The Effect of Concentrated Microwave Field (CMF) on Selected Physical and Rheological Properties of Liquid Egg Products
Abstract
:1. Introduction
2. Material and Method
2.1. Preparing the Egg Raw Material
2.2. Device for Microwave Treatment
2.3. Microwave Process Parameters
2.4. Measurements of the Temperature, pH and Color
2.5. Analysis of Apparent Viscosity
2.6. Statistical Analysis
3. Results
3.1. Egg White (Albumen)
3.2. Yolk
3.3. Liquid Whole Eggs
3.4. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vadivambal, R.; Jayas, D.S. Non-uniform temperature distribution during microwave heating of food materials—A review. Food Bioprocess Technol. 2010, 3, 161–171. [Google Scholar] [CrossRef]
- Dolińska, R.; Warchlewski, J.R. Przyszłościowe technologie żywności z udziałem mikrofal i ich wpływ na składniki żywności. Przemysł Spożywczy 2003, 11, 2–6. [Google Scholar]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing–A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, R.F.; Kudra, T. Uniformity issue in microwave drying. Dry. Technol. 2011, 29, 652–660. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, C.; Martin-Gonzalez, M.F.S.; Lopez-Malo, A.; Sosa-Morales, M.E. Recent Studies Related to Microwave Processing of Fluid Foods. Food Bioprocess Technol. 2012, 5, 31–46. [Google Scholar] [CrossRef]
- Dev, S.R.S.; Raghavan, G.S.V.; Gariepy, Y. Dielectric properties of egg components and microwave heating for in-shell pasteurization of eggs. J. Food Eng. 2008, 86, 207–214. [Google Scholar] [CrossRef]
- Shenga, E.; Singh, R.P.; Yadav, A.S. Effect of pasteurization of shell egg on its quality characteristics under ambient storage. J. Food Sci. Technol. Mysore 2010, 47, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.N.; Vincente, A.A. Environmental impact of novel thermal and non-thermal technologies in food processing. Food Res. Int. 2010, 43, 1936–1943. [Google Scholar] [CrossRef] [Green Version]
- Oziembłowski, M.; Dróżdż, M.; Kiełbasa, P.; Dróżdż, T.; Gliniak, M.; Nawara, P.; Ostafin, M. Impact of pulsed electric field on the quality of unpasteurized beer. Prog. Appl. Electr. Eng. 2017, 1–4. [Google Scholar] [CrossRef]
- Ostafin, M.; Miernik, A.; Dróżdż, T.; Nawara, P.; Gliniak, M.; Kiełbasa, P.; Tabor, S. The effect of alternating magnetic field on biofilm formation by Saccharomyces cerevisiae yeast. Prog. Appl. Electr. Eng. 2017, 1–5. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Dróżdż, T.; Kiełbasa, P.; Ostafin, M.; Buski, K.; Oziembłowski, M. Effect of pulsed electric field treatment on shelf life and nutritional value of apple juice. J. Food Sci. Technol. 2019, 56, 1184–1191. [Google Scholar] [CrossRef] [Green Version]
