Assessment of Daytime HONO Emission Source from Asphalt Surface to Urban Air
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Setup
2.2. Measurements
2.3. Conversion Rate of NO2 to HONO
3. Results
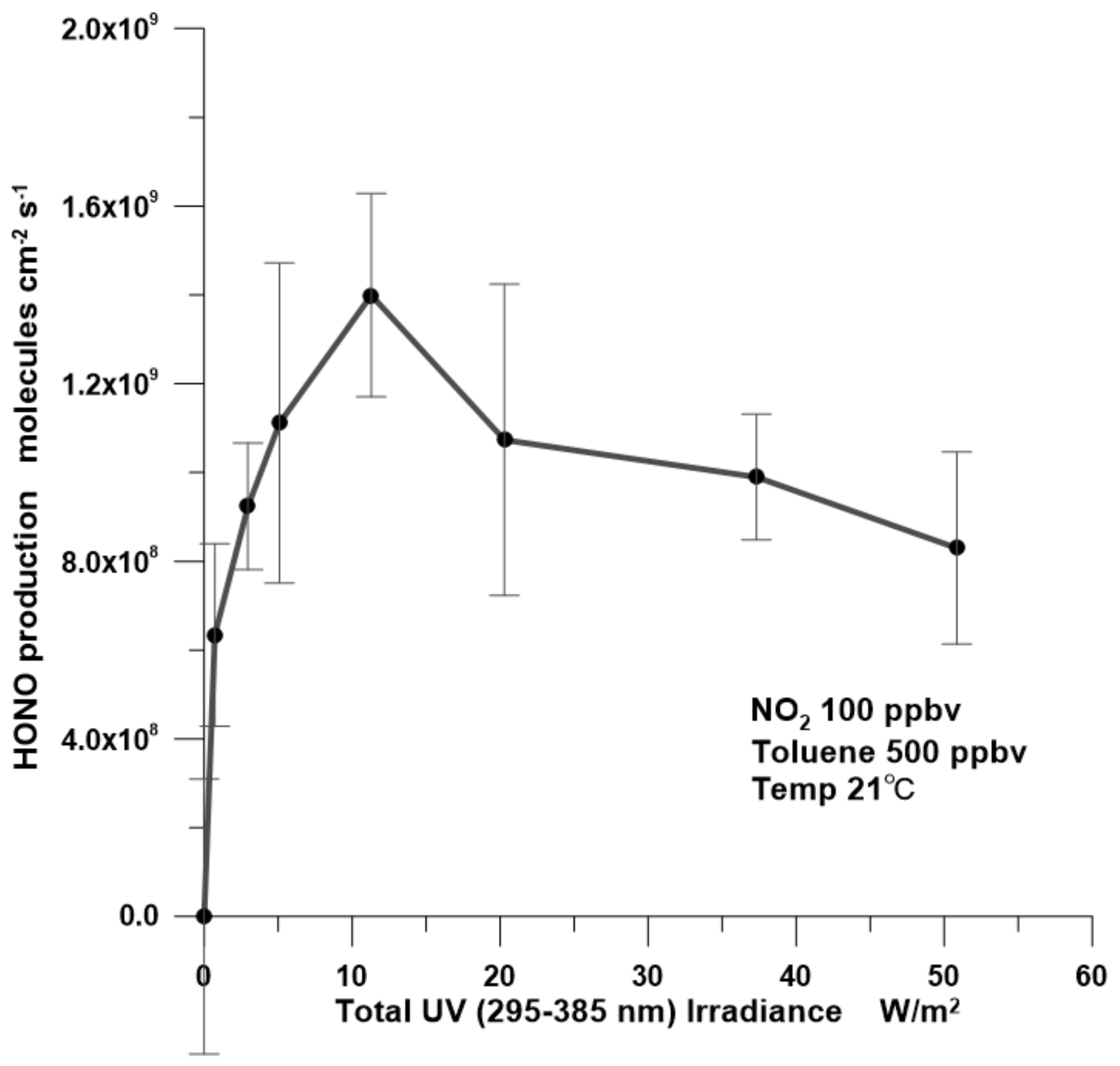
3.1. UV Irradiance Dependency
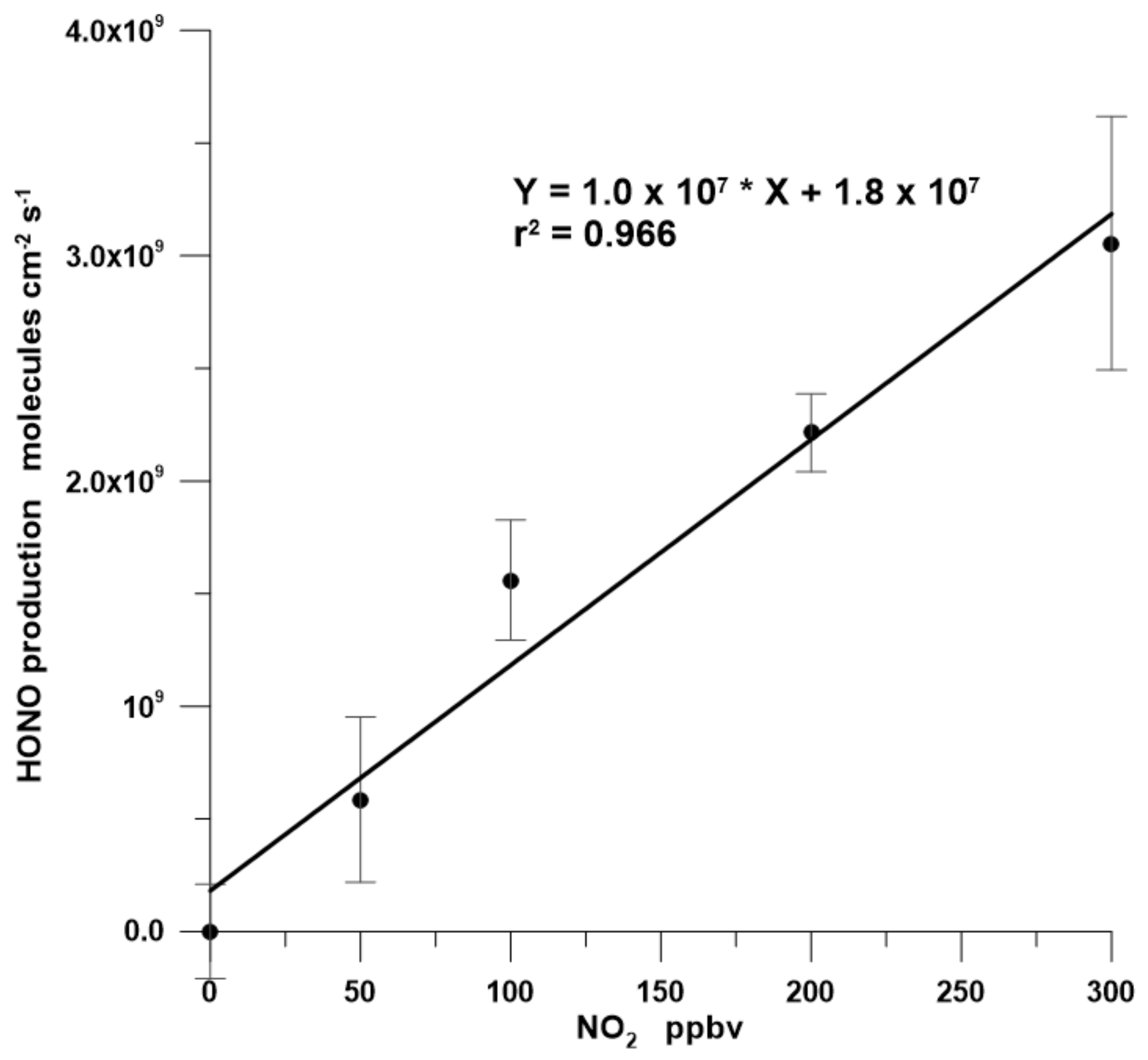
3.2. NO2 Dependency
3.3. Conversion Rate of NO2 to HONO
3.4. VOCs Dependency
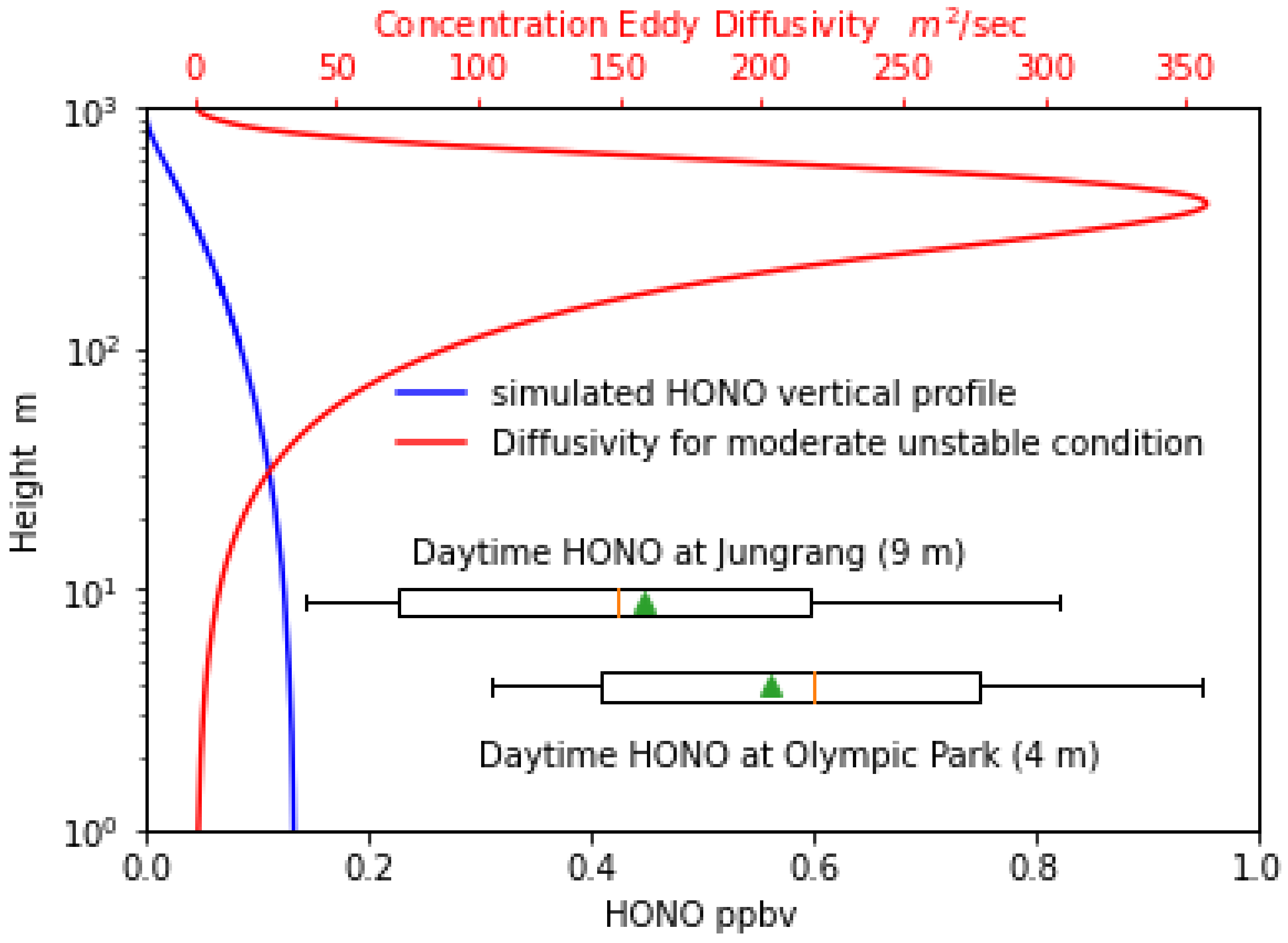
3.5. Vertical Profile of HONO above the Asphalt Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hofzumahaus, A.; Rohrer, F.; Lu, K.; Bohn, B.; Brauers, T.; Chang, C.C.; Fuchs, H.; Holland, F.; Kita, K.; Kondo, Y.; et al. Amplified trace gas removal in the troposphere. Science 2009, 324, 1702–1704. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Lei, W.; Zavala, M.; Volkamer, R.; Dusanter, S.; Stevens, P.; Molina, L.T. Impacts of HONO sources on the photochemistry in Mexico City during the MCMA-2006/MILAGO Campaign. Atmos. Chem. Phys. 2010, 10, 6551–6567. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhou, R.; Zhao, H.; Wang, Z.; Chen, L.; Zhou, B. Long-term observation of atmospheric nitrous acid (HONO) and its implication to local NO2 levels in Shanghai, China. Atmos. Environ. 2013, 77, 718–724. [Google Scholar] [CrossRef]
- Li, D.; Xue, L.; Wen, L.; Wang, X.; Chen, T.; Mellouki, A.; Chen, J.; Wang, W. Characteristics and sources of nitrous acid in an urban atmosphere of northern China: Results from 1-yr continuous observations. Atmos. Environ. 2018, 182, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Jiang, N.; Zhang, R.; Yang, L.; Li, S. Characteristics, sources, and reactions of nitrous acid during winter at an urban site in the Central Plains Economic Region in China. Atmos. Chem. Phys. 2020, 20, 7087–7102. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Lian, C.; Yan, C.; Feng, Z.; Zheng, F.; Fan, X.; Chen, Y.; Wang, W.; Chu, B.; et al. The promotion effect of nitrous acid on aerosol formation in wintertime Beijing: Possible contribution of traffic-related emission. Atmos. Chem. Phys. 2020, 1–43. [Google Scholar] [CrossRef]
