Nanostructured Strontium-Doped Calcium Phosphate Cements: A Multifactorial Design
Abstract
1. Introduction
2. Materials and Methods
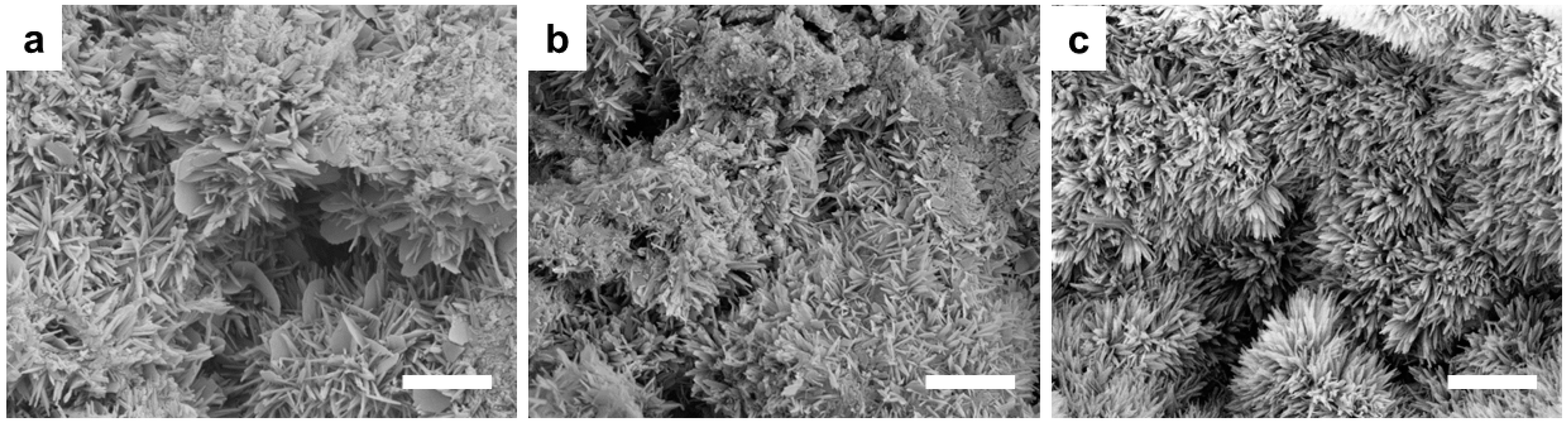
3. Results
3.1. Powder Thermal Treatment
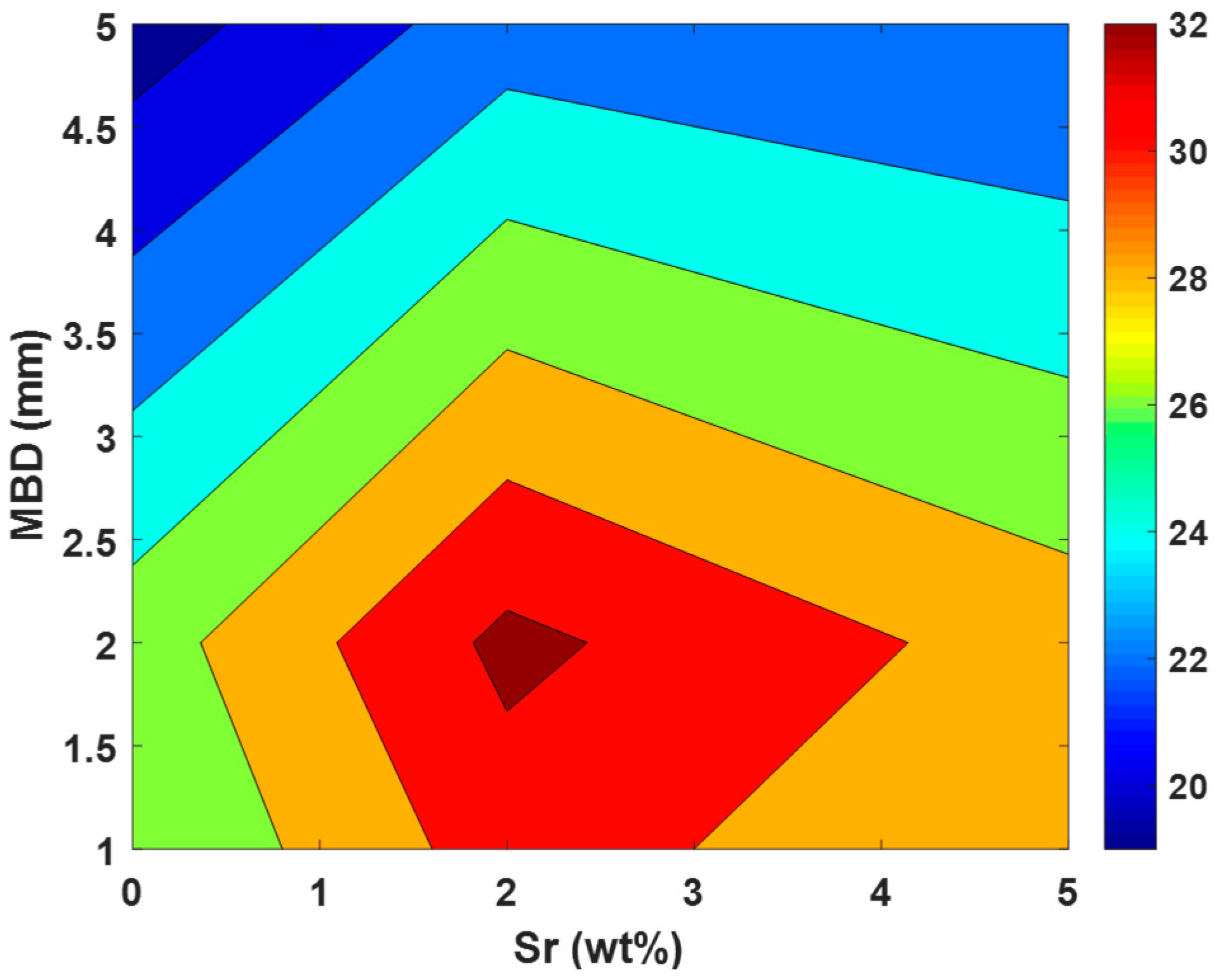
3.2. Powder Planetary Milling
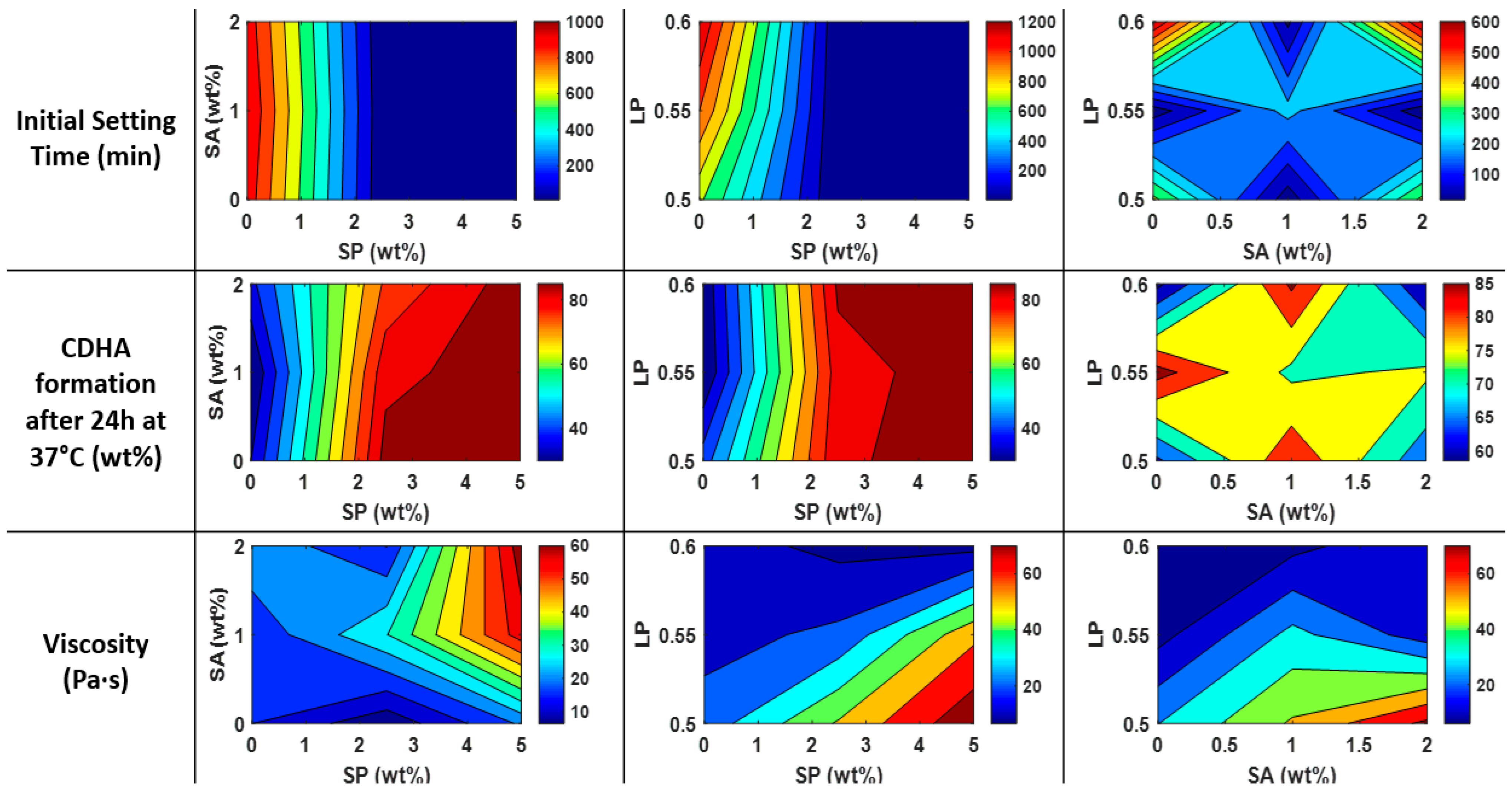
3.3. Setting Solution and Mixing
3.4. Effect of Different Mixing Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kona, S.; Wadajkar, A.S.; Nguyen, K.T. 6—Tissue engineering applications of injectable biomaterials. In Injectable Biomaterials; Vernon, B., Ed.; Woodhead Publishing Series in Biomaterials: Cambridge, UK, 2011; pp. 142–182. [Google Scholar]
- Deramond, H.; Depriester, C.; Galibert, P.; Le Gars, D. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol. Clin. N. Am. 1998, 36, 533–546. [Google Scholar] [CrossRef]
- Rehman, I.; Harper, E.J.; Bonfield, W. In situ analysis of the degree of polymerization of bone cement by using FT-Raman spectroscopy. Biomaterials 1996, 17, 1615–1619. [Google Scholar] [CrossRef]
- Bettencourt, A.; Calado, A.; Amaral, J.; Vale, F.M.; Rico, J.M.T.; Monteiro, J.; Lopes, A.; Pereira, L.; Castro, M. In vitro release studies of methylmethacrylate liberation from acrylic cement powder. Int. J. Pharm. 2000, 197, 161–168. [Google Scholar] [CrossRef]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31 (Suppl. 4), 37–47. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef]
- Driessens, F.C.M.; Planell, J.A.; Boltong, M.G.; Khairoun, I.; Ginebra, M.P. Osteotransductive bone cements. Proc. Inst. Mech. Eng. H 1998, 212, 427–435. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S.; Sandri, M.; Logroscino, G. Sr-substituted hydroxyapatites for osteoporotic bone replacement. Acta Biomater. 2007, 3, 961–969. [Google Scholar] [CrossRef]
