Splatters and Aerosols Contamination in Dental Aerosol Generating Procedures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Statistical Analysis
3. Results
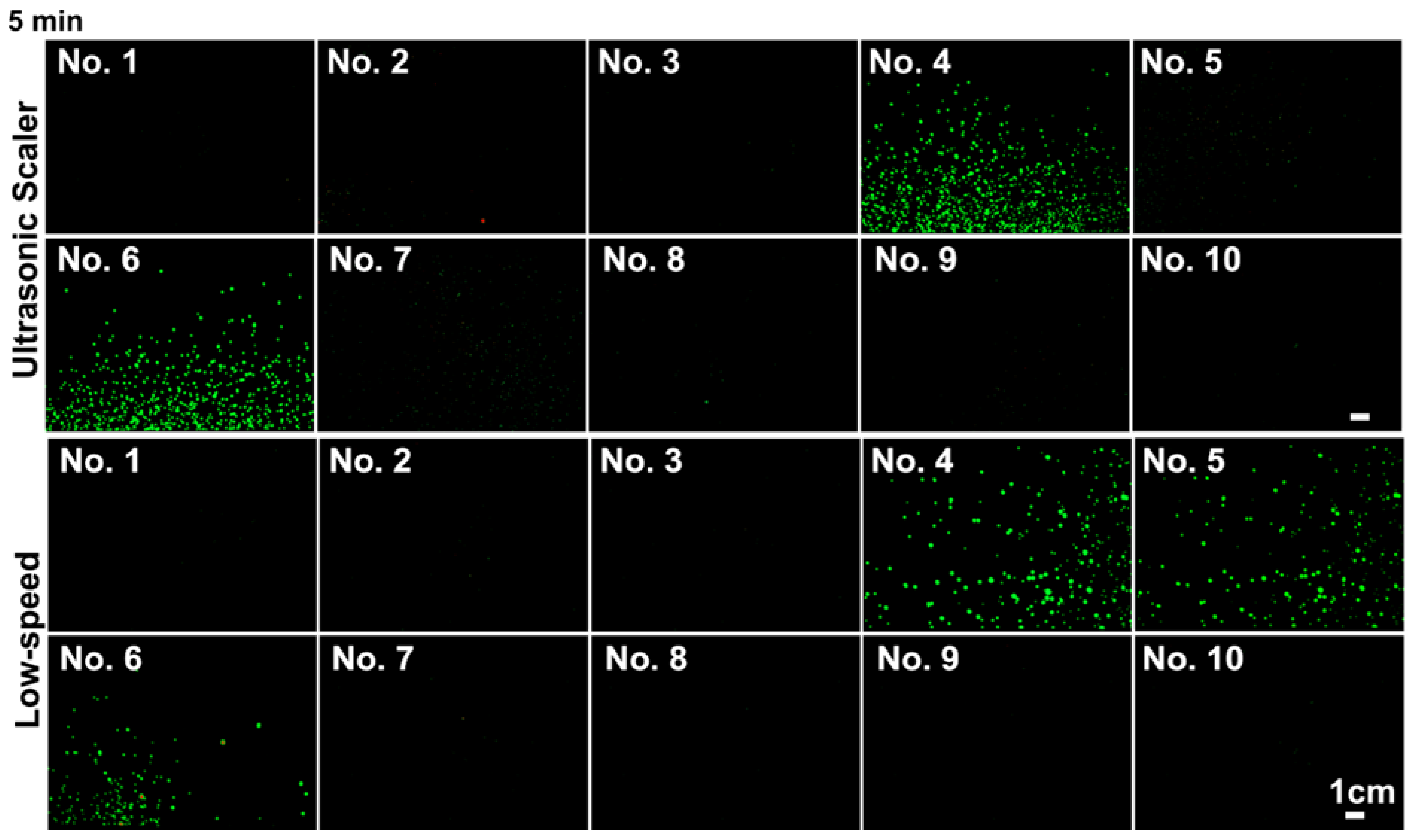
3.1. Splatter Distribution Generated during 15 s Mock Procedures
3.2. Presence of Aerosols, 120 cm away from the Source
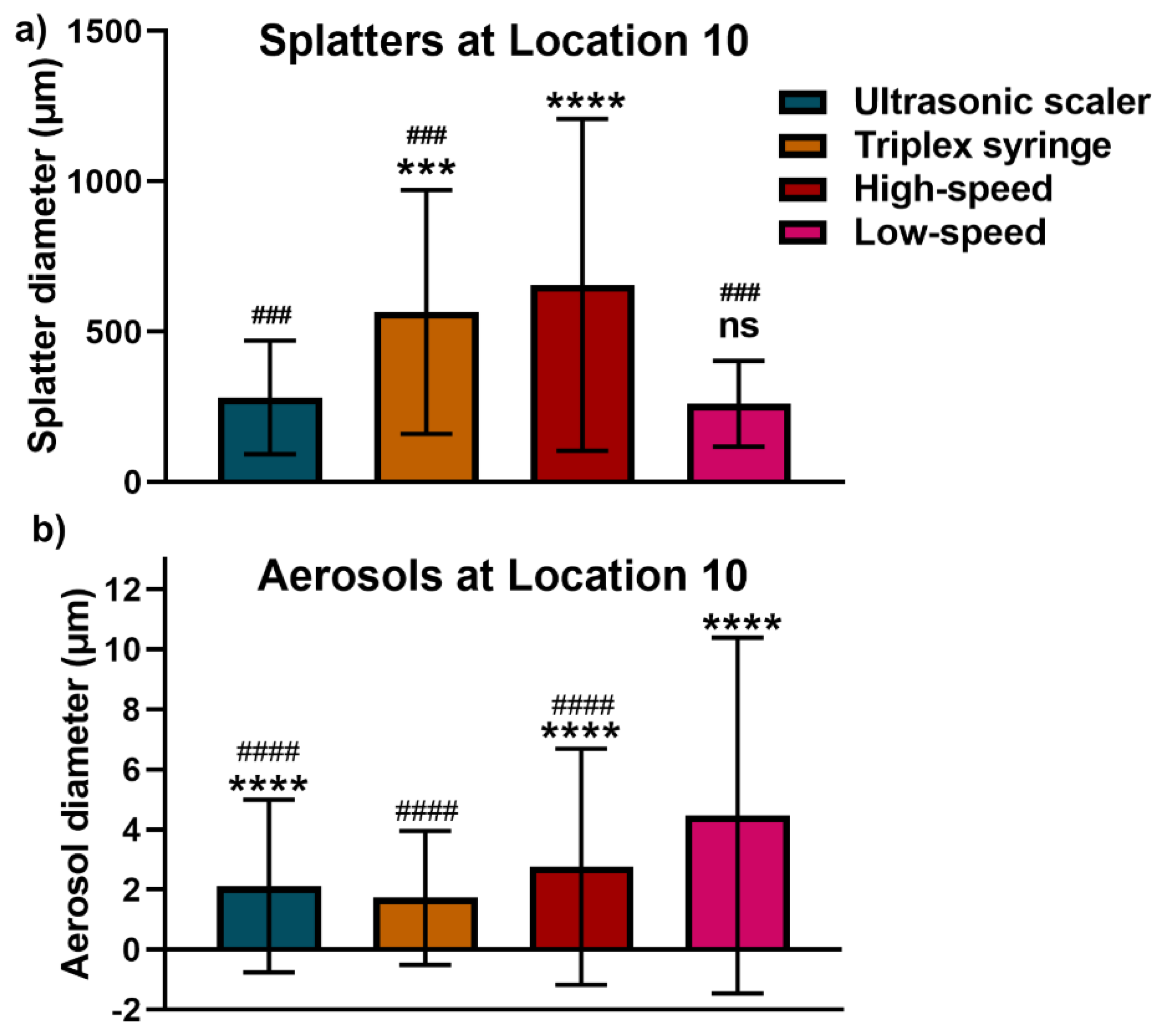
3.3. Comparison of Splatter and Aerosol Particle Size at Location 10
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrel, S.K.; Molinari, J. Aerosols and splatter in dentistry: A brief review of the literature and infection control implications. J. Am. Dent. Assoc. 2004, 135, 429–437. [Google Scholar] [CrossRef]
- Volgenant, C.M.C.; de Soet, J.J. Cross-transmission in the dental office: Does this make you ill? Curr. Oral Health Rep. 2018, 5, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Zemouri, C.; de Soet, H.; Crielaard, W.; Laheij, A. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS ONE 2017, 12, e0178007. [Google Scholar] [CrossRef] [PubMed]
- Dahlke, W.O.; Cottam, M.R.; Herring, M.C.; Leavitt, J.M.; Ditmyer, M.M.; Walker, R.S. Evaluation of the spatter-reduction effectiveness of two dry-field isolation techniques. J. Am. Dent. Assoc. 2012, 143, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Ivanovski, S. Saliva - friend and foe in the COVID-19 outbreak. Diagnostics 2020, 10, 290. [Google Scholar] [CrossRef]