- Laufs, S.; Kleffmann, J. Investigations on HONO formation from photolysis of adsorbed HNO3 on quartz glass surfaces. Phys. Chem. Chem. Phys. 2016, 18, 9616–9625. [Google Scholar] [CrossRef] [Green Version]
- Czader, B.H.; Rappengï Uck, B.; Percell, P.; Byun, D.W.; Ngan, F.; Kim, S. Modeling nitrous acid and its impact Atmospheric Chemistry and Physics Discussions Modeling nitrous acid and its impact on ozone and hydroxyl radical during the Texas Air Quality Study 2006 Modeling nitrous acid and its impact Modeling nitrous acid and its impact. Atmos. Chem. Phys. Discuss 2012, 12, 5851–5880. [Google Scholar] [CrossRef]
- Gonçalves, M.; Dabdub, D.; Chang, W.L.; Jorba, O.; Baldasano, J.M. Impact of HONO sources on the performance of mesoscale air quality models. Atmos. Environ. 2012, 54, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Kleffmann, J. Daytime sources of nitrous acid (HONO) in the atmospheric boundary layer. ChemPhysChem. 2007, 8, 1137–1144. [Google Scholar] [CrossRef]
- Spataro, F.; Ianniello, A. Sources of atmospheric nitrous acid: State of the science, current research needs, and future prospects. J. Air Waste Manag. Assoc. 2014, 64, 1232–1250. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, V.; Sörgel, M.; Gligorovski, S.; Alvarez, E.G.; Gandolfo, A.; Strekowski, R.; Quivet, E.; Held, A.; Zetzsch, C.; Wortham, H. Formation of indoor nitrous acid (HONO) by light-induced NO2 heterogeneous reactions with white wall paint. Environ. Sci. Pollut. Res. 2014, 21, 9259–9269. [Google Scholar] [CrossRef] [PubMed]
- Langridge, J.M.; Gustafsson, R.J.; Griffiths, P.T.; Cox, R.A.; Lambert, R.M.; Jones, R.L. Solar driven nitrous acid formation on building material surfaces containing titanium dioxide: A concern for air quality in urban areas? Atmos. Environ. 2009, 43, 5128–5131. [Google Scholar] [CrossRef]
