Entomopathogenic Fungi Biomass Production and Extracellular Biosynthesis of Silver Nanoparticles for Bioinsecticide Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Source of Fungus and Insect Breeding
2.3. Entomopathogenic Fungi Pathogenicity
2.4. Fungal Biomass Production
2.5. Silver Nanoparticle Synthesis
2.6. Silver Nanoparticle Characterization
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.R.; Fromm, K.M.; Rizvi, T.F.; Giese, B.; Ahamad, F.; Turner, R.J.; Füeg, M.; Marsili, E. Metal Nanoparticle–Microbe Interactions: Synthesis and Antimicrobial Effects. Part. Part. Syst. Charact. 2020, 1900419. [Google Scholar] [CrossRef]
- Moraga, E.Q. Entomopathogenic fungi as endophytes: Their broader contribution to IPM and crop production. Biocontrol Sci. Technol. 2020, 1–14. [Google Scholar] [CrossRef]
- Mora, M.A.E.; Castilho, A.M.C.; Fraga, M.E. Classification and infection mechanism of entomopathogenic fungi. Arq. Inst. Biol. 2017, 84. [Google Scholar] [CrossRef] [Green Version]
- Al-Ani, L.K.T.; Aguilar-Marcelino, L.; Fiorotti, J.; Sharma, V.; Sarker, M.S.; Furtado, E.L.; Wijayawardene, N.N.; Herrera-Estrella, A. Biological Control Agents and Their Importance for the Plant Health. In Microbial Services in Restoration Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–36. [Google Scholar]
- Das Chagas Bernardo, C.; Pereira-Junior, R.A.; Luz, C.; Mascarin, G.M.; Fernandes, É.K.K. Differential susceptibility of blastospores and aerial conidia of entomopathogenic fungi to heat and UV-B stresses. Fungal Biol. 2020. [Google Scholar] [CrossRef]
- Ghazanfar, M.U.; Hagenbucher, S.; Romeis, J.; Grabenweger, G.; Meissle, M. Fluctuating temperatures influence the susceptibility of pest insects to biological control agents. J. Pest Sci. 2020, 1–12. [Google Scholar] [CrossRef]