- Sprio, S.; Tampieri, A.; Landi, E.; Sandri, M.; Martorana, S.; Celotti, G.; Logroscino, G. Physico-chemical properties and solubility behaviour of multi-substituted hydroxyapatite powders containing silicon. Mater. Sci. Eng. C 2008, 28, 179–187. [Google Scholar] [CrossRef]
- Supova, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Laskus, A.; Kolmas, J. Ionic substitutions in non-apatitic calcium phosphates. Int. J. Mol. Sci. 2017, 18, 2542. [Google Scholar] [CrossRef] [PubMed]
- Braux, J.; Velard, F.; Guillaume, C.; Bouthors, S.; Jallot, E.; Nedelec, J.M.; Laurent-Maquin, D.; Laquerriere, P. A new insight into the dissociating effect of strontium on bone resorption and formation. Acta Biomater. 2011, 7, 2593–2603. [Google Scholar] [CrossRef]
- Schumacher, M.; Gelinsky, M. Strontium modified calcium phosphate cements—Approaches towards targeted stimulation of bone turnover. J. Mater. Chem. B 2015, 3, 4626–4640. [Google Scholar] [CrossRef] [PubMed]
- Montesi, M.; Panseri, S.; Dapporto, M.; Tampieri, A.; Sprio, S. Sr-substituted bone cements direct mesenchymal stem cells, osteoblasts and osteoclasts fate. PLoS ONE 2017, 12, e0172100. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Wagner, A.S.; Kokesch-Himmelreich, J.; Bernhardt, A.; Rohnke, M.; Wenisch, S.; Gelinsky, M. Strontium substitution in apatitic CaP cements effectively attenuates osteoclastic resorption but does not inhibit osteoclastogenesis. Acta Biomater. 2016, 37, 184–194. [Google Scholar] [CrossRef]
- Canal, C.; Ginebra, M.P. Fibre-reinforced calcium phosphate cements: A review. J. Mech. Behav. Biomed. 2011, 4, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Geffers, M.; Groll, J.; Gbureck, U. Reinforcement strategies for load-bearing calcium phosphate biocements. Materials 2015, 8, 2700–2717. [Google Scholar] [CrossRef]
- O’Neill, R.; McCarthy, H.O.; Montufar, E.B.; Ginebra, M.P.; Wilson, D.I.; Lennon, A.; Dunne, N. Critical review: Injectability of calcium phosphate pastes and cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Baroud, G. Injectability of calcium phosphate pastes. Biomaterials 2005, 26, 1553–1563. [Google Scholar] [CrossRef]