- Dróżdż, T.; Bieńkowski, P.; Kiełbasa, P.; Nawara, P.; Popardowski, E. The research stand to stimulation of biological materials by the various electromagnetic field. Przegląd Elektrotechniczny 2019, 95, 66–69. [Google Scholar]
- Guillard, V.; Mauricio-Iglesias, M.; Gontard, N. Effect of novel food processing methods on packaging: Structure, composition, and migration properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 969–988. [Google Scholar] [CrossRef] [PubMed]
- Oziembłowski, M.; Korzycki, M. Liquid food preserved by unconventional methods. Pol. J. Food Nutr. Sci. 2007, 57, 423–427. [Google Scholar]
- Oziembłowski, M.; Dróżdż, T.; Bobak, Ł.; Nęcka, K.; Lis, S.; Nawara, P.; Wrona, P. Skoncentrowane pole mikrofalowe (CMF) jako niekonwencjonalna metoda utrwalania płynnych produktów spożywczych w ramach “teorii płotkowej”. Przegląd Elektrotechniczny 2016, 92, 113–116. [Google Scholar]
- Oziembłowski, M. Wpływ Pulsacyjnych pól Elektrycznych (PEF) Oraz Skoncentrowanego Pola Mikrofalowego (CMF) na Wybrane Cechy Płynnych Produktów Jajczarskich; Wydawnictwo Uniwersytetu Przyrodnicznego we Wrocławiu: Wrocław, Poland, 2019. [Google Scholar]
- Karisma, A.D.; Hamaba, T.; Fukasawa, T.; Huang, A.N.; Segawa, T.; Fukui, K. Validation of measured microwave absorption and temperature change for development of a single-mode-type microwave heating thermogravimetry apparatus. Rev. Sci. Instrum. 2017, 88, 024101. [Google Scholar] [CrossRef] [PubMed]
- Ignatenko, M.; Motohiko, T. Numerical analysis of the microwave heating of compacted copper powders in single-mode cavity. Jpn. J. Appl. Phys. 2011, 50, 097302. [Google Scholar] [CrossRef]
- Savary, E.; Thuault, A.; Hornez, J.C.; Descamps, M.; Marinel, S.; Leriche, A. Frittage micro-ondes en cavit´e monomode de bioc´eramiques (Microwaves sintering of bioceramics in a single mode cavity). MATEC Web Conf. 2013, 7, 04017. [Google Scholar] [CrossRef] [Green Version]
- Molinari, F.; Maignan, A.; Marinel, S.; Savary, E. Fast synthesis of SrFe12O19 hexaferrite in a single-mode microwave cavity. Ceram. Int. 2017, 43, 4229–4234. [Google Scholar] [CrossRef]
- Ozkoc, S.; Sumnu, G.; Sahin, S. Recent developments in microwave heating, chapter 20. In Emerging Technologies for Food Processing; Sun, D., Ed.; Academic Press: London, UK, 2014; pp. 361–383. [Google Scholar]
