Ultra-Deep Oxidative Desulfurization of Fuel with H2O2 Catalyzed by Mesoporous Silica-Supported Molybdenum Oxide Modified by Ce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Characterization of the Samples
2.4. Catalytic Oxidative Desulfurization
3. Result and Discussion
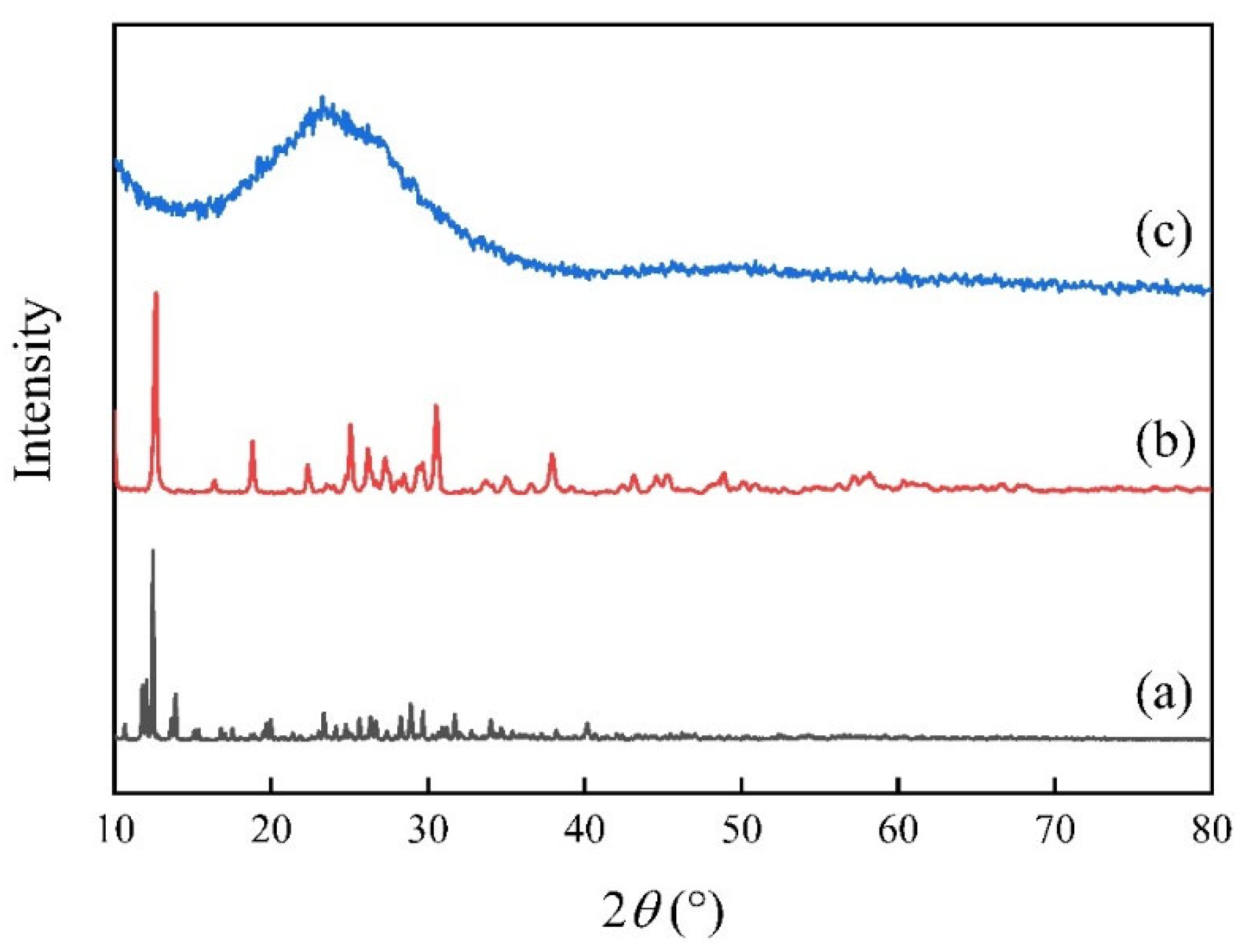
3.1. Characterization of Catalysts
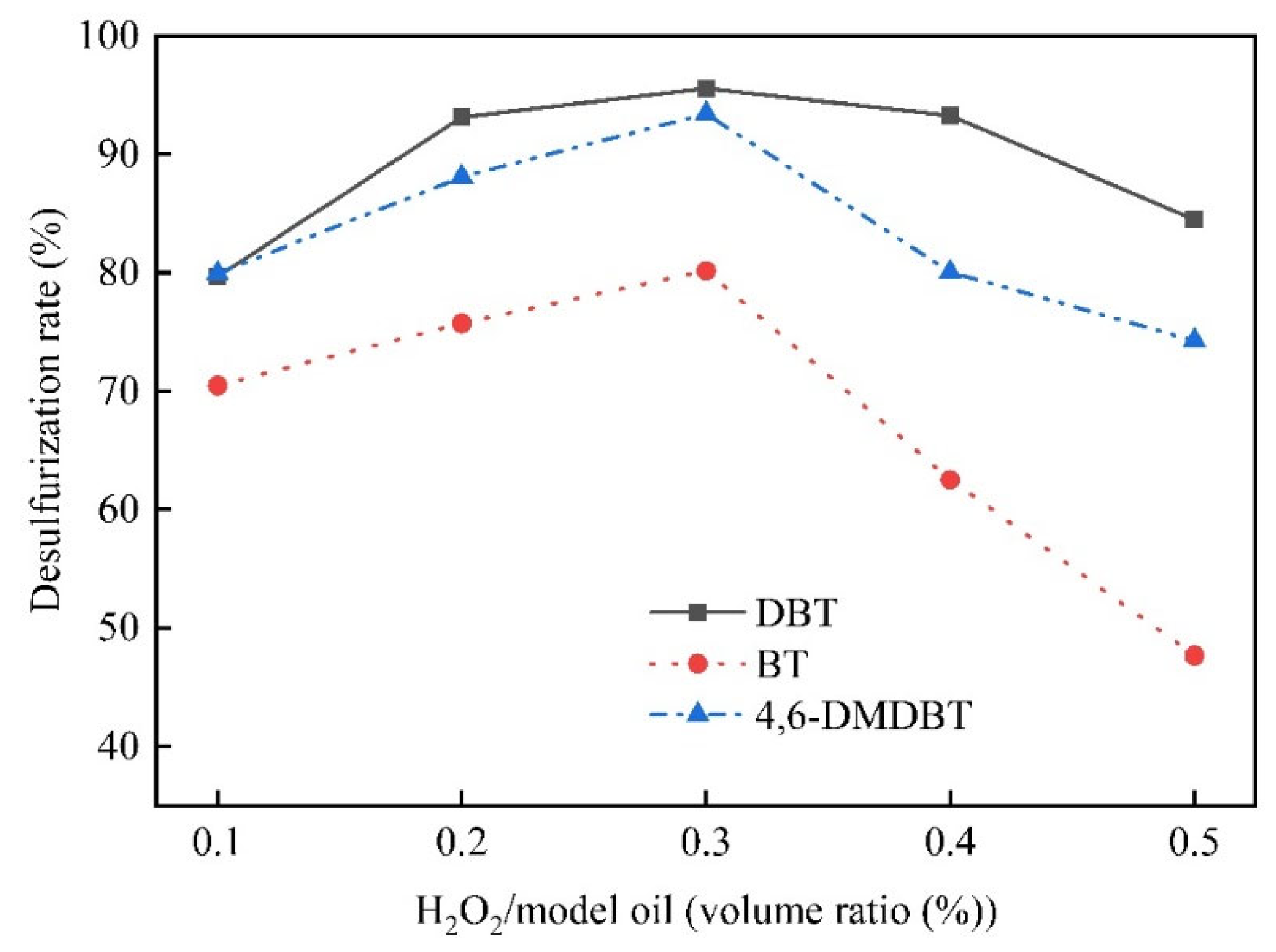
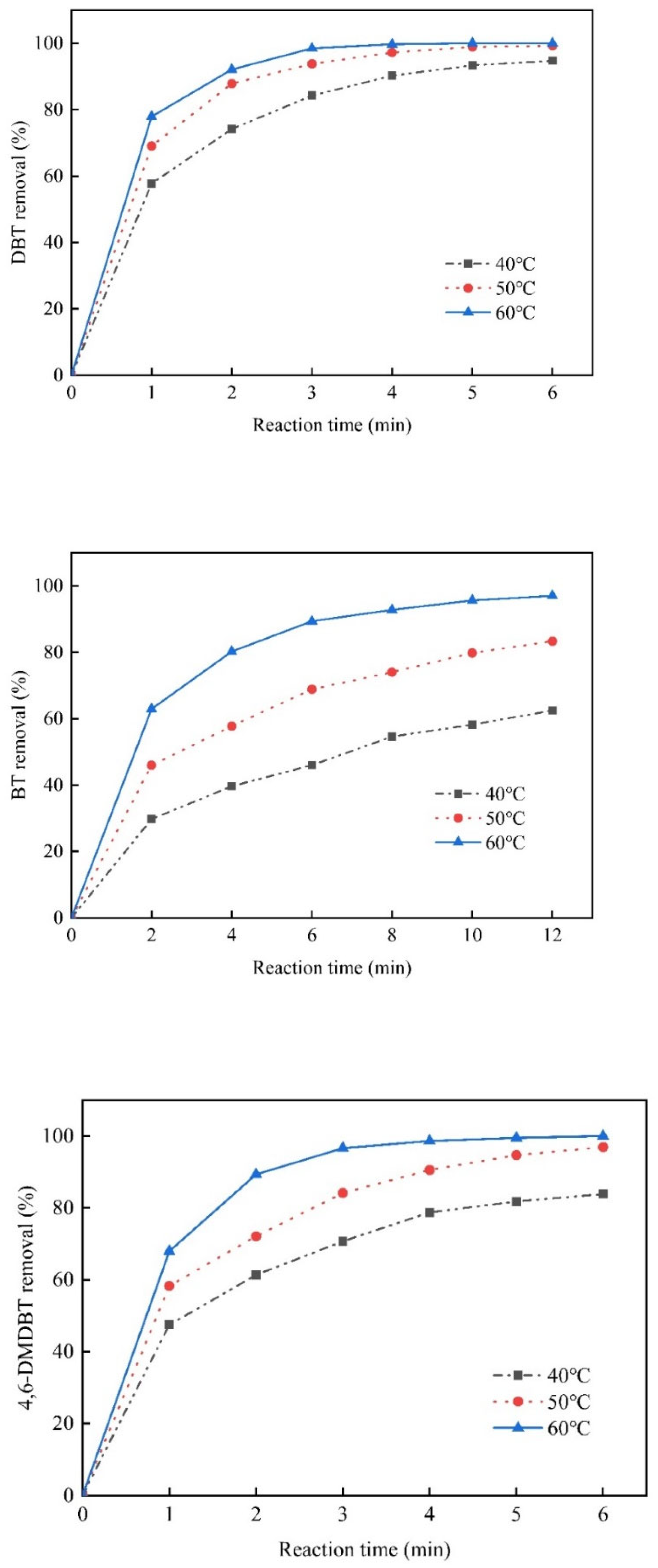
3.2. Oxidative Desulfurization of the Model Oil
3.3. Apparent Activation Energy and Reaction Rate Constant
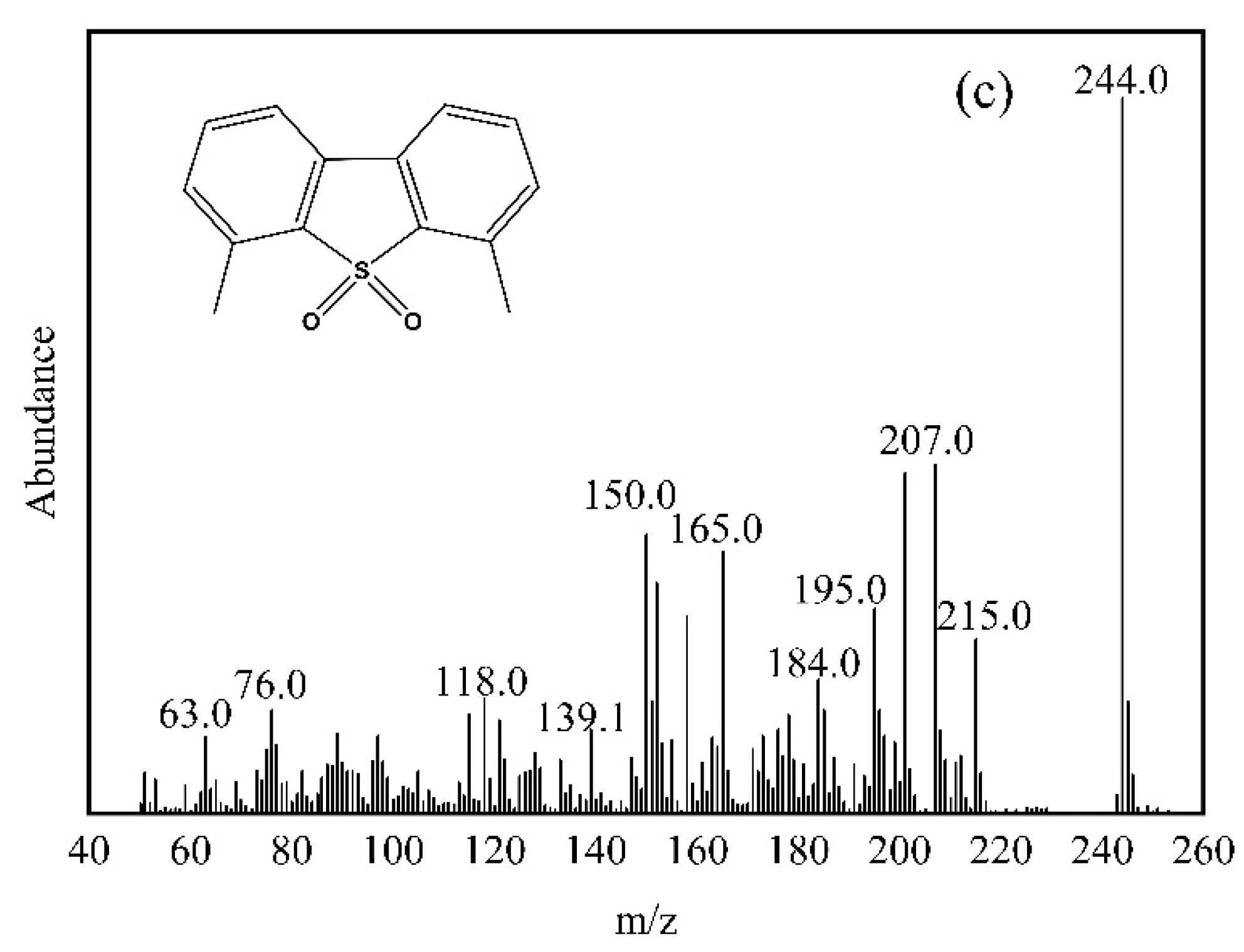
3.4. Analysis of Oxidation Product
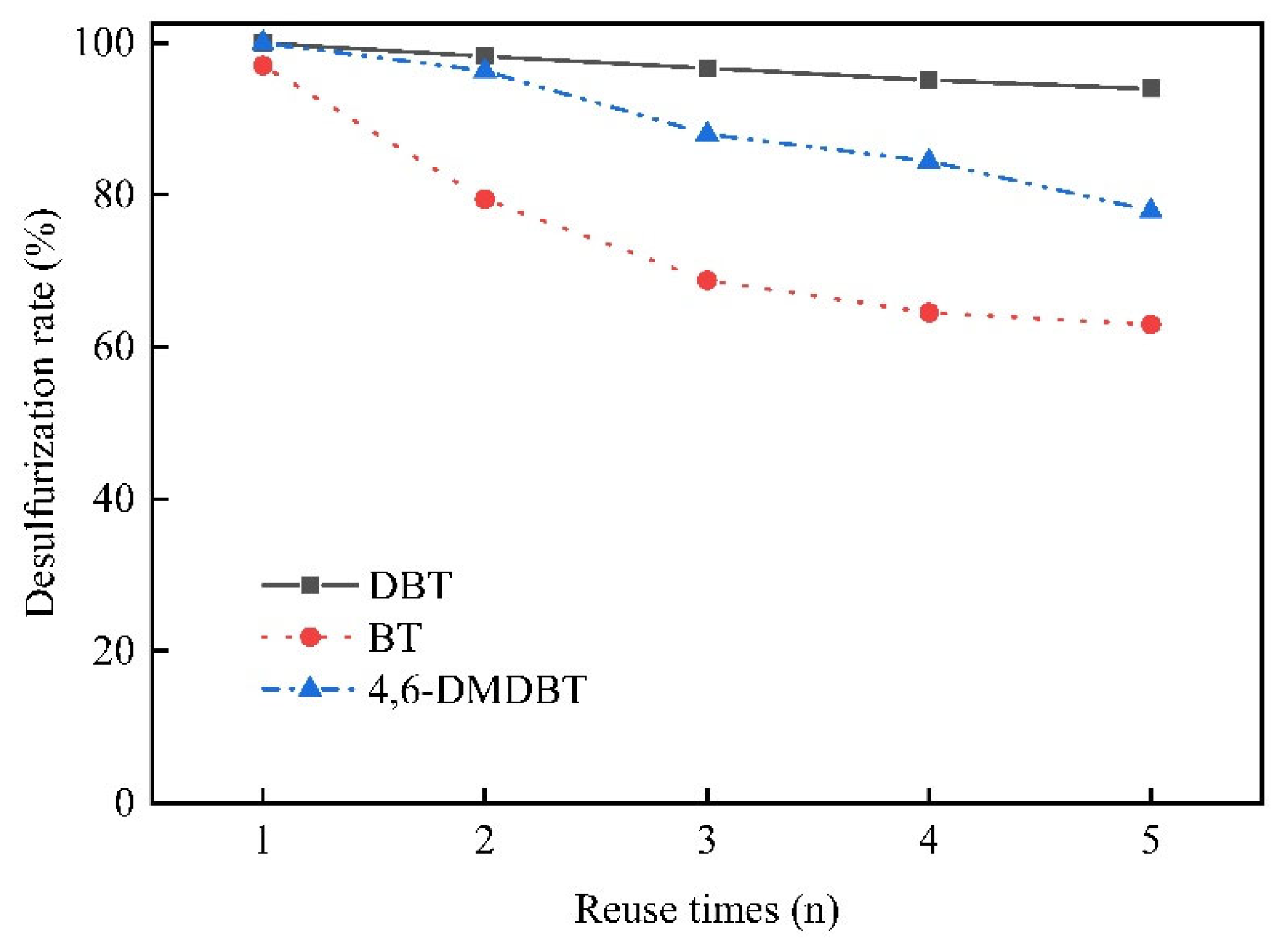
3.5. Reuse Cycles of the Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.N.; Park, H.C.; Choi, H.S. A Comprehensive Review on Catalytic Oxidative Desulfurization of Liquid Fuel Oil. Catalysts 2019, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhao, K.; Cheng, Y.; Zeng, G.; Zhang, M.; Shao, J.; Lu, L. Catalytic oxidative desulfurization of BT and DBT from n-octane using cyclohexanone peroxide and catalyst of molybdenum supported on 4A molecular sieve. Sep. Purif. Technol. 2016, 163, 153–161. [Google Scholar] [CrossRef]