- Shiu, E.Y.C.; Leung, N.H.L.; Cowling, B.J. Controversy around airborne versus droplet transmission of respiratory viruses: Implication for infection prevention. Curr. Opin. Infect. Dis. 2019, 32, 372–379. [Google Scholar] [CrossRef]
- Yan, J.; Grantham, M.; Pantelic, J.; Bueno de Mesquita, P.J.; Albert, B.; Liu, F.; Ehrman, S.; Milton, D.K.; Consortium, E. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA 2018, 115, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Leggat, P.A.; Kedjarune, U. Bacterial aerosols in the dental clinic: A review. Int. Dent. J. 2001, 51, 39–44. [Google Scholar] [CrossRef]
- James, R.; Mani, A. Dental aerosols: A silent hazard in dentistry! Int. J. Sci. Res. 2016, 5, 1761–1763. [Google Scholar]
- Tellier, R.; Li, Y.; Cowling, B.J.; Tang, J.W. Recognition of aerosol transmission of infectious agents: A commentary. BMC Infect. Dis 2019, 19, 101. [Google Scholar] [CrossRef]
- WHO/2019-nCoV/Oral_health/2020.1. Available online: https://www.who.int/publications/i/item/who-2019-nCoV-oral-health-2020.1 (accessed on 21 December 2020).