- Li, S.; Matthews, J.; Sinha, A. Atmospheric hydroxyl radical production from electronically excited NO2 and H2O. Science 2008, 319, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Meusel, H.; Tamm, A.; Kuhn, U.; Wu, D.; Leifke, A.L.; Fiedler, S.; Ruckteschler, N.; Yordanova, P.; Lang-Yona, N.; Pöhlker, M.; et al. Emission of nitrous acid from soil and biological soil crusts represents an important source of HONO in the remote atmosphere in Cyprus. Atmos. Chem. Phys 2018, 18, 799–813. [Google Scholar] [CrossRef] [Green Version]
- Oswald, R.; Behrendt, T.; Ermel, M.; Wu, D.; Su, H.; Cheng, Y.; Breuninger, C.; Moravek, A.; Mougin, E.; Delon, C.; et al. HONO emissions from soil bacteria as a major source of atmospheric reactive nitrogen. Science 2013, 341, 1233–1235. [Google Scholar] [CrossRef]
- Kleffmann, J.; Kurtenbach, R.; Lörzer, J.; Wiesen, P.; Kalthoff, N.; Vogel, B.; Vogel, H. Measured and simulated vertical profiles of nitrous acid - Part I: Field measurements. Atmos. Environ. 2003, 37, 2949–2955. [Google Scholar] [CrossRef]
- Ren, X.; Sanders, J.E.; Rajendran, A.; Weber, R.J.; Goldstein, A.H.; Pusede, S.E.; Browne, E.C.; Min, K.-E.; Cohen, R.C. A relaxed eddy accumulation system for measuring vertical fluxes of nitrous acid. Atmos. Meas. Tech. 2011, 4, 2093–2103. [Google Scholar] [CrossRef] [Green Version]
- VandenBoer, T.C.; Markovic, M.Z.; Sanders, J.E.; Ren, X.; Pusede, S.E.; Browne, E.C.; Cohen, R.C.; Zhang, L.; Thomas, J.; Brune, W.H.; et al. Evidence for a nitrous acid (HONO) reservoir at the ground surface in Bakersfield, CA, during CalNex 2010. J. Geophys. Res. Atmos. 2014, 119, 9093–9106. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, N.; Teravest, M.; Tang, D.; Hou, J.; Bertman, S.; Alaghmand, M.; Shepson, P.B.; Carroll, M.A.; Griffith, S.; et al. Nitric acid photolysis on forest canopy surface as a source for tropospheric nitrous acid. Nat. Geosci. 2011, 4, 440–443. [Google Scholar] [CrossRef]