- Thaochan, N.; Benarlee, R.; Prabhakar, C.S.; Hu, Q. Impact of temperature and relative humidity on effectiveness of Metarhizium guizhouense PSUM02 against longkong bark eating caterpillar Cossus chloratus Swinhoe under laboratory and field conditions. J. Asia-Pac. Entomol. 2020, 23, 285–290. [Google Scholar] [CrossRef]
- Banu, A.N.; Balasubramanian, C. Optimization and synthesis of silver nanoparticles using Isaria fumosorosea against human vector mosquitoes. Parasitol. Res. 2014, 113, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Kamil, D.; Prameeladevi, T.; Ganesh, S.; Prabhakaran, N.; Nareshkumar, R.; Thomas, S.P. Green Synthesis of Silver Nanoparticles by Entomopathogenic Fungus Beauveria bassiana and Their Bioefficacy Against Mustard Aphid (Lipaphis erysimi Kalt.); NISCAIR-CSIR: New Delhi, India, 2017. [Google Scholar]
- Diniz, F.R.; Maia, R.C.A.; Rannier, L.; Andrade, L.N.; VChaud, M.; Da Silva, C.F.; Corrêa, C.B.; De Albuquerque, R.L.C., Jr.; Da Costa, P.L.; Shin, S.R.; et al. Silver nanoparticles-composing alginate/gelatin hydrogel improves wound healing in vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Alves, S.B.; Lopes, R.B. Culture media selection for mass production of Isaria fumosorosea and Isaria farinosa. Braz. Arch. Biol. Technol. 2010, 53, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Amerasan, D.; Nataraj, T.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, M.; Nicoletti, G. Benelli, Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae). J. Pest Sci. 2016, 89, 249–256. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Adamski, Z.; Bufo, S.A.; Chowański, S.; Falabella, P.; Lubawy, J.; Marciniak, P.; Pacholska-Bogalska, J.; Salvia, R.; Scrano, L.; Słocińska, M.; et al. Beetles as Model Organisms in Physiological, Biomedical and Environmental Studies—A Review. Front. Physiol. 2019, 10, 319. [Google Scholar] [CrossRef] [Green Version]
