatments, i.e., at the level of about 4.0–4.4 kJ.
3.4. Correlations
Table 7 shows the correlations between the studied parameters within individual egg raw materials subjected to CMF treatment. Calculations were performed on the mean values included in the “response curve” models. Nine statistically significant pairs of discriminants were found, including for four pairs of parameters the correlation was almost complete according to J. Guilford’s classification, i.e., within the range (0.9–1.0), for two pairs—very high (0.7–0.9) and for three pairs—high (0.5–0.7). Almost complete positive correlations were found between the intensity of the yellow color (parameter b*) and the brightness (L*) in the case of the yolk and the liquid whole eggs. Almost complete correlation, but negative, was found in the case of the egg white between the parameters a* and b* and between the temperature increase and the active acidity. Slightly weaker, but still high correlations occurred in the case of pH and a* parameter of the egg white and the pH and lightness of the yolk (both negative correlations), as well as for the b* color parameter and temperature increase in relation to the LWE (positive correlation).

Table 7.
Correlation coefficients R between the studied parameters within individual egg materials subjected to the effects of the concentrated microwave CMF field (n = 9). Significant correlation coefficients (p < 0.05) are marked in bold with an asterisk.
4. Discussion
Based on the experiment, it was found that both the amount of energy supplied to the system and the intervals between the CMF pulses had a statistically significant impact on the differentiation of the analyzed parameters. Part of the individual variants of CMF treatment was also statistically differentiated in relation to the control sample, which was shown by the analysis of variance and Duncan’s test with regard to the active acidity, color (L*, a*, b*) and apparent viscosity. In 16 out of 18 cases, a statistically significant impact of the amount of energy, intervals between impulses or the interaction of these variables on the “response surface” models, which were an estimation of the values of the studied parameters in the analyzed variability intervals, was found. No such influence on the “response surface” model was found only in the case of the b* yolk color parameter and the apparent viscosity of the egg mass.
The temperature increase of all three analyzed raw materials was higher the shorter the intervals were between the CMF pulses and the higher the energy dose delivered to the system. Earlier own research noted that the use of the impulse mode of CMF treatments, with a similar energy emission to the system (7.2 kJ) as in the case of the continuous mode, leads to stronger heating of the liquid whole eggs [16]. For this reason, the amount of energy emitted into the samples was reduced and the interval between CMF pulses was increased. It allowed a reduction in the temperature increment of LWE to 43.9 °C. The same reduced CMF treatment parameters were used for albumen and yolk where the temperature increments were, respectively 48.4 °C and 56.3 °C. Earlier own research showed that too short intervals between CMF pulses result in a faster increase in the temperature of the egg raw material, despite the total amount of energy supplied to the system. Due to the different density of individual batches of raw material, heat transport was limited in some places, which caused excessive heating of many places (in an archipelagic manner), leading to local denaturation and protein precipitation observed in previous experiments. The phenomenon of uneven heating of various food products treated with a microwave field is a known problem in industrial and gastronomic processing [25]. The temperature increase was positively correlated with the b * color parameter (R = 0.695) in the case of LWE and negatively correlated with the active acidity of the egg white (R = −0.917). The specific and complicated chemical composition of the albumen, yolk and LWE as well as their different dry weights are the reasons why empirical measurements are more reliable than theoretical calculations. Nevertheless, different model studies mainly use density, heat capacity and thermal conductivity of liquid egg products [26].