- Veena, H.R.; Mahantesha, S.; Joseph, P.A.; Patil, S.R.; Patil, S.H. Dissemination of aerosol and splatter during ultrasonic scaling: A pilot study. J. Infect. Public Health 2015, 8, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Bartold, P.M.; Salomon, C.; Ivanovski, S. Salivary small extracellular vesicles associated miRNAs in periodontal status - a pilot study. Int J. Mol. Sci. 2020, 21, 2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, P.; Ivanovski, S. Effect of saliva collection methods on the detection of periodontium-related genetic and epigenetic biomarkers - a pilot study. Int J. Mol. Sci. 2019, 20, 4729. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Lai, A.; Salomon, C.; Ivanovski, S. Detection of salivary small extracellular vesicles associated inflammatory cytokines gene methylation in gingivitis. Int. J. Mol. Sci. 2020, 21, 5273. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Maurino, V.; Baj, A.; Dani, M.; d’Aiuto, A.; Fasano, M.; Lualdi, M.; Sessa, F.; Alberio, T. Diagnostic salivary tests for SARS-CoV-2. J. Dent. Res. 2020, 100. [Google Scholar] [CrossRef]
- National Research Council. Rapid Expert Consultation on the Possibility of Bioaerosol Spread of SARS-CoV-2 for the COVID-19 Pandemic (April 1, 2020); The National Academies Press: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- Tang, S.; Mao, Y.; Jones, R.M.; Tan, Q.; Ji, J.S.; Li, N.; Shen, J.; Lv, Y.; Pan, L.; Ding, P.; et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int 2020, 144, 106039. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1. [Google Scholar] [CrossRef]
- Meng, L.; Hua, F.; Bian, Z. Coronavirus disease 2019 (COVID-19): Emerging and future challenges for dental and oral medicine. J. Dent. Res. 2020, 98. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, A.C.; Cagetti, M.G.; Ferracane, J.L.; Garcia-Godoy, F.; Brambilla, E. Topographic aspects of airborne contamination caused by the use of dental handpieces in the operative environment. J. l Am. Dent. Assn. 2020, 151, 660–667. [Google Scholar] [CrossRef]
- Gund, M.; Isack, J.; Hannig, M.; Thieme-Ruffing, S.; Gärtner, B.; Boros, G.; Rupf, S. Contamination of surgical mask during aerosol-producing dental treatments. Clin. Oral Invest. 2020. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.R.; Currie, C.C.; Edwards, D.C.; Bowes, C.; Coulter, J.; Pickering, K.; Kozhevnikova, E.; Durham, J.; Nile, C.J.; Jakubovics, N.; et al. Evaluating aerosol and splatter following dental procedures: Addressing new challenges for oral health care and rehabilitation. J. Oral Rehabil. 2020, 48, 61–72. [Google Scholar] [CrossRef]
- Kumbargere Nagraj, S.; Eachempati, P.; Paisi, M.; Nasser, M.; Sivaramakrishnan, G.; Verbeek, J.H. Interventions to reduce contaminated aerosols produced during dental procedures for preventing infectious diseases. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Lu, J.; Gu, J.; Li, K.; Xu, C.; Su, W.; Lai, Z.; Zhou, D.; Yu, C.; Xu, B.; Yang, Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020, 26, 1628–1631. [Google Scholar] [CrossRef]
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rautemaa, R.; Nordberg, A.; Wuolijoki-Saaristo, K.; Meurman, J.H. Bacterial aerosols in dental practice—A potential hospital infection problem? J. Hospital Infect. 2006, 64, 76–81. [Google Scholar] [CrossRef]
- Alsved, M.; Matamis, A.; Bohlin, R.; Richter, M.; Bengtsson, P.E.; Fraenkel, C.J.; Medstrand, P.; Löndahl, J. Exhaled respiratory particles during singing and talking. Aerosol Sci. Technol. 2020, 54, 1245–1248. [Google Scholar] [CrossRef]
- Morawska, L.; Milton, D.K. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2311–2313. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, P.; Li, H.; Walsh, L.J.; Ivanovski, S. Splatters and Aerosols Contamination in Dental Aerosol Generating Procedures. Appl. Sci. 2021, 11, 1914. https://doi.org/10.3390/app11041914
Han P, Li H, Walsh LJ, Ivanovski S. Splatters and Aerosols Contamination in Dental Aerosol Generating Procedures. Applied Sciences. 2021; 11(4):1914. https://doi.org/10.3390/app11041914
Chicago/Turabian StyleHan, Pingping, Honghui Li, Laurence J. Walsh, and Sašo Ivanovski. 2021. "Splatters and Aerosols Contamination in Dental Aerosol Generating Procedures" Applied Sciences 11, no. 4: 1914. https://doi.org/10.3390/app11041914
APA StyleHan, P., Li, H., Walsh, L. J., & Ivanovski, S. (2021). Splatters and Aerosols Contamination in Dental Aerosol Generating Procedures. Applied Sciences, 11(4), 1914. https://doi.org/10.3390/app11041914