- De Souza, P.C.; Morey, A.T.; Castanheira, G.M.; Bocate, K.P.; Panagio, L.A.; Ito, F.A.; Furlaneto, M.C.; Yamada-Ogatta, S.F.; Costa, I.N.; Mora-Montes, H.M.; et al. Tenebrio molitor (Coleoptera: Tenebrionidae) as an alternative host to study fungal infections. J. Microbiol. Methods 2015, 118, 182–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oreste, M.; Bubici, G.; Poliseno, M.; Triggiani, O.; Tarasco, E. Pathogenicity of Beauveria bassiana (Bals.-Criv.) Vuill. and Metarhizium anisopliae (Metschn.) Sorokin against Galleria mellonella L. and Tenebrio molitor L. in laboratory assays. Redia 2012, 95, 43–48. [Google Scholar]
- Bhattarai, M.K.; Bhattarai, U.R.; Masoudi, A.; Feng, J.-N.; Wang, D. Pathogenicity and virulence of the entomopathogenic fungi depend on selective suppression of anti-oxidative and detoxification enzymes in Tenebriomolitor (Coleoptera: Tenebrionidae) larvae. Biochem. Cell Arch 2018, 1, 861–874. [Google Scholar]
- Santos, T.S.; De Freitas, A.C.; Poderoso, J.C.M.; Hernandez-Macedo, M.L.; Ribeiro, G.T.; Da Costa, L.P.; Da Costa Mendonça, M. Evaluation of isolates of entomopathogenic fungi in the genera Metarhizium, Beauveria, and Isaria, and their virulence to Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae). Fla. Entomol. 2018, 101, 597–602. [Google Scholar] [CrossRef] [Green Version]
- Senthamizhlselvan, P.; Sujeetha, J.A.R.; Jeyalakshmi, C. Growth, sporulation and biomass production of native entomopathogenic fungal isolates on a suitable medium. J. Biopestic. 2010, 3, 466. [Google Scholar]
- Bhadauria, B.P.; Puri, S.; Singh, P. Mass production of entomopathogenic fungi using agricultural products. Bioscan 2012, 7, 229–232. [Google Scholar]
- Soundarapandian, P.; Chandra, R. Mass production of entomopathogenic fungus Metarhizium anisopliae (Deuteromycota; Hyphomycetes) in the laboratory. Res. J. Microbiol. 2007, 2, 690–705. [Google Scholar]