- Wong, K.W.; Tsai, C.; Lefer, B.; Haman, C.; Grossberg, N.; Brune, W.H.; Ren, X.; Luke, W.; Stutz, J. Daytime HONO vertical gradients during SHARP 2009 in Houston, TX. Atmos. Chem. Phys. 2012, 12, 635–652. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Zhou, X.; Bertman, S.; Tang, D.; Alaghmand, M.; Shepson, P.B.; Carroll, M.A. Measurements of ambient HONO concentrations Atmospheric Chemistry and Physics Discussions Measurements of ambient HONO concentrations and vertical HONO flux above a northern Michigan forest canopy Measurements of ambient HONO concentrations. Atmos. Chem. Phys. Discuss 2012, 12, 7273–7304. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, Y.; Sadanaga, Y.; Saito, S.; Hoshi, J.; Ueno, H. Contributions of vehicular emissions and secondary formation to nitrous acid concentrations in ambient urban air in Tokyo in the winter. Sci. Total Environ. 2017, 592, 178–186. [Google Scholar] [CrossRef]
- Stemmler, K.; Ammann, M.; Donders, C.; Kleffmann, J.; George, C. Photosensitized reduction of nitrogen dioxide on humic acid as a source of nitrous acid. Nature 2006, 440, 195–198. [Google Scholar] [CrossRef]
- Guan, C.; Li, X.; Zhang, W.; Huang, Z. Identification of nitration products during heterogeneous reaction of NO2 on soot in the dark and under simulated sunlight. J. Phys. Chem. A 2017, 121, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Gao, H.; Zhang, N.; Zhou, X. Photolysis of Nitric Acid and Nitrate on Natural and Artificial Surfaces. Environ. Sci. Technol. 2016, 50, 46. [Google Scholar] [CrossRef]
- Bao, F.; Li, M.; Zhang, Y.; Chen, C.; Zhao, J. Photochemical Aging of Beijing Urban PM2.5: HONO Production. Environ. Sci. Technol. 2018, 52, 6309–6316. [Google Scholar] [CrossRef] [PubMed]