- Tao, X.; Zhou, Y.; Wei, Q.; Ding, S.; Zhou, W.; Liu, T.; Li, X. Inhibiting effects of nitrogen compounds on deep hydrodesulfurization of straight-run gas oil over a NiW/Al2O3 catalyst. Fuel 2017, 188, 401–407. [Google Scholar] [CrossRef]
- De Luna, M.D.G.; Futalan, C.M.; Duavis, A.G.; Wan, M.-W. Optimization and kinetics of the desulfurization of diesel fuel via high shear mixing oxidation assisted by adsorption of sulfones onto chitosan-coated bentonite. Int. J. Green Energy 2018, 15, 930–940. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Z.; Li, J.; Zhang, Y.; Lin, F.; Liu, Y.; Li, C. Catalytic oxidation of thiophene and its derivatives via dual activation for ultra-deep desulfurization of fuels. J. Catal. 2012, 287, 5–12. [Google Scholar] [CrossRef]
- Lai, W.; Chen, Z.; Zhu, J.; Yang, L.; Zheng, J.; Yi, X.; Fang, W. A NiMoS flower-like structure with self-assembled nanosheets as high-performance hydrodesulfurization catalysts. Nanoscale 2016, 8, 3823–3833. [Google Scholar] [CrossRef]
- Hajjar, Z.; Kazemeini, M.; Rashidi, A.; Bazmi, M. Graphene based catalysts for deep hydrodesulfurization of naphtha and diesel fuels: A physiochemical study. Fuel 2016, 165, 468–476. [Google Scholar] [CrossRef]
- Tavizón-Pozos, J.A.; Suárez-Toriello, V.A.; Reyes, J.A.D.L.; Guevara-Lara, A.; Pawelec, B.; Fierro, J.L.G.; Vrinat, M.; Geantet, C. Deep Hydrodesulfurization of Dibenzothiophenes over NiW Sulfide Catalysts Supported on Sol–Gel Titania–Alumina. Top. Catal. 2015, 59, 241–251. [Google Scholar] [CrossRef]
- Song, S.; Yang, X.; Wang, B.; Zhou, X.; Duan, A.; Chi, K.; Zhao, Z.; Xu, C.; Chen, Z.; Li, J. Al-modified mesocellular silica foam as a superior catalyst support for dibenzothiophene hydrodesulfurization. Chin. J. Catal. 2017, 38, 1347–1359. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, M.; Zhou, Y.; Wei, Q.; Zhang, Q.; Ding, S.; Zhang, Y.; Yu, T.; You, Q. 4,6-Dimethyldibenzothiophene Hydrodesulfurization on Nickel-Modified USY-Supported NiMoS Catalysts: Effects of Modification Method. Energy Fuels 2017, 31, 7445–7455. [Google Scholar] [CrossRef]