- El-Enshasy, H.A. Filamentous fungal cultures–Process characteristics, products, and applications. Bioprocess. Value Added Prod. Renew. Resour. 2007, 225–261. [Google Scholar] [CrossRef]
- Badi’ah, H.; Seedeh, F.; Supriyanto, G.; Zaidan, A. Synthesis of Silver Nanoparticles and the Development in Analysis Method. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 012005. [Google Scholar]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Murei, A.; Ayinde, W.B.; Gitari, M.W.; Samie, A. Functionalization and antimicrobial evaluation of ampicillin, penicillin and vancomycin with Pyrenacantha grandiflora Baill and silver nanoparticles. Sci. Rep. 2020, 10, 11596. [Google Scholar] [CrossRef] [PubMed]
- Qamandar, M.A.; Shafeeq, M.A.A. Biosynthesis and properties of silver nanoparticles of fungus Beauveria bassiana. Int. J. Chem. Tech. Res. 2017, 10, 1073–1083. [Google Scholar]
- Peeran, M.; Deeba, K.; Lakshman, P. Extracellular myco-synthesis of silver nanoparticles from and Trichoderma virens and Metarhizzium anisopliae. J. Mycol. Plant Pathol. 2017, 47, 424–429. [Google Scholar]
- Sadeghi, R.; Etemad, S.G.; Keshavarzi, E.; Haghshenasfard, M. Investigation of alumina nanofluid stability by UV–Vis spectrum. Microfluid. Nanofluidics 2015, 18, 1023–1030. [Google Scholar] [CrossRef]
- Mukaka, M. Statistics corner: A guide to appropriate use of correlation in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Mondal, S.; Baksi, S.; Koris, A.; Vatai, G. Journey of enzymes in entomopathogenic fungi. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Guilger-Casagrande, M.; Lima, R.D. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]

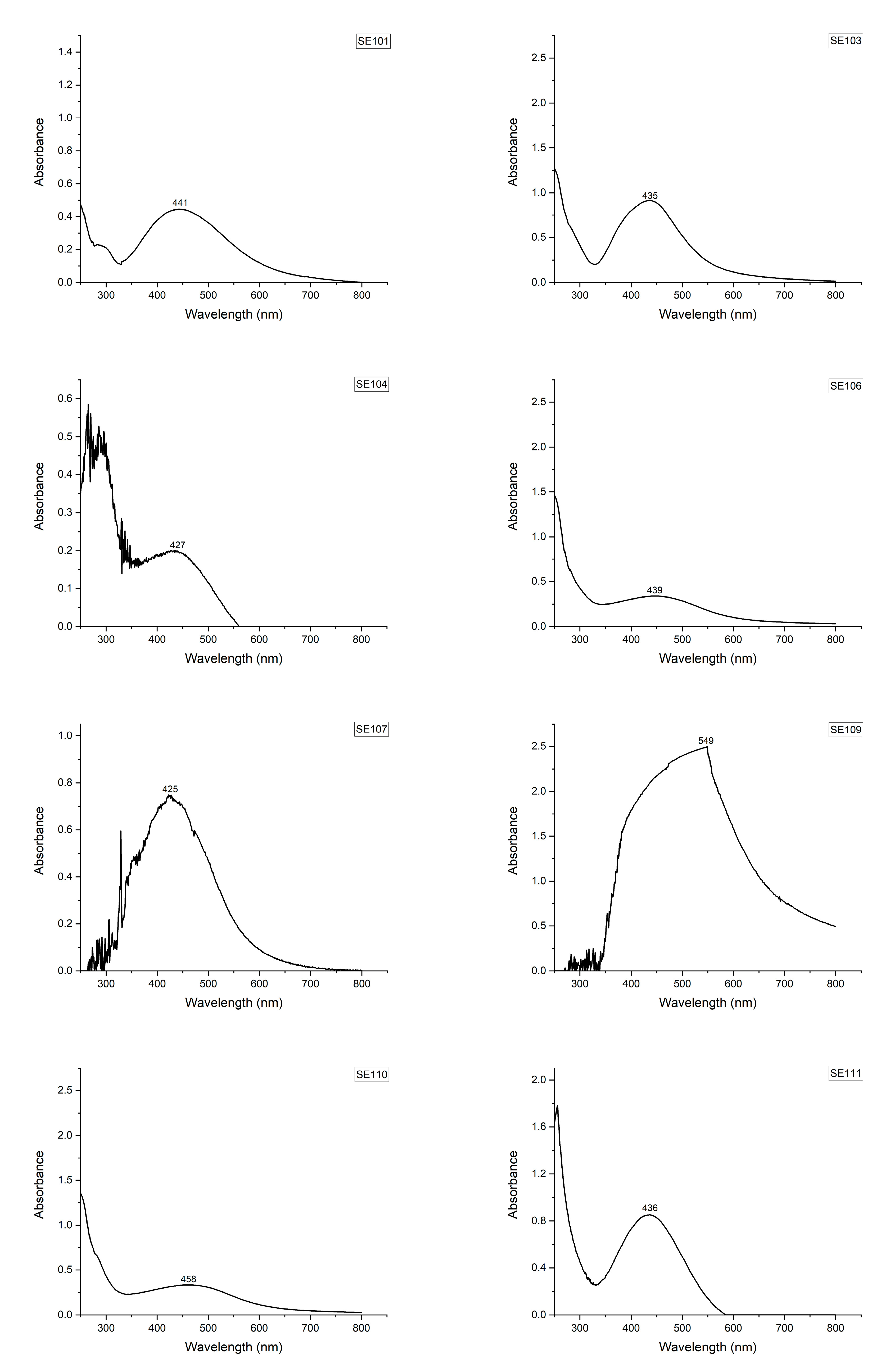
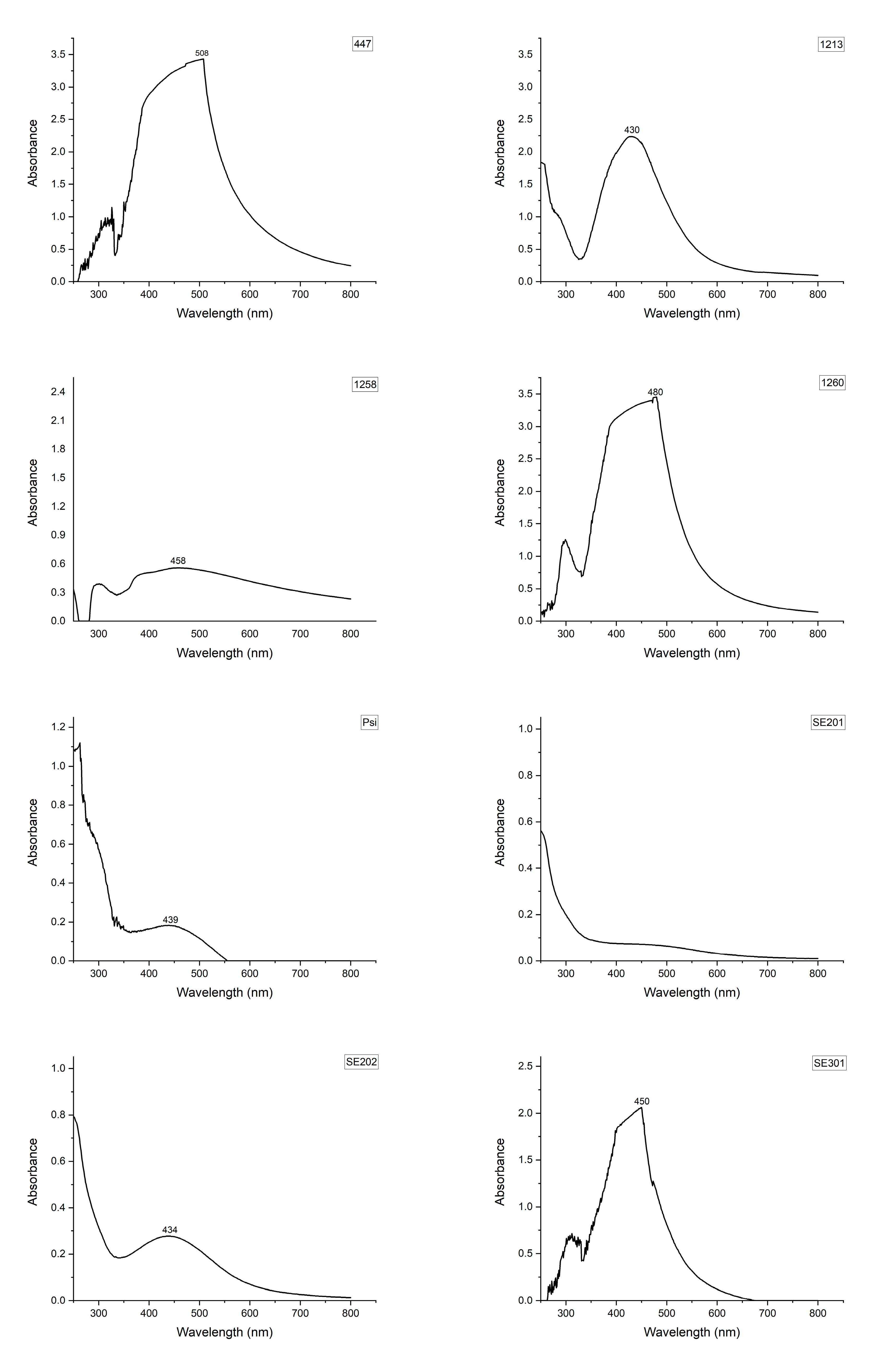
| Fungi/Isolate | Mortality (%) * | LT50 ** | Standard Error • | Lower Limit • | Upper Limit • | Biomass Production (g/100 mL) *