CMF treatment had a relatively small but statistically significant effect on the reduction of the active acidity value in the case of using the most energetically powerful variants of CMF treatments—mainly in relation to albumen (from approximately 9.2 to approximately 9.0–9.1) and liquid whole eggs (from about 8.1 to about 7.9). Active acidity (pH) may be one of the factors in “hurdle technology” to better inactivate undesirable microbes in liquid foods. For example, studies by Somolinos et al. [27] showed that inactivation of Escherichia coli in orange juice with different pH, subjected to Pulsed Electric Field (PEF) treatment, was statistically greater at higher pH values. Relationships between the impact of new preservation technologies, including PEF, on the pH value of a liquid food product in the context of effective microbiological inactivation have been analyzed by various research teams [28]. This has practical implications for the risk assessment of using new technologies, especially for more acidic products. There is no certainty in which pH ranges of egg raw materials the impact of new technologies could have a stronger inactivation effect of individual microorganisms. Earlier own research showed a significant positive, very high correlation (0.704) between the pH value and the degree of inactivation of Escherichia coli after the PEF treatment, and no such correlation was found after CMF treatment [16]. The pH value, as well as the addition of lysozyme, may potentially be one of the additional factors in “hurdle technologies”, also in the context of pH−lysozyme interactions, as analyzed in the Otosu study [29]. In our study, a statistically significant slight decrease in the pH value after the CMF treatment was found for most variants of the albumen, yolk and liquid whole eggs, which can be considered a favorable phenomenon, especially since there is more ovomucin in fresh egg raw material. Alkalization of albumen is a natural phenomenon associated with the release of carbon dioxide from egg content [30]. According to the data presented by Wang et al. [31], there is a negative correlation between the content of ovomucin and pH. Considering the above, it may be concluded that changes in the albumen alkalinity will significantly reduce the value of other egg quality assessment parameters [32]. Own research showed that CMF treatment did not alkalize the egg raw material, on the contrary, it influenced its slight acidification, which may positively influence the extension of the shelf life of the final product. It is a particularly valuable property of CMF treatment, especially since other methods usually alkalize the egg raw material, e.g., after ultrasound treatment the pH value increases by approximately 0.7, and after heat treatment (64 °C/3 min) by approximately 0.4 [33].
Color and aroma are probably the most relevant quality attributes for fresh eggs and liquid egg products because they are simple indicators of microbiological spoilage [34]. The color of the liquid whole eggs depends primarily on the chemical composition of the yolk, which is closely related to the feeding of laying hens and the natural pigments contained in the feed, mainly xanthophylls and carotenoids present in dried and green fodder, e.g., from alfalfa or maize [35]. Technological processes may also affect the color of the LWE, which is usually associated with denaturation processes (even partial ones) due to temperature or other treatment factors. Thermal treatment on the LWE may lead to a darkening of the samples, e.g., by 6 L* units, with no significant effect on the a* and b* parameters [33]. From the consumer point of view, it cannot be clearly stated whether the darker egg product is a favorable phenomenon, as it depends on local preferences. In the studies by the same team, ultrasound treatment led to greater color changes, as it had a LWE brightening of over 11 L* units, as well as a statistically significant increase in yellow saturation and a decrease in red saturation [33]. In our own research, after the CMF treatment, there was a slight darkening of the LWE samples by about 3 L* units and a slight decrease in the saturation with yellow color by about 2 units, with similar values of the a* parameter of red color. Similar relationships were found in the case of the yolk. The color differences after CMF treatment were smaller in egg white. It was also found that the color of egg products after CMF treatment is significantly negatively correlated with active acidity, although for different raw materials and color components, i.e., for egg white pH and parameter a* (R = −0.670), for yolk pH and L* parameter (R = −0.669) and for the pH of the LWE the parameter L* (R = −0,858) and the parameter b* (R = −0.807). These correlations indicate specific color components that are most susceptible to change in different egg raw materials after CMF treatment. The main reason for this may be the different chemical composition and the way of complexing individual chemical compounds within the egg white, yolk and liquid whole eggs.
Liquid egg products are typical non-Newtonian fluids. Their apparent viscosity depends on shear rates, which is connected with shear-thinning characteristics [36]. The greatest changes in the value of apparent viscosity (from approximately 104 mPa·s to approximately 120–130 mPa·s) were observed in the case of the yolk subjected to the high-energy CMF treatment (at the level above 3.5 kJ) and at the same time shorter intervals between pulses (0.5–0.8 s). The smallest effect of CMF on apparent viscosity was found for liquid whole eggs. LWE and yolk are composed of proteins, phospholipids and triacylglycerols, whose interactions are easily destroyed by a high shear force [37]. Nevertheless the shape of the apparent viscosity curves of all egg raw materials are similar at a high shear rate. It is well known that the shear molecules are oriented in one direction, so the resistance to fluid is reduced [38]. CMF treatment significantly influenced the thinning of the egg white while the yolk had thickened and there was no significant effect of CMF on the apparent viscosity of LWE. It follows that CMF interactions did not break down the protein−lipid complexes in the yolk, therefore, along with the increase in the intensity of the process and the higher final temperature of the yolk, its apparent viscosity increased due to denaturing processes. In order to reduce the apparent viscosity of the yolk, an additional preservation process should be used in the “hurdle technology”, e.g., high pressure carbon dioxide (HPCD), the effectiveness of which in lowering the viscosity of LWE was proven by Sheng et al. [39]. On the other hand, our research shows the possible effectiveness of CMF in thinning the egg white. The apparent viscosity was not correlated with other analyzed factors.