- Kiri, L.; Boyd, D. Predicting composition-property relationships for glass ionomer cements: A multifactor central composite approach to material optimization. J. Mech. Behav. Biomed. 2015, 46, 285–291. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, R.M.; Dunne, N.J.; Orr, J.F.; Buchanan, F.J.; Wilcox, R.K.; Barton, D.C. Optimisation of the mechanical and handling properties of an injectable calcium phosphate cement. J. Mater. Sci. Mater. M 2010, 21, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, S.M.; Baseri, H. Prediction of the setting properties of calcium phosphate bone cement. Comput. Intell. Neurosc. 2012, 2012, 809235. [Google Scholar] [CrossRef]
- O’Neill, R.; McCarthy, H.O.; Cunningham, E.; Montufar, E.; Ginebra, M.P.; Wilson, D.I.; Lennon, A.; Dunne, N. Extent and mechanism of phase separation during the extrusion of calcium phosphate pastes. J. Mater. Sci. Mater. M 2016, 27, 29. [Google Scholar] [CrossRef]
- Bohner, M.; Doebelin, N.; Baroud, G. Theoretical and experimental approach to test the cohesion of calcium phosphate pastes. Eur. Cells Mater. 2006, 12, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Kim, H.W.; Ginebra, M.P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012, 3, 2041731412439555. [Google Scholar] [CrossRef] [PubMed]
- Khairoun, I.; Driessens, F.C.M.; Boltong, M.G.; Planell, J.A.; Wenz, R. Addition of cohesion promoters to calcium phosphate cements. Biomaterials 1999, 20, 393–398. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A. Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: A kinectic analysis. Biomaterials 2004, 25, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- Montufar, E.B.; Maazouz, Y.; Ginebra, M.P. Relevance of the setting reaction to the injectability of tricalcium phosphate pastes. Acta Biomater. 