- Stemmler, K.; Ndour, M.; Elshorbany, Y.; Kleffmann, J.; D’Anna, B.; George, C.; Bonn, B.; Ammann, M. Light induced conversion of nitrogen dioxide into nitrous acid on submicron humic acid aerosol. Atmos. Chem. Phys. 2007, 7, 4237–4248. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, G.; Roselle, S.J.; Mathur, R.; Appel, W.; Dennis, R.L.; Vogel, B. A comparison of CMAQ HONO predictions with observations from the Northeast Oxidant and Particle Study. Atmos. Environ. 2008, 42, 5760–5770. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Zhang, Q.; Zheng, J.; Xu, Z.; Lv, M. Potential sources of nitrous acid (HONO) and their impacts on ozone: A WRF-Chem study in a polluted subtropical region. J. Geophys. Res. 2016, 121, 3645–3662. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Wood, E.C.; Wormhoudt, J.; Shorter, J.H.; Herndon, S.C.; Zahniser, M.S.; Munger, J.W. Effective line strengths of trans-nitrous acid near 1275cm -1 and cis-nitrous acid at 1660cm -1. J. Quant. Spectrosc. Radiat. Transf. 2012, 113, 1905–1912. [Google Scholar] [CrossRef]
- Wall, K.J.; Schiller, C.L.; Harris, G.W. Measurements of the HONO photodissociation constant. J. Atmos. Chem. 2006, 55, 31–54. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Nanayakkara, C.E.; Grassian, V.H. Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef] [PubMed]
- Laufs, S.; Burgeth, G.; Duttlinger, W.; Kurtenbach, R.; Maban, M.; Thomas, C.; Wiesen, P.; Kleffmann, J. Conversion of nitrogen oxides on commercial photocatalytic dispersion paints. Atmos. Environ. 2010, 44, 2341–2349. [Google Scholar] [CrossRef]
- Gómez Alvarez, E.; Sörgel, M.; Gligorovski, S.; Bassil, S.; Bartolomei, V.; Coulomb, B.; Zetzsch, C.; Wortham, H. Light-induced nitrous acid (HONO) production from NO2 heterogeneous reactions on household chemicals. Atmos. Environ. 2014, 95, 391–399. [Google Scholar] [CrossRef]