- Abdi, G.; Ashokkumar, M.; Alizadeh, A. Ultrasound-assisted oxidative-adsorptive desulfurization using highly acidic graphene oxide as a catalyst-adsorbent. Fuel 2017, 210, 639–645. [Google Scholar] [CrossRef]
- Bonde, S.E.; Gore, W.; Dolbear, G.E.; Skov, E.R. Selective oxidation and extraction of sulfur-containing compounds to economically achieve ultra-low proposed diesel fuel sulfur requirements. Prepr. Am. Chem. Soc. Div. Pet. Chem. 2000, 45, 364–366. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, C.; Hu, H.; Ye, Z.; Chen, X. A modified homogeneous surface diffusion model for the fixed-bed adsorption of 4,6-DMDBT on Ag–CeOx/TiO2–SiO2. RSC Adv. 2016, 6, 112899–112907. [Google Scholar] [CrossRef]
- Sudhir, N.; Yadav, P.; Nautiyal, B.; Singh, R.; Rastogi, H.; Chauhan, H. Extractive desulfurization of fuel with methyltriphenyl phosphonium bromide- tetraethylene glycol-based eutectic solvents. Sep. Sci. Technol. 2020, 55, 554–563. [Google Scholar] [CrossRef]
- Kang, Y.; Shao, D.; Li, N.; Yang, G.; Zhang, Q.; Zeng, L.; Luo, J.; Zhong, W. Cancer risk assessment of human exposure to polycyclic aromatic hydrocarbons (PAHs) via indoor and outdoor dust based on probit model. Environ. Sci. Pollut. Res. 2014, 22, 3451–3456. [Google Scholar] [CrossRef]