2013, 9, 6188–6198. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shao, H.; Chen, F.; Zheng, H. Effects of the granularity of raw materials on the hydration and hardening process of calcium phosphate cement. Biomaterials 2003, 24, 4103–4113. [Google Scholar] [CrossRef]
- Kuang, G.M.; Yau, W.P.; Lam, W.M.; Wu, J.; Chiu, K.Y.; Lu, W.W.; Pan, H.B. An effective approach by a chelate reaction in optimizing the setting process of strontium-incorporated calcium phosphate bone cement. J. Biomed. Mater. Res. B 2012, 100B, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Henss, A.; Rohnke, M.; Gelinsky, M. A novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013, 9, 7536–7544. [Google Scholar] [CrossRef]
- Bohner, M.; Luginbuhl, R.; Reber, C.; Doebelin, N.; Baroud, G.; Conforto, E. A physical approach to modify the hydraulic reactivity of alpha-tricalcium phosphate powder. Acta Biomater. 2009, 5, 3524–3535. [Google Scholar] [CrossRef] [PubMed]
- Jack, V.; Buchanan, F.J.; Dunne, N.J. Particle attrition of alpha-tricalcium phosphate: Effect on mechanical, handling, and injectability properties of calcium phosphate cements. Proc. Inst. Mech. Eng. H 2008, 222, 19–28. [Google Scholar] [CrossRef]
- Bergmann, C.J.D.; Odekerken, J.C.E.; Welting, T.J.M.; Jungwirth, F.; Devine, D.; Boure, L.; Zeiter, S.; van Rhijn, L.W.; Telle, R.; Fischer, H.; et al. Calcium phosphate based three-dimensional cold plotted bone scaffolds for critical size bone defects. Biomed. Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.J.; Grass, R.N.; Bohner, M.; Stark, W.J. Effect of particle size, crystal phase and crystallinity on the reactivity of tricalcium phosphate cements for bone reconstruction. J. Mater. Chem. 2007, 17, 4072–4078. [Google Scholar] [CrossRef]