- Tlili, M.; Deshours, F.; Alquié, G.; Kokabi, H.; Hardinata, S.; Koskas, F. Microwave resonant sensor for non-invasive characterization of biological tissues. IRBM 2018, 39, 445–450. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C.; Zhang, Y.; Niu, D.; Du, J. Effects of aqueous chlorine dioxide treatment on enzymatic browning and shelflife of fresh-cut asparagus lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2010, 58, 232–238. [Google Scholar] [CrossRef]
- Necidová, L.; Bursová, S.; Ježek, F.; Haruštiaková, D.; Vorlová, L.; Golian, J. Effect of preservatives on the shelf-life and sensory characteristics of pasteurized liquid whole egg stored at 4 °C. Poult. Sci. 2019, 98, 5940–5948. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Ramaswamy, H.S. Microwave pasteurization and sterilization of foods. In Handbook of Food Preservation, 2nd ed.; Rahman, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 691–711. [Google Scholar]
- Coimbra, J.S.R.; Gabas, A.L.; Minim, L.A.; Rojas, E.E.G.; Telis, V.R.N.; Telis-Romero, J. Density, heat capacity and thermal conductivity of liquid egg products. J. Food Eng. 2006, 74, 186–190. [Google Scholar] [CrossRef]
- Somolinos, M.; García, D.; Manas, P.; Condon, S.; Pagan, R. Organic acids make Escherichia coli more resistant to pulsed electric fields at acid pH. Int. J. Food Microbiol. 2010, 136, 381–384. [Google Scholar] [CrossRef]
- Sagarzazu, N.; Cebrian, G.; Pagan, R.; Condon, S.; Manas, P. Resistance of Campylobacter jejuni to heat and to pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2010, 11, 283–289. [Google Scholar] [CrossRef]
- Otosu, T.; Kobayashi, K.; Yamaguchi, S. Local pH at the surface of hen egg white lysozyme. Chem. Phys. Lett. 2018, 693, 165–169. [Google Scholar] [CrossRef]
- Monira, K.; Salahuddin, M.; Miah, G. Effect of breed and holding period on egg quality characteristics of chicken. Int. J. Poult. Sci. 2003, 4, 261–263. [Google Scholar]
- Wang, Y.; Wang, Z.; Shan, Y. Assessment of the relationship between ovomucin and albumen quality of shell eggs during storage. Poult. Sci. 2019, 98, 473–479. [Google Scholar] [CrossRef]
- Drabik, K.; Batkowska, J.; Próchniak, T.; Horecka, B. Citric acid as a factor limiting changes in the quality of table eggs during their storage. Poult. Sci. 2021. [Google Scholar] [CrossRef]
- Bi, X.; Wang, X.; Chen, Y.; Chen, L.; Xing, Y.; Che, Z. Effects of combination treatments of lysozyme and high power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochemistry 2020, 60, 104763. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.M.; Fernández, A. Consumer acceptance of UV-C treated liquid egg products and preparations with UV-C treated eggs. Innov. Food Sci. Emerg. Technol. 2012, 14, 107–114. [Google Scholar] [CrossRef]