- Dou, S.-Y.; Wang, R. Recent Advances in New Catalysts for Fuel Oil Desulfurization. Curr. Org. Chem. 2017, 21, 1019–1036. [Google Scholar] [CrossRef]
- Jin, W.; Tian, Y.; Wang, G.; Zeng, D.; Xu, Q.; Cui, J. Ultra-deep oxidative desulfurization of fuel with H2O2 catalyzed by molybdenum oxide supported on alumina modified by Ca2+. RSC Adv. 2017, 7, 48208–48213. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Wang, A.; Liu, J.; Li, X.; Hu, Y. Oxidation of dibenzothiophene with cumene hydroperoxide on MoO3/SiO2 modified with alkaline earth metals. Catal. Today 2010, 149, 122–126. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, W.; Li, H.; Xun, S.; Li, M.; Li, Y.; Wei, Y.; Li, H. Fabrication and characterization of tungsten-containing mesoporous silica for heterogeneous oxidative desulfurization. Chin. J. Catal. 2016, 37, 971–978. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, G.; Zhang, Y.; Zeng, D.; Chen, Y. Direct synthesis of mesoporous H3PMo12O40/SiO2 and its catalytic performance in oxidative desulfurization of fuel oil. Fuel 2015, 147, 195–202. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, G.; Long, J.; Cui, J.; Jin, W.; Zeng, D. Ultra-deep oxidative desulfurization of fuel with H2O2 catalyzed by phosphomolybdic acid supported on silica. Chin. J. Catal. 2016, 37, 2098–2105. [Google Scholar] [CrossRef]
- Seguin, L.; Figlarz, M.; Cavagnat, R.; Lassègues, J.-C. Infrared and Raman spectra of MoO3 molybdenum trioxides and MoO3 xH2O molybdenum trioxide hydrates. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 1995, 51, 1323–1344. [Google Scholar] [CrossRef]
- Ravikumar, R.; Sundeep, D.; Krishna, A.G.; Ephraim, S.D.; Ali, A.; Ahmed, S.I.; Manikanta, K.; Kumar, T.V. Spectral Investigation of Structural and Optical Properties of Mechanically Synthesized TiO2-V2O5 Nanocomposite Powders. Mater. Today: Proc. 2016, 3, 31–38. [Google Scholar] [CrossRef]
- Du, K.; Wei, R.H.; Bai, Y.N.; Gu, Y.; Ben Li, L. Synthesis of MoO3 Nanostructures by a Solution Method. Adv. Mater. Res. 2012, 554, 494–497. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Chary, K.V.R. Structure and catalytic properties of molybdenum oxide catalysts supported on zirconia. J. Catal. 2004, 226, 283–291. [Google Scholar] [CrossRef]