- Camire, C.L.; Gbureck, U.; Hirsiger, W.; Bohner, M. Correlating crystallinity and reactivity in an alpha-tricalcium phosphate. Biomaterials 2005, 26, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Kano, J.; Mio, H.; Saito, F. Correlation of size reduction rate of inorganic materials with impact energy of balls in planetary ball milling. J. Chem. Eng. Jpn. 1999, 32, 445–448. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Bohner, M.; Zhou, W.; Winnubst, A.J.A.; Wolke, J.G.C.; Jansen, J.A. The effect of ball milling grinding pathways on the bulk and reactivity properties of calcium phosphate cements. J. Biomed. Mater. Res. B 2011, 98B, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Serraj, S.; Boudeville, P.; Pauvert, B.; Terol, A. Effect on composition of dry mechanical grinding of calcium phosphate mixtures. J. Biomed. Mater. Res. 2001, 55, 566–575. [Google Scholar] [CrossRef]
- Clements, J.; Walker, G.; Pentlavalli, S.; Dunne, N. Optimisation of a two-liquid component pre-filled acrylic bone cement system: A design of experiments approach to optimise cement final properties. J. Mater. Sci. Mater. M 2014, 25, 2287–2296. [Google Scholar] [CrossRef][Green Version]
- Sprio, S.; Dapporto, M.; Montesi, M.; Panseri, S.; Lattanzi, W.; Pola, E.; Logroscino, G.; Tampieri, A. Novel osteointegrative sr-substituted apatitic cements enriched with alginate. Materials 2016, 9, 763. [Google Scholar] [CrossRef]
- Czitrom, V. One-factor-at-a-time versus designed experiments. Am. Stat. 1999, 53, 126–131. [Google Scholar]
- Carrodeguas, R.G.; De Aza, S. Alpha-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Fernandez, E.; DeMaeyer, E.A.P.; Verbeeck, R.M.H.; Boltong, M.G.; Ginebra, J.; Driessens, F.C.M.; Planell, J.A. Setting reaction and hardening of an apatitic calcium phosphate cement. J. Dent. Res. 1997, 76, 905–912. [Google Scholar] [CrossRef]