It is worth mentioning that the mechanism of microbial inactivation in food during exposure to the microwave field is not entirely clear. There are several theories on this subject. One of them, called the theory of selective heating, assumes that microorganisms are heated more selectively by the microwave field compared to the surrounding environment, which leads to their inactivation. According to the theory of electroporation, the potential difference on both sides of the bacterial cell membrane causes damage and the formation of the so-called “pores” through which cellular content passes out, which leads to the death of the bacterial cell [16]. In turn, a similar theory of rupture of the cell membrane assumes that the destruction of the cell membrane through ruptures occurs as a result of the generated electrical voltage. A slightly different reason for microbial inactivation is indicated by the coupled magnetic field theory, according to which it is electromagnetic energy, through coupled influence on important bacteria, i.e., protein or DNA molecules, that causes its death [3]. Although the aforementioned theories refer rather to the lethal effect of a nonthermal cause, various studies indicate a synergistic effect of microwave interactions with factors such as pH or moderate temperature, without which the microwave treatment alone did not lead to significant inactivation of microorganisms [40]. Regardless of the actual causes of the phenomenon, it can be concluded that the impact of the microwave field is effective in destroying various microorganisms (including not only bacteria, but also yeasts and mold) and inactivating enzymes. Thus, in microwave treatments it is possible to use not only a strong thermal effect, but also partly its nonthermal effect as part of the “hurdle technology” with the simultaneous use of other technologies. Such an approach should ensure the microbiological safety of final products with less deterioration of the quality of the food, both in terms of the technology and for the consumer.
5. Conclusions
CMF treatment significantly influenced most of the analyzed parameters of liquid egg raw materials. The use of shorter intervals between CMF pulses results in a greater increase in the temperature of the preserved raw materials, which was highly correlated with the b* yolk color parameter (yellow color saturation) and almost completely negatively correlated with the active acidity of the egg white. Changes in the active acidity of the liquid egg raw materials after the CMF treatment were at least highly negatively correlated with the individual color parameters, especially with regard to LWE. CMF treatment caused egg white thinning, yolk thickening, and had no significant effect on the apparent viscosity of liquid whole eggs.
Taking into account the overall impact of CMF on the physical and rheological properties of egg material, it can be concluded that the amount of energy introduced into the system had a greater impact than the intervals between pulses. In industrial practice (as part of the “hurdle technology”), it can be recommended to use lower CMF treatment (at the level of 2.2–2.6 kJ) for the yolk, medium (3.2–3.6 kJ) for egg white and higher (4.0–4.4 kJ) for the liquid whole eggs. The “response surface” models may have a practical application, e.g., in industrial conditions, in determining the influence of individual technological parameters on the final quality of various liquid food products, also described by physical and rheological characteristics.
Author Contributions
Conceptualization, M.O., A.N.-O. and D.M.; methodology, M.O., A.N.-O. and D.M.; software, M.O. and A.M.; validation, D.M. and A.B.; formal analysis, M.O., D.M.; investigation, D.M., M.T., A.B. and D.K.; resources, M.O. and A.N.-O.; data curation, M.O. and A.N.-O.; writing—original draft preparation, M.O., D.M. and D.K.; writing—review and editing, M.O., M.T. and A.M.; visualization, M.O., A.N.-O. and D.M.; supervision, M.O. and D.M.; project administration, D.K. and A.M.; funding acquisition M.O., A.N.-O. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting reported results are stored at Department of Functional Food Products Development, 51-630 Wrocław, Poland. Raw research data can be obtained from a corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vadivambal, R.; Jayas, D.S. Non-uniform temperature distribution during microwave heating of food materials—A review. Food Bioprocess Technol. 2010, 3, 161–171. [Google Scholar] [CrossRef]