- Eighmy, T.T. Hinsdalite (PbAl3PO4SO4(OH)6) Characterized by XPS: An Environmentally Important Secondary Mineral. Surf. Sci. Spectra 1999, 6, 184–192. [Google Scholar] [CrossRef]
- Long, Z.; Yang, C.; Zeng, G.; Peng, L.; Dai, C.; He, H. Catalytic oxidative desulfurization of dibenzothiophene using catalyst of tungsten supported on resin D152. Fuel 2014, 130, 19–24. [Google Scholar] [CrossRef]
- Guo, Z.; Du, Y.; Lei, J.; Zhou, L.; Du, X. Oxidative Desulfurization of Fuel Oil at Room Temperature Catalyzed by Ordered Meso-macroporous HPW/SiO2. J. Wuhan Univ. Technol. Sci. Ed. 2019, 34, 1071–1076. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Meng, H.; Li, C. Lipophilicity of amphiphilic phosphotungstates matters in catalytic oxidative desulfurization of oil by H2O2. Fuel 2019, 253, 802–810. [Google Scholar] [CrossRef]
- Jatav, S.; Srivastava, V.C. Ce/Al2O3 as an efficient catalyst for oxidative desulfurization of liquid fuel. Pet. Sci. Technol. 2019, 37, 633–640. [Google Scholar] [CrossRef]




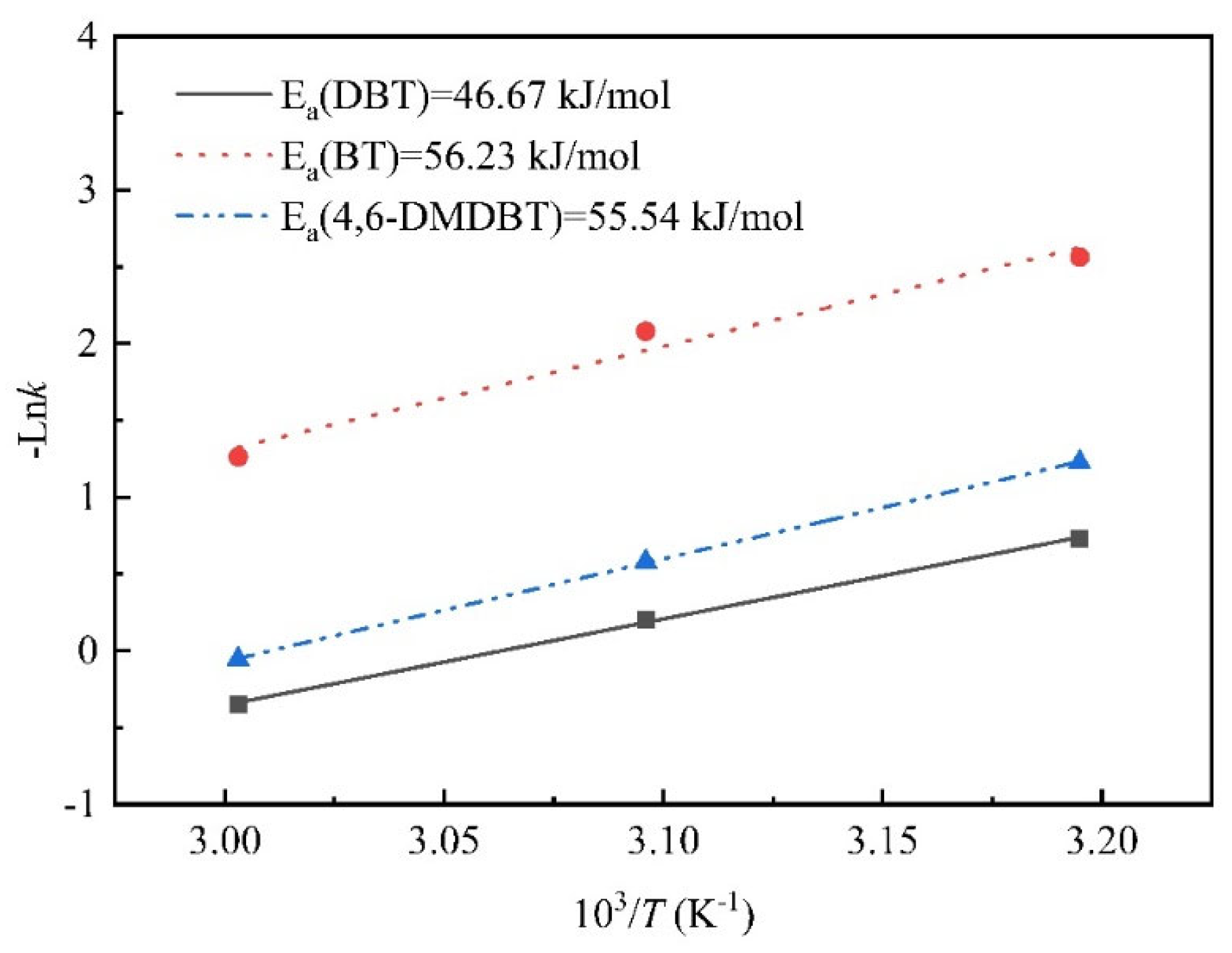

| Sample | t(°C) | k(min−1) | R2 |