- Gandolfo, A.; Rouyer, L.; Wortham, H.; Gligorovski, S. The influence of wall temperature on NO2 removal and HONO levels released by indoor photocatalytic paints. Appl. Catal. B Environ. 2017, 209, 429–436. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Mekic, M.; Jiang, H.; Zhou, W.; Loisel, G.; Song, W.; Wang, X.; Gligorovski, S. Photoenhanced Uptake of NO2 and HONO Formation on Real Urban Grime. Environ. Sci. Technol. Lett. 2019, 6, 413–417. [Google Scholar] [CrossRef]
- Laufs, S.; Cazaunau, M.; Stella, P.; Kurtenbach, R.; Cellier, P.; Mellouki, A.; Loubet, B.; Kleffmann, J. Diurnal fluxes of HONO above a crop rotation. Atmos. Chem. Phys. 2017, 17, 6907–6923. [Google Scholar] [CrossRef] [Green Version]
- Sörgel, M.; Trebs, I.; Wu, D.; Held, A. A comparison of measured HONO uptake and release with calculated source strengths in a heterogeneous forest environment. Atmos. Chem. Phys. 2015, 15, 9237–9251. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Brauers, T.; Häseler, R.; Bohn, B.; Fuchs, H.; Hofzumahaus, A.; Holland, F.; Lou, S.; Lu, K.D.; Rohrer, F.; et al. Exploring the atmospheric chemistry of nitrous acid (HONO) at a rural site in Southern China. Atmos. Chem. Phys. 2012, 12, 1497–1513. [Google Scholar] [CrossRef] [Green Version]
- Arens, F.; Gutzwiller, L.; Baltensperger, U.; Gäggeler, H.W.; Ammann, M. Heterogeneous reaction of NO2 on diesel soot particles. Environ. Sci. Technol. 2001, 35, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, W.; Yang, H.; Xue, X. Enhanced photochemical conversion of NO2 to HONO on humic acids in the presence of benzophenone. Environ. Pollut. 2017, 231, 979–986. [Google Scholar] [CrossRef]
- Lee, J.D.; Whalley, L.K.; Heard, D.E.; Stone, D.; Dunmore, R.E.; Hamilton, J.F.; Young, D.E.; Allan, J.D.; Laufs, S.; Kleffmann, J. Central London HONO budget analysis Detailed budget analysis of HONO in central London reveals a missing daytime source Central London HONO budget analysis. Atmos. Chem. Phys. Discuss 2015, 15, 22097–22139. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Harder, H.; Martinez, M.; Lesher, R.L.; Oliger, A.; Simpas, J.B.; Brune, W.H.; Schwab, J.J.; Demerjian, K.L.; He, Y.; et al. OH and HO2 chemistry in the urban atmosphere of New York City. Atmos. Environ. 