- Seemann, M. Factors which influence pigmentation. Lohmann Inf. 2000, 24, 20–24. [Google Scholar]
- Severa, L.; Nedomová, Š.; Buchar, J. Influence of storing time and temperature on the viscosity of an egg yolk. J. Food Eng. 2010, 96, 266–269. [Google Scholar] [CrossRef]
- Atılgan, M.; Unluturk, S. Rheological properties of liquid egg products (LEPs). Int. J. Food Prop. 2008, 11, 296–309. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Munro, P.A.; Mccarthy, O.J. Rheology of whey protein concentrate solutions as a function of concentration, temperature, pH and salt concentration. J. Dairy Res. 1993, 60, 349–361. [Google Scholar] [CrossRef]
- Sheng, L.; Zu, L.; Ma, M. Study of high pressure carbon dioxide on the physicochemical, interfacial and rheological properties of liquid whole egg. Food Chem. 2021, 337, 127989. [Google Scholar] [CrossRef]
- Kozempel, M.F.; Cook, R.D.; Scullen, O.J.; Annous, B.A. Development of a process for detecting nonthermal effects of microwave energy on microorganisms at low temperature. J. Food Process. Preserv. 2000, 24, 287–301. [Google Scholar] [CrossRef]



| Variant | ΔT ( °C) | pH | L* | a* | b* | Apparent Viscosity (mPa·s) |
|---|---|---|---|---|---|---|
| Control | - | 9.21b,c | 54.46 b,c | −2.47 b,c | 4.77 a,b | 5.053 a |
| 2.40kJ (0.5s) | 30.8 e | 9.28 a | 54.28 b,c | −2.62 c | 5.22 a | 3.760 d |
| 3.36kJ (0.5s) | 42.8 b | 9.05 e | 54.99 a,b,c | −2.36 a,b,c | 4.76 a,b | 4.223 c,d |
| 4.32kJ (0.5s) | 48.4 a | 8.99 f | 57.12 a,b | −2.24 a,b | 3.91 d,e | 4.383 b,c |
| 2.40kJ (1.0s) | 24.0 g | 9.23 b | 56.06 a,b,c | −2.68 c | 5.21 a | 4.507 a,b,c |
| 3.36kJ (1.0s) | 30.9 e | 9.19 c | 55.54 a,b,c | −2.37 a,b,c | 4.22 b,c,d,e | 4.937 a,b |
| 4.32kJ (1.0s) | 36.8 c | 9.16 d | 54.13 b,c | −2.27 a,b | 4.13 c,d,e | 5.050 a |
| 2.40kJ (1.5s) | 20.2 h | 9.27 a | 55.56 a,b,c | −2.50 b,c | 4.68 a,b,c | 4.927 a,b |
| 3.36kJ (1.5s) | 29.0 f | 9.19 c | 57.64 a | −2.56 b,c | 4.47 b,c,d | 4.943 a,b |
| 4.32kJ (1.5s) | 35.2 d | 9.14 d | 53.38 c | −2.12 a | 3.68 e | 4.950 a,b |
| Variables | ΔT [ °C] | pH | L* | a* | b* | Apparent Viscosity [mPa·s] |
|---|---|---|---|---|---|---|
| Energy (L) | 25.184 * | −7.580 * | −0.515 | 4.578 * | −6.648 * | 2.460 * |
| Interval (L) | −20.857 * | 4.288 * | 0.076 | 0.092 | −2.070 * | 5.072 * |
| Energy (Q) | −3.069 * | 1.637 | −1.372 | 0.070 | 0.371 | −0.871 |
| Interval (Q) | 8.347 * | −2.804 * | 0.500 | 0.339 | 0.016 | −2.380 * |