- Dolińska, R.; Warchlewski, J.R. Przyszłościowe technologie żywności z udziałem mikrofal i ich wpływ na składniki żywności. Przemysł Spożywczy 2003, 11, 2–6. [Google Scholar]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing–A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, R.F.; Kudra, T. Uniformity issue in microwave drying. Dry. Technol. 2011, 29, 652–660. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, C.; Martin-Gonzalez, M.F.S.; Lopez-Malo, A.; Sosa-Morales, M.E. Recent Studies Related to Microwave Processing of Fluid Foods. Food Bioprocess Technol. 2012, 5, 31–46. [Google Scholar] [CrossRef]
- Dev, S.R.S.; Raghavan, G.S.V.; Gariepy, Y. Dielectric properties of egg components and microwave heating for in-shell pasteurization of eggs. J. Food Eng. 2008, 86, 207–214. [Google Scholar] [CrossRef]
- Shenga, E.; Singh, R.P.; Yadav, A.S. Effect of pasteurization of shell egg on its quality characteristics under ambient storage. J. Food Sci. Technol. Mysore 2010, 47, 420–425. [Google Scholar] [CrossRef]
- Pereira, R.N.; Vincente, A.A. Environmental impact of novel thermal and non-thermal technologies in food processing. Food Res. Int. 2010, 43, 1936–1943. [Google Scholar] [CrossRef]
- Oziembłowski, M.; Dróżdż, M.; Kiełbasa, P.; Dróżdż, T.; Gliniak, M.; Nawara, P.; Ostafin, M. Impact of pulsed electric field on the quality of unpasteurized beer. Prog. Appl. Electr. Eng. 2017, 1–4. [Google Scholar] [CrossRef]
- Ostafin, M.; Miernik, A.; Dróżdż, T.; Nawara, P.; Gliniak, M.; Kiełbasa, P.; Tabor, S. The effect of alternating magnetic field on biofilm formation by Saccharomyces cerevisiae yeast. Prog. Appl. Electr. Eng. 2017, 1–5. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Dróżdż, T.; Kiełbasa, P.; Ostafin, M.; Buski, K.; Oziembłowski, M. Effect of pulsed electric field treatment on shelf life and nutritional value of apple juice. J. Food Sci. Technol. 2019, 56, 1184–1191. [Google Scholar] [CrossRef]
- Dróżdż, T.; Bieńkowski, P.; Kiełbasa, P.; Nawara, P.; Popardowski, E. The research stand to stimulation of biological materials by the various electromagnetic field. Przegląd Elektrotechniczny 2019, 95, 66–69. [Google Scholar]
- Guillard, V.; Mauricio-Iglesias, M.; Gontard, N. Effect of novel food processing methods on packaging: Structure, composition, and migration properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 969–988. [Google Scholar] [CrossRef] [PubMed]
- Oziembłowski, M.; Korzycki, M. Liquid food preserved by unconventional methods. Pol. J. Food Nutr. Sci. 2007, 57, 423–427. [Google Scholar]
- Oziembłowski, M.; Dróżdż, T.; Bobak, Ł.; Nęcka, K.; Lis, S.; Nawara, P.; Wrona, P. Skoncentrowane pole mikrofalowe (CMF) jako niekonwencjonalna metoda utrwalania płynnych produktów spożywczych w ramach “teorii płotkowej”. Przegląd Elektrotechniczny 2016, 92, 113–116. [Google Scholar]
- Oziembłowski, M. Wpływ Pulsacyjnych pól Elektrycznych (PEF) Oraz Skoncentrowanego Pola Mikrofalowego (CMF) na Wybrane Cechy Płynnych Produktów Jajczarskich; Wydawnictwo Uniwersytetu Przyrodnicznego we Wrocławiu: Wrocław, Poland, 2019. [Google Scholar]
- Karisma, A.D.; Hamaba, T.; Fukasawa, T.; Huang, A.N.; Segawa, T.; Fukui, K. Validation of measured microwave absorption and temperature change for development of a single-mode-type microwave heating thermogravimetry apparatus. Rev. Sci. Instrum. 2017, 88, 024101. [Google Scholar] [CrossRef] [PubMed]
- Ignatenko, M.; Motohiko, T. Numerical analysis of the microwave heating of compacted copper powders in single-mode cavity. Jpn. J. Appl. Phys. 2011, 50, 097302. [Google Scholar] [CrossRef]
- Savary, E.; Thuault, A.; Hornez, J.C.; Descamps, M.; Marinel, S.; Leriche, A. Frittage micro-ondes en cavit´e monomode de bioc´eramiques (Microwaves sintering of bioceramics in a single mode cavity). MATEC Web Conf. 2013, 7, 04017. [Google Scholar] [CrossRef]