- Mathew, M.; Schroeder, L.W.; Dickens, B.; Brown, W.E. The crystal structure of [alpha]-Ca3(PO4)2. Acta Crystallogr. B 1977, 33, 1325–1333. [Google Scholar] [CrossRef]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.M.; Hart, J.N.; Gross, K.A. Structural and chemical analysis of well-crystallized hydroxyfluorapatites. J. Phys. Chem. B 2003, 107, 8316–8320. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. A 2003, 65A, 188–195. [Google Scholar] [CrossRef]
- Ross, S.M. Peirce’s criterion for the elimination of suspect experimental data. J. Eng. Technol. 2003, 20, 38–41. [Google Scholar]
- Compton, B.G.; Lewis, J.A. 3D-printing of lightweight cellular composites. Adv. Mater. 2014, 26, 5930–5935. [Google Scholar] [CrossRef]
- Araujo, P.W.; Brereton, R.G. Experimental design .2. Optimization. Trac. Trend Anal. Chem. 1996, 15, 63–70. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Saint-Jean, S.J.; Camire, C.L.; Nevsten, P.; Hansen, S.; Ginebra, M.P. Study of the reactivity and in vitro bioactivity of Sr-substituted alpha-TCP cements. J. Mater. Sci. Mater. M 2005, 16, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Khairoun, I.; Boltong, M.G.; Driessens, F.C.; Planell, J.A. Some factors controlling the injectability of calcium phosphate bone cements. J. Mater. Sci. Mater. M 1998, 9, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Gbureck, U.; Barralet, J.E.; Spatz, K.; Grover, L.M.; Thull, R. Ionic modification of calcium phosphate cement viscosity. Part I: Hypodermic injection and strength improvement of apatite cement. Biomaterials 2004, 25, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Gbureck, U.; Barralet, J.E.; Radu, L.; Klinger, H.G.; Thull, R. Amorphous alpha-tricalcium phosphate: Preparation and aqueous setting reaction. J. Am. Ceram. Soc. 2004, 87, 1126–1132. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; de Groot, K. Hydrolysis and phase transition of alpha-tricalcium phosphate. Biomaterials 1997, 18, 737–741. [Google Scholar] [PubMed]