| Interaction (L·L) | −1.766 | 3.001 * | −2.520 * | 0.000 | 0.730 | −1.519 |
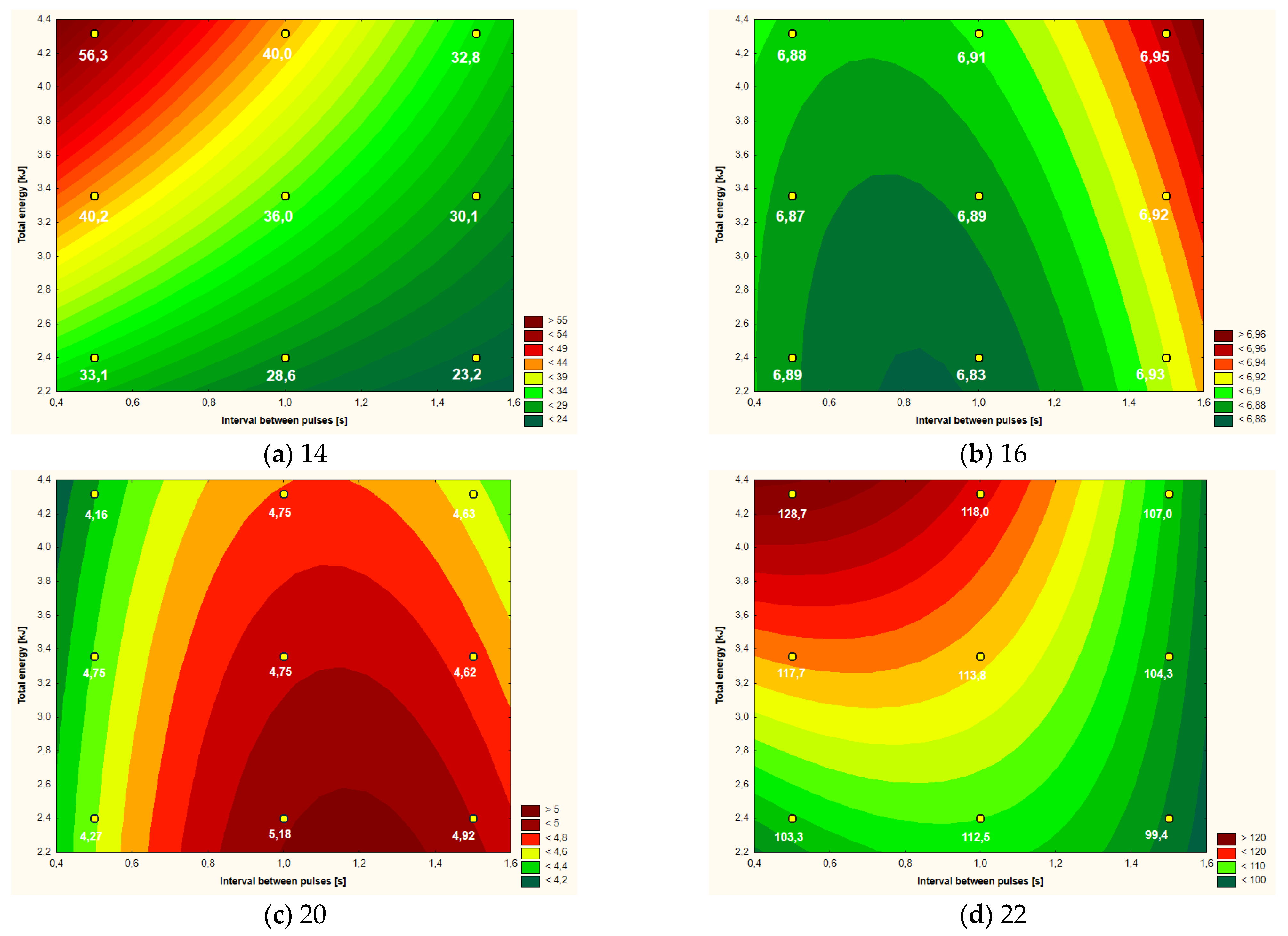
| Variant | ΔT (oC) | pH | L* | a* | b* | Apparent Viscosity (mPa·s) |
|---|---|---|---|---|---|---|
| Control | - | 6.94 a,b | 46.21 a,b | 4.20 d | 20.62 a,b | 103.7 b |
| 2.40 kJ (0.5 s) | 33.1 d | 6.89 e,f | 46.59 a,b | 4.27 c,d | 19.99 a,b | 103.3 b |
| 3.36 kJ (0.5 s) | 40.2 b | 6.87 g | 49.39 a | 4.75 b | 23.28 a | 117.7 a,b |
| 4.32 kJ (0.5 s) | 56.3 a | 6.88 f,g | 45.68 b | 4.16 d | 19.05 a,b | 128.7 a |
| 2.40 kJ (1.0 s) | 28.6 f | 6.83 h | 46.72 a,b | 5.18 a | 20.52 a,b | 112.5 a,b |
| 3.36 kJ (1.0 s) | 36.0 c | 6.89 e,f | 45.10 b | 4.75 b | 19.98 a,b | 113.8 a,b |
| 4.32 kJ (1.0 s) | 40.0 b | 6.91 d,e | 46.33 a,b | 4.75 b | 21.53 a,b | 118.0 a,b |
| 2.40 kJ (1.5 s) | 23.2 g | 6.93 b,c | 43.90 b | 4.92 a,b | 18.45 b | 99.4 b |
| 3.36 kJ (1.5 s) | 30.1 e | 6.92 c,d | 45.10 b | 4.62 b,c | 19.55 a,b | 104.3 b |
| 4.32 kJ (1.5 s) | 32.8 d | 6.95 a | 44.15 b | 4.63 b,c | 18.01 b | 107.0 b |
| Variables | ΔT ( °C) | pH | L* | a* | b* | Apparent Viscosity (mPa·s) |
|---|---|---|---|---|---|---|
| Energy (L) | 12.755 * | 2.331 * | −0.359 | −2.034 | −0.104 | 2.556 * |
| Interval (L) | −12.553 * | 4.144 * | −2.920 * | 2.418 * | −1.781 | 2.589 * |
| Energy (Q) | −0.023 | 0.312 | −0.803 | −0.177 | −1.065 | −0.051 |
| Interval (Q) | 0.895 | 2.734 * | 0.121 | −2.699 * | −0.648 | −1.103 |
| Interaction (L·L) | −4.807 * | 0.952 | 0.492 | −0.510 | 0.175 | −1.445 |
| Variant | ΔT [oC] | pH | L* | a* | b* | Apparent Viscosity [mPa·s] |
|---|---|---|---|---|---|---|
| Control | - | 8.12 a | 48.08 a,b | 2.32 d | 18.23 a,b | 7.483 a,b |
| 2.40 kJ (0.5 s) | 28.4 f | 8.06 b | 45.14 c | 1.96 e,f | 15.78 c | 7.240 b |
| 3.36 kJ (0.5 s) | 37.2 c | 7.98 f | 46.94 a,b,c | 2.00 e | 17.36 a,b,c | 7.587 a,b |
| 4.32 kJ (0.5 s) | 43.9 a | 8.03 c,d | 47.61 a,b,c | 3.10 a | 18.21 a,b | 7.397 a,b |
| 2.40 kJ (1.0 s) | 24.9 g | 8.01 c,d,e | 46.96 a,b,c | 1.83 f | 17.42 a,b,c | 7.263 a,b |
| 3.36 kJ (1.0 s) | 31.2 d | 7.98 f | 48.10 a,b | 2.49 c | 18.36 a,b | 7.473 a,b |
| 4.32 kJ (1.0 s) | 41.0 b | 7.90 g | 49.20 a | 2.64 b | 19.22 a | 7.607 a,b |
| 2.40 kJ (1.5 s) | 21.3 h | 8.03 c | 46.47 b,c | 2.44 c,d | 16.39 b,c | 7.490 a,b |
| 3.36 kJ (1.5 s) | 28.8 f | 8.01 d,e | 46.40 b,c | 2.08 e | 16.72 b,c | 7.667 a |
| 4.32 kJ (1.5 s) | 29.4 e | 8.00 e,f | 46.81 a,b,c | 2.63 b | 17.32 a,b,c | 7.287 a,b |
| Variables | ΔT ( °C) | pH | L* | a* | b* | Apparent Viscosity (mPa·s) |
|---|---|---|---|---|---|---|