2003, 37, 3639–3651. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Costabile, F.; Amoroso, A.; Zhao, C.; Huey, L.G.; Stickel, R.; Liao, J.; Zhu, T. Evidence of aerosols as a media for rapid daytime HONO production over China. Environ. Sci. Technol. 2014, 48, 14386–14391. [Google Scholar] [CrossRef]
- Hendrick, F.; Müller, J.-F.; Clémer, K.; Wang, P.; De Mazière, M.; Fayt, C.; Gielen, C.; Hermans, C.; Ma, J.Z.; Pinardi, G.; et al. Atmospheric Chemistry and Physics Four years of ground-based MAX-DOAS observations of HONO and NO2 in the Beijing area. Atmos. Chem. Phys. 2014, 14, 765–781. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wen, L.; Xu, C.; Chen, J.; Wang, X.; Yang, L.; Wang, W.; Yang, X.; Sui, X.; Yao, L.; et al. HONO and its potential source particulate nitrite at an urban site in North China during the cold season. Sci. Total Environ. 2015, 538, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Saylor, R.D. The Atmospheric Chemistry and Canopy Exchange Simulation System (ACCESS): Model description and application to a temperate deciduous forest canopy. Atmos. Chem. Phys. 2012, 13, 693–715. [Google Scholar] [CrossRef] [Green Version]
- Li, J.G. A multiple-cell flat-level model for atmospheric tracer dispersion over complex terrain. Boundary-Layer Meteorol. 2003, 107, 289–322. [Google Scholar] [CrossRef]
- Tapp, M.C.; White, P.W. A non-hydrostatic mesoscale model. Q. J. R. Meteorol. Soc. 1976, 102, 277–296. [Google Scholar] [CrossRef]
- Na, K. Determination of VOC source signature of vehicle exhaust in a traffic tunnel. J. Environ. Manag. 2006, 81, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.-S.; Lee, T. Study on Risk Assessment of Vehicle Emission on the Road–Based on the Tunnel Study; NIER Report; Ministry of Environment of Korea: Seoul, Korea, 2018; pp. 42–45.