- Cicek, G.; Aksoy, E.A.; Durucan, C.; Hasirci, N. Alpha-tricalcium phosphate (alpha-TCP): Solid state synthesis from different calcium precursors and the hydraulic reactivity. J. Mater. Sci. Mater. M 2011, 22, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Dahlquist, K.; Banerjee, A.; Bandyopadhyay, A.; Bose, S. Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants. J. Mater. Sci. Mater. M 2008, 19, 2669–2677. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, M.J. Introduction to Particle Technology; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Tao, Y.; Li, D.X.; Li, Y.B. Effect of substitutional Sr ion on mechanical properties of calcium phosphate bone cement. J. Wuhan Univ. Technol. 2013, 28, 741–745. [Google Scholar] [CrossRef]
- Lee, G.S.; Park, J.H.; Won, J.E.; Shin, U.S.; Kim, H.W. Alginate combined calcium phosphate cements: Mechanical properties and in vitro rat bone marrow stromal cell responses. J. Mater. Sci. Mater. M 2011, 22, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.A.; De Oliveria, L.C.; Rigo, E.C.; Carrodeguas, R.G.; Boschi, A.O.; De Arruda, A.C. Influence of polymeric additives on the mechanical properties of alpha-tricalcium phosphate cement. Bone 1999, 25, 99S–102S. [Google Scholar] [CrossRef]
- Driessens, F.C.M.; Verbeeck, R.K. Biominerals; Taylor & Francis: Abingdon, UK, 1990. [Google Scholar]
- Williams, D.A.; Saak, A.W.; Jennings, H.M. The influence of mixing on the rheology of fresh cement paste. Cem. Concr. Res. 1999, 29, 1491–1496. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed]
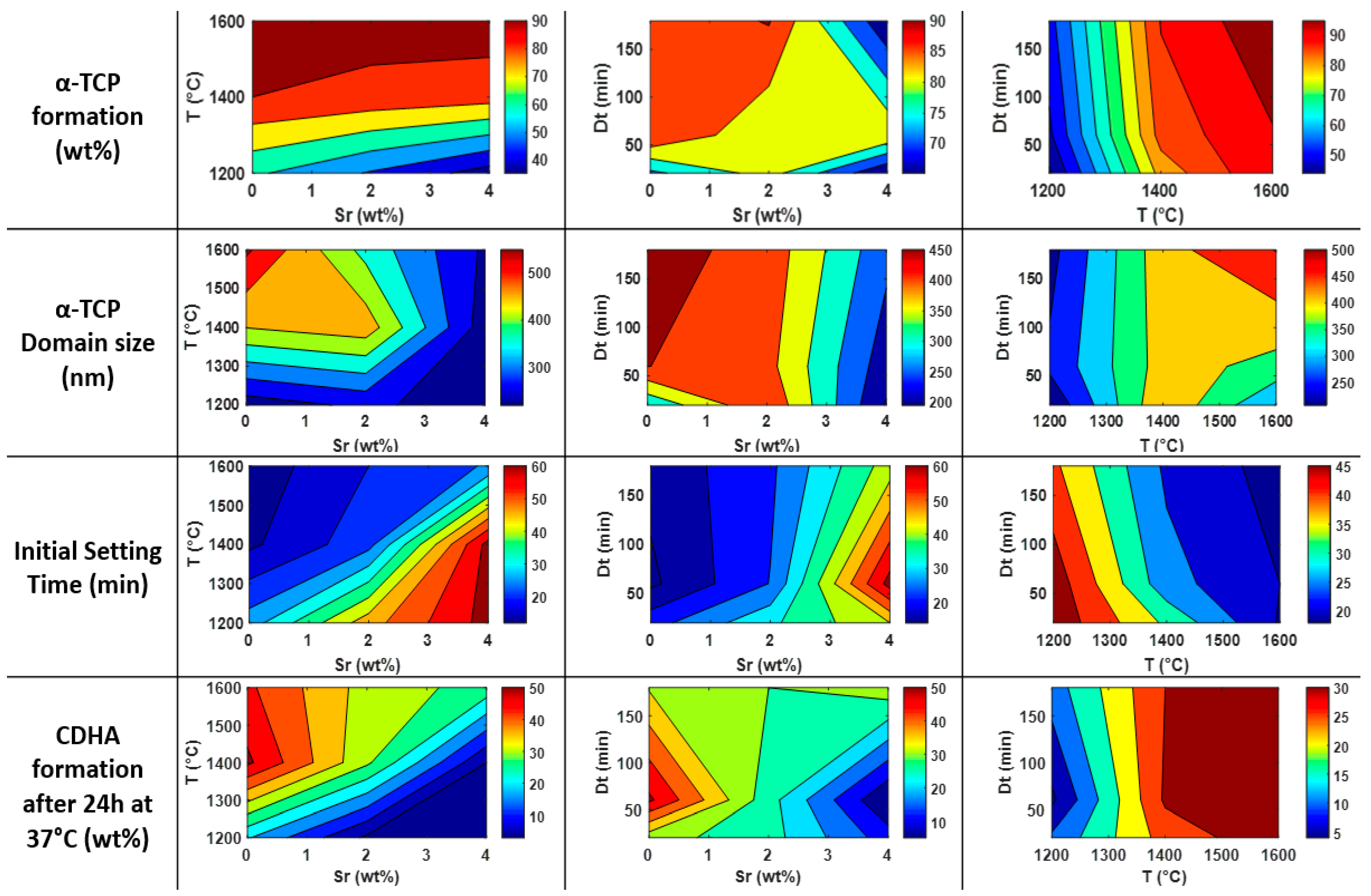
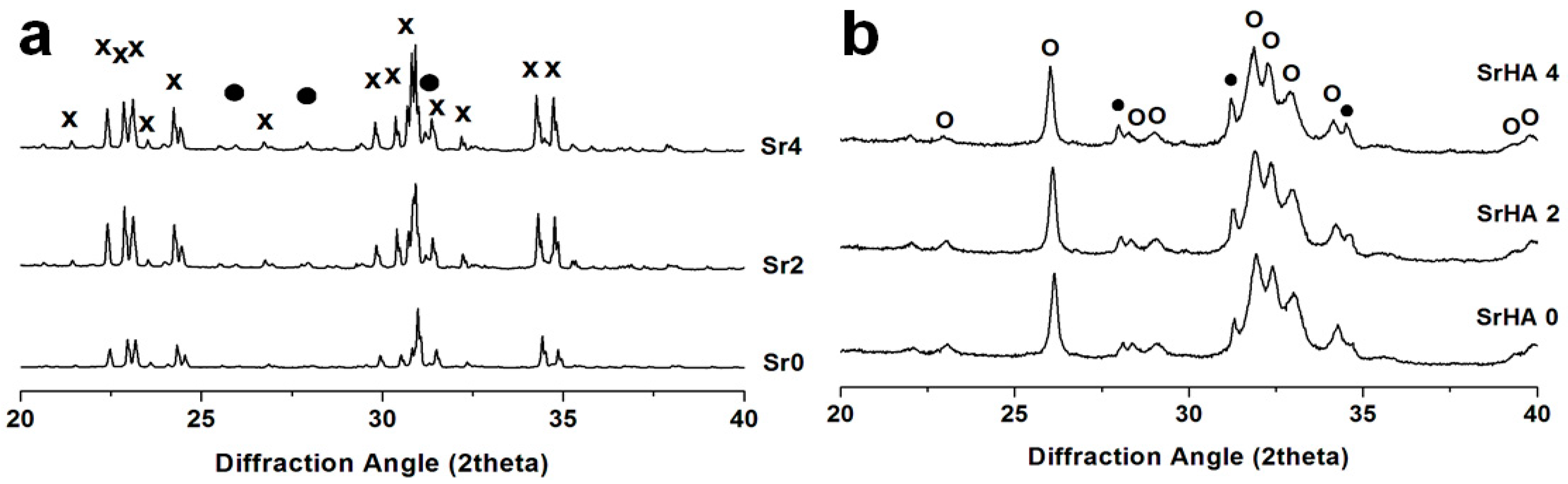

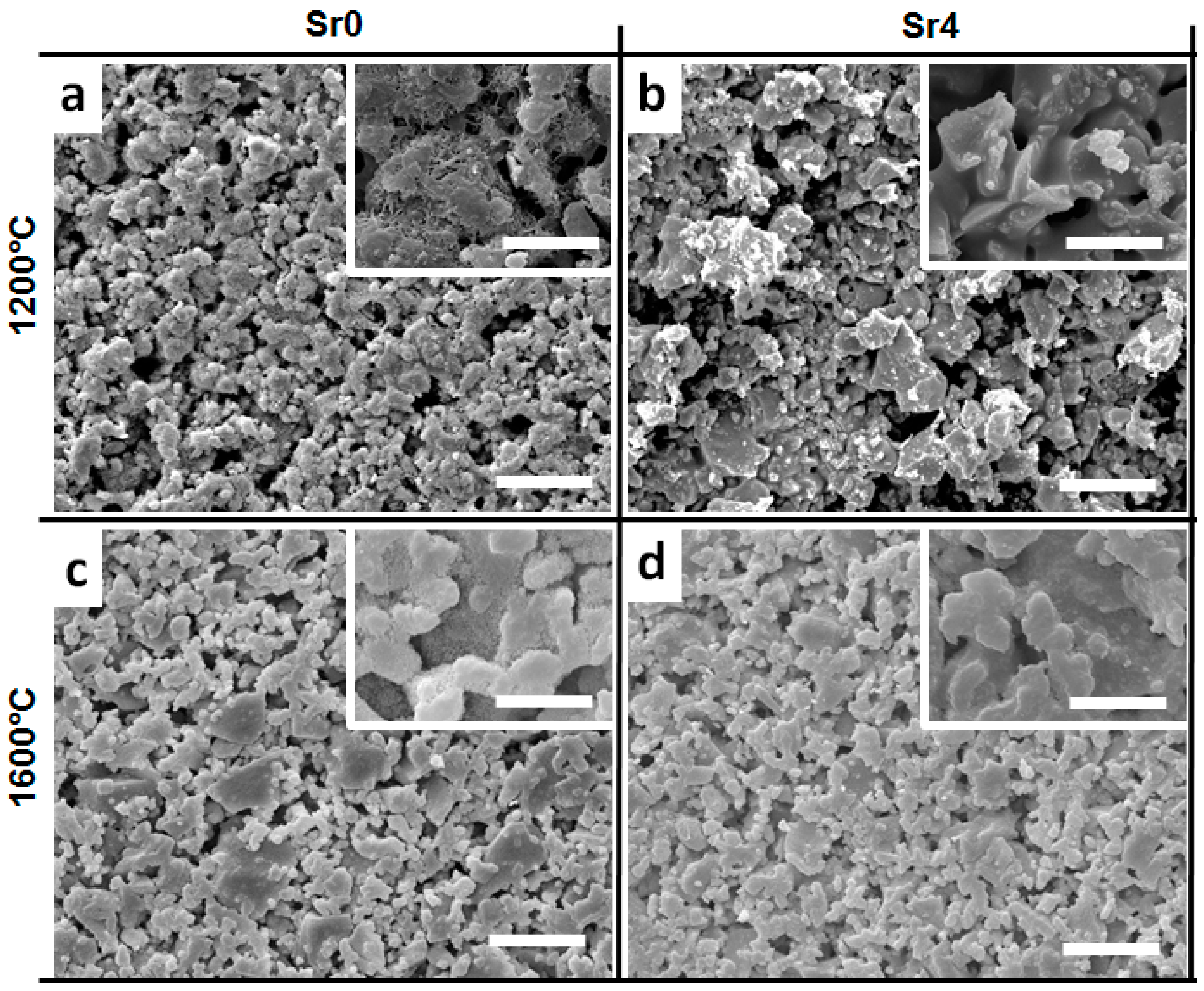
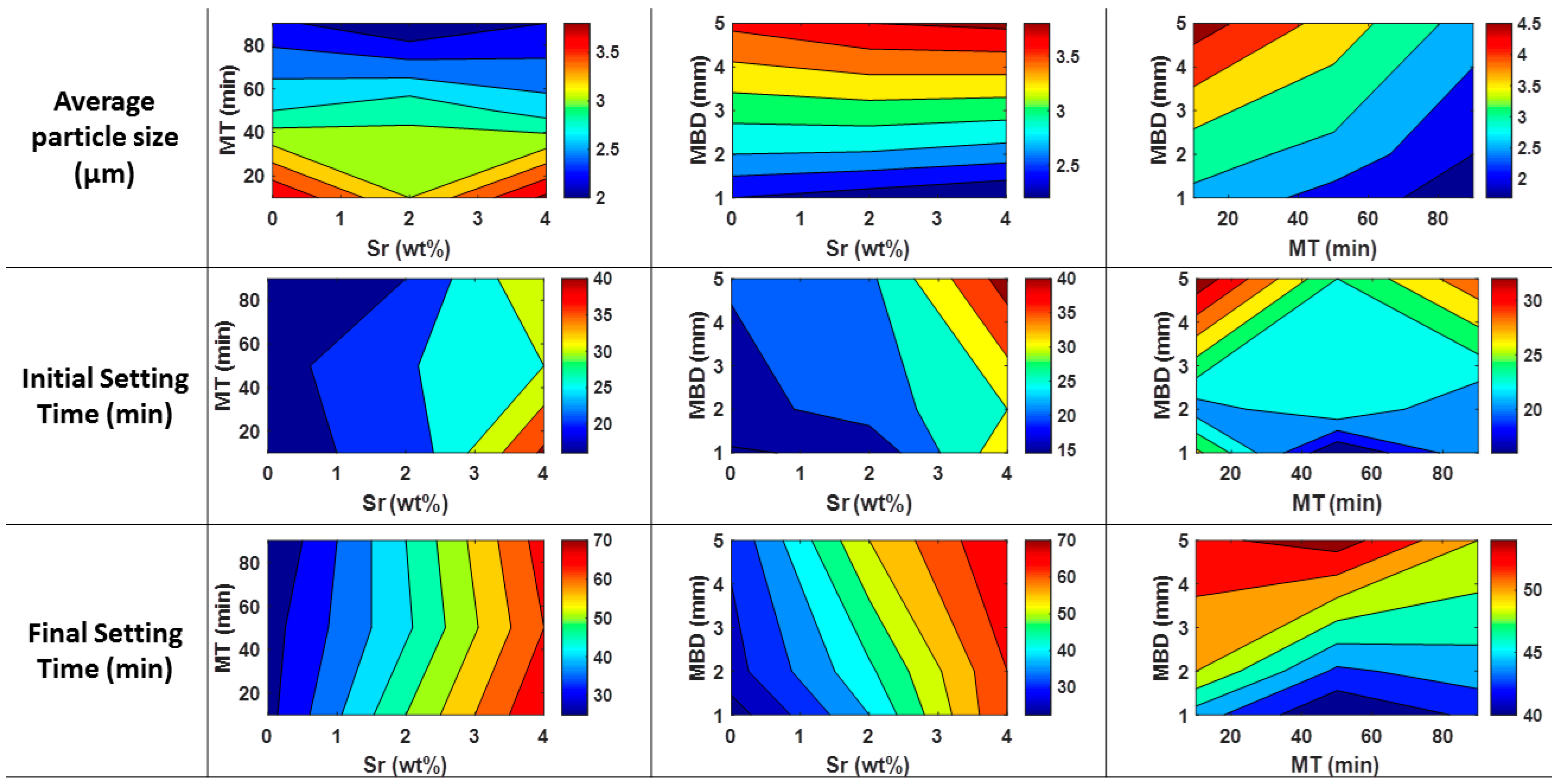

| Factor | Factor Symbol | Coded Levels | Output | Output Symbol | |||
|---|---|---|---|---|---|---|---|
| −1 | 0 | +1 | |||||
| Powder Thermal Treatment | Sr/(Ca + Sr) (mol%) | Sr | 0 | 2 | 4 |
| α αDs tin HA |