|---|---|---|---|
| DBT | 40 | 0.482 | 0.973 |
| DBT | 50 | 0.815 | 0.987 |
| DBT | 60 | 1.416 | 0.995 |
| BT | 40 | 0.077 | 0.955 |
| BT | 50 | 0.140 | 0.960 |
| BT | 60 | 0.283 | 0.974 |
| 4,6-DMDBT | 40 | 0.293 | 0.943 |
| 4,6-DMDBT | 50 | 0.559 | 0.994 |
| 4,6-DMDBT | 60 | 1.056 | 0.998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tian, Q.; Tian, Y.; Cui, J.; Wang, G. Ultra-Deep Oxidative Desulfurization of Fuel with H2O2 Catalyzed by Mesoporous Silica-Supported Molybdenum Oxide Modified by Ce. Appl. Sci. 2021, 11, 2018. https://doi.org/10.3390/app11052018
Chen Y, Tian Q, Tian Y, Cui J, Wang G. Ultra-Deep Oxidative Desulfurization of Fuel with H2O2 Catalyzed by Mesoporous Silica-Supported Molybdenum Oxide Modified by Ce. Applied Sciences. 2021; 11(5):2018. https://doi.org/10.3390/app11052018
Chicago/Turabian StyleChen, Yang, Qi Tian, Yongsheng Tian, Jiawei Cui, and Guanghui Wang. 2021. "Ultra-Deep Oxidative Desulfurization of Fuel with H2O2 Catalyzed by Mesoporous Silica-Supported Molybdenum Oxide Modified by Ce" Applied Sciences 11, no. 5: 2018. https://doi.org/10.3390/app11052018