| Material | Emission Rate (Molecules cm−2 s−1) | NO2 (ppb) | RH (%) | Irradiance (W m−2) | Wavelength (nm) | RF | |
|---|---|---|---|---|---|---|---|
| Paint | White wall paint | 1.3 × 1010 | 50 | 50 | 10.6 | 300–400 | [35] |
| Photocatalytic paint (3.5% TiO2 nanoparticles) | 2.1 × 1010 | 40 | 40 | 8.5 | 340–400 | [36] | |
| Glass | Clean | 1.3 × 109 | 50 | 50 | 10.6 | 300–400 | [35] |
| Urban grime | 1.5 × 1010 | 46 | 90 | 8.0 | 300–400 | [37] | |
| Aerosol | 2.5 × 109 | 60 | 400 < λ | [27] | |||
| Soil | 2.2 × 1011 | [37] | |||||
| 1.3 × 108 | [38] | ||||||
| 1.5 × 109 | [39] | ||||||
| Forest canopy | 2.4 × 109 | [39] | |||||
| 6.2 × 109 | [40] | ||||||
| 6.0 × 108 | [18] | ||||||
| 6.7 × 109 | [26] | ||||||
| 3.3 × 109 | [20] | ||||||
| Asphalt | 0.5–3.1× 109 | 50–300 | 50 | 11.3 | 295–385 | This study | |
| Location | Sampling Period | Instrument | Min | Mean | Max | Reference |
|---|---|---|---|---|---|---|
| London, UK | July–Aug 2012 | LOPAP a | 0.20 | 0.44 | 0.60 | [43] |
| New York, USA | July–Aug 2001 | HPLC b | 0.40 | 0.46 | 1.40 | [44] |
| Houston, USA | Apr 21, 2009 | LP-DOAS c | 0.05 | 0.1 | 0.15 | [21] |
| Bakersfield, USA | May–July, 2010 | AIM d | 0.03 | 0.08 | 0.13 | [19] |
| Beijing, China | Aug 2007 | UV-Vis | 0.80 | - | 1.60 | [45] |
| July 2008–April 2009 | MAX-DOAS e | 0.1 | 0.36 | 0.8 | [46] | |
| Jinan, China | Nov 2013–Jan 2014 | WRD f | 0.16 | - | 0.58 | [47] |
| Sep 2015–Aug 2016 | LOPAP | 0.02 | 0.99 | 7.39 | [4] | |
| Seoul, Korea | May–June 2016 | QC-TILDAS g | 0.20 | 0.60 | 1.47 | This study |
| May–June 2019 | QC-TILDAS | 0.01 | 0.45 | 1.20 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, J.; Lee, M.; Ahn, J.Y.; Lee, G. Assessment of Daytime HONO Emission Source from Asphalt Surface to Urban Air. Appl. Sci. 2021, 11, 1930. https://doi.org/10.3390/app11041930
Kim D, Kim J, Lee M, Ahn JY, Lee G. Assessment of Daytime HONO Emission Source from Asphalt Surface to Urban Air. Applied Sciences. 2021; 11(4):1930. https://doi.org/10.3390/app11041930
Chicago/Turabian StyleKim, Deokyoon, Jeonghwan Kim, Meehye Lee, Joon Young Ahn, and Gangwoong Lee. 2021. "Assessment of Daytime HONO Emission Source from Asphalt Surface to Urban Air" Applied Sciences 11, no. 4: 1930. https://doi.org/10.3390/app11041930
APA StyleKim, D., Kim, J., Lee, M., Ahn, J. Y., & Lee, G. (2021). Assessment of Daytime HONO Emission Source from Asphalt Surface to Urban Air. Applied Sciences, 11(4), 1930. https://doi.org/10.3390/app11041930