| Temperature (°C) | T | 1200 | 1400 | 1600 | |||
| Dwell time (min) | Dt | 20 | 60 | 180 | |||
| Powder Milling | Sr/(Ca + Sr) (mol%) | Sr | 0 | 2 | 4 |
| D50 tin tfin |
| Milling Time (min) | MT | 10 | 50 | 90 | |||
| Milling Balls Diameter (mm) | MBD | 1 | 2 | 5 | |||
| Setting Solution | Na2HPO4 (wt%) | SP | 0 | 2.5 | 5 |
| tin HA η |
| Sodium Alginate (wt%) | SA | 0 | 1 | 2 | |||
| Liquid-to-Powder ratio (-) | LP | 0.50 | 0.55 | 0.60 | |||
| Outputs | |||||||
|---|---|---|---|---|---|---|---|
| Increasing Input Factor | α-TCP Amount | α-TCP Domain Size | Average Particle Size | Initial Setting Time | Final Setting Time | HA Amount | Viscosity |
| Temperature | ↑↑ | ↑↑ | ↓ | ↑↑ | |||
| Dwell Time | ~ | ~ | ~ | ~ | |||
| Strontium Amount | ↓ | ↓↓ | ~ | ↑↑ | ↑↑ | ↓ | |
| Milling Time | ↓↓ | ~ | ~ | ||||
| Milling Balls Diameter | ↑ | ↑ | ↑ | ||||
| Sodium Phosphate | ↓↓ | ↑↑ | ↑ | ||||
| Sodium Alginate | ~ | ~ | ~ | ||||
| Liquid-to-powder ratio | ↑ | ~ | ↓ | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dapporto, M.; Gardini, D.; Tampieri, A.; Sprio, S. Nanostructured Strontium-Doped Calcium Phosphate Cements: A Multifactorial Design. Appl. Sci. 2021, 11, 2075. https://doi.org/10.3390/app11052075
Dapporto M, Gardini D, Tampieri A, Sprio S. Nanostructured Strontium-Doped Calcium Phosphate Cements: A Multifactorial Design. Applied Sciences. 2021; 11(5):2075. https://doi.org/10.3390/app11052075
Chicago/Turabian StyleDapporto, Massimiliano, Davide Gardini, Anna Tampieri, and Simone Sprio. 2021. "Nanostructured Strontium-Doped Calcium Phosphate Cements: A Multifactorial Design" Applied Sciences 11, no. 5: 2075. https://doi.org/10.3390/app11052075
APA StyleDapporto, M., Gardini, D., Tampieri, A., & Sprio, S. (2021). Nanostructured Strontium-Doped Calcium Phosphate Cements: A Multifactorial Design. Applied Sciences, 11(5), 2075. https://doi.org/10.3390/app11052075