- Molinari, F.; Maignan, A.; Marinel, S.; Savary, E. Fast synthesis of SrFe12O19 hexaferrite in a single-mode microwave cavity. Ceram. Int. 2017, 43, 4229–4234. [Google Scholar] [CrossRef]
- Ozkoc, S.; Sumnu, G.; Sahin, S. Recent developments in microwave heating, chapter 20. In Emerging Technologies for Food Processing; Sun, D., Ed.; Academic Press: London, UK, 2014; pp. 361–383. [Google Scholar]
- Tlili, M.; Deshours, F.; Alquié, G.; Kokabi, H.; Hardinata, S.; Koskas, F. Microwave resonant sensor for non-invasive characterization of biological tissues. IRBM 2018, 39, 445–450. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C.; Zhang, Y.; Niu, D.; Du, J. Effects of aqueous chlorine dioxide treatment on enzymatic browning and shelflife of fresh-cut asparagus lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2010, 58, 232–238. [Google Scholar] [CrossRef]
- Necidová, L.; Bursová, S.; Ježek, F.; Haruštiaková, D.; Vorlová, L.; Golian, J. Effect of preservatives on the shelf-life and sensory characteristics of pasteurized liquid whole egg stored at 4 °C. Poult. Sci. 2019, 98, 5940–5948. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Ramaswamy, H.S. Microwave pasteurization and sterilization of foods. In Handbook of Food Preservation, 2nd ed.; Rahman, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 691–711. [Google Scholar]
- Coimbra, J.S.R.; Gabas, A.L.; Minim, L.A.; Rojas, E.E.G.; Telis, V.R.N.; Telis-Romero, J. Density, heat capacity and thermal conductivity of liquid egg products. J. Food Eng. 2006, 74, 186–190. [Google Scholar] [CrossRef]
- Somolinos, M.; García, D.; Manas, P.; Condon, S.; Pagan, R. Organic acids make Escherichia coli more resistant to pulsed electric fields at acid pH. Int. J. Food Microbiol. 2010, 136, 381–384. [Google Scholar] [CrossRef]
- Sagarzazu, N.; Cebrian, G.; Pagan, R.; Condon, S.; Manas, P. Resistance of Campylobacter jejuni to heat and to pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2010, 11, 283–289. [Google Scholar] [CrossRef]
- Otosu, T.; Kobayashi, K.; Yamaguchi, S. Local pH at the surface of hen egg white lysozyme. Chem. Phys. Lett. 2018, 693, 165–169. [Google Scholar] [CrossRef]
- Monira, K.; Salahuddin, M.; Miah, G. Effect of breed and holding period on egg quality characteristics of chicken. Int. J. Poult. Sci. 2003, 4, 261–263. [Google Scholar]
- Wang, Y.; Wang, Z.; Shan, Y. Assessment of the relationship between ovomucin and albumen quality of shell eggs during storage. Poult. Sci. 2019, 98, 473–479. [Google Scholar] [CrossRef]
- Drabik, K.; Batkowska, J.; Próchniak, T.; Horecka, B. Citric acid as a factor limiting changes in the quality of table eggs during their storage. Poult. Sci. 2021. [Google Scholar] [CrossRef]
- Bi, X.; Wang, X.; Chen, Y.; Chen, L.; Xing, Y.; Che, Z. Effects of combination treatments of lysozyme and high power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochemistry 2020, 60, 104763. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.M.; Fernández, A. Consumer acceptance of UV-C treated liquid egg products and preparations with UV-C treated eggs. Innov. Food Sci. Emerg. Technol. 2012, 14, 107–114. [Google Scholar] [CrossRef]
- Seemann, M. Factors which influence pigmentation. Lohmann Inf. 2000, 24, 20–24. [Google Scholar]
- Severa, L.; Nedomová, Š.; Buchar, J. Influence of storing time and temperature on the viscosity of an egg yolk. J. Food Eng. 2010, 96, 266–269. [Google Scholar] [CrossRef]
- Atılgan, M.; Unluturk, S. Rheological properties of liquid egg products (LEPs). Int. J. Food Prop. 2008, 11, 296–309. [Google Scholar] [CrossRef]
- Tang, Q.; Munro, P.A.; Mccarthy, O.J. Rheology of whey protein concentrate solutions as a function of concentration, temperature, pH and salt concentration. J. Dairy Res. 1993, 60, 349–361. [Google Scholar] [CrossRef]
- Sheng, L.; Zu, L.; Ma, M. Study of high pressure carbon dioxide on the physicochemical, interfacial and rheological properties of liquid whole egg. Food Chem. 2021, 337, 127989. [Google Scholar] [CrossRef]
- Kozempel, M.F.; Cook, R.D.; Scullen, O.J.; Annous, B.A. Development of a process for detecting nonthermal effects of microwave energy on microorganisms at low temperature. J. Food Process. Preserv. 2000, 24, 287–301. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).