| Energy (L) | 14.349 * | −3.597* | 2.702 * | 7.008 * | 3.071 * | 0.943 |
| Interval (L) | −10.843 * | −0.583 | −0.002 | 0.294 | −0.549 | 0.699 |
| Energy (Q) | −0.696 | 0.702 | −0.208 | 2.167* | −0.183 | −2.053 |
| Interval (Q) | −0.628 | 3.430 * | −3.031 * | −0.280 | −3.025 * | 0.227 |
| Interaction (L·L) | −3.276 * | 0.000 | −1.400 | −3.764 * | −1.100 | −1.401 |
| Delta T | pH | L* | a* | b* | Viscosity | |
|---|---|---|---|---|---|---|
| Egg white (albumen) | ||||||
| DeltaT | 1.000 | −0.917 * | −0.016 | 0.665 | −0.504 | −0.262 |
| pH | 1.000 | −0.192 | −0.670 * | 0.565 | 0.055 | |
| L* | 1.000 | −0.370 | 0.098 | 0.050 | ||
| a* | 1.000 | −0.909 * | 0.366 | |||
| b* | 1.000 | −0.592 | ||||
| Viscosity | 1.000 | |||||
| Yolk | ||||||
| DeltaT | 1.000 | −0.229 | 0.317 | −0.644 | 0.211 | 0.115 |
| pH | 1.000 | −0.669 * | −0.219 | −0.551 | −0.598 | |
| L* | 1.000 | 0.028 | 0.922 * | 0.282 | ||
| a* | 1.000 | 0.213 | −0.095 | |||
| b* | 1.000 | 0.137 | ||||
| Viscosity | 1.000 | |||||
| Liquid whole eggs | ||||||
| DeltaT | 1.000 | −0.471 | 0.600 | 0.562 | 0.695 * | 0.302 |
| pH | 1.000 | −0.858 * | −0.206 | −0.807 * | −0.550 | |
| L* | 1.000 | 0.547 | 0.978 * | 0.420 | ||
| a* | 1.000 | 0.562 | 0.076 | |||
| b* | 1.000 | 0.314 | ||||
| Viscosity | 1.000 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oziembłowski, M.; Nawirska-Olszańska, A.; Maksimowski, D.; Trenka, M.; Break, A.; Kulig, D.; Miernik, A. The Effect of Concentrated Microwave Field (CMF) on Selected Physical and Rheological Properties of Liquid Egg Products. Appl. Sci. 2021, 11, 1832. https://doi.org/10.3390/app11041832
Oziembłowski M, Nawirska-Olszańska A, Maksimowski D, Trenka M, Break A, Kulig D, Miernik A. The Effect of Concentrated Microwave Field (CMF) on Selected Physical and Rheological Properties of Liquid Egg Products. Applied Sciences. 2021; 11(4):1832. https://doi.org/10.3390/app11041832
Chicago/Turabian StyleOziembłowski, Maciej, Agnieszka Nawirska-Olszańska, Damian Maksimowski, Magdalena Trenka, Artur Break, Dominika Kulig, and Anna Miernik. 2021. "The Effect of Concentrated Microwave Field (CMF) on Selected Physical and Rheological Properties of Liquid Egg Products" Applied Sciences 11, no. 4: 1832. https://doi.org/10.3390/app11041832
APA StyleOziembłowski, M., Nawirska-Olszańska, A., Maksimowski, D., Trenka, M., Break, A., Kulig, D., & Miernik, A. (2021). The Effect of Concentrated Microwave Field (CMF) on Selected Physical and Rheological Properties of Liquid Egg Products. Applied Sciences, 11(4), 1832. https://doi.org/10.3390/app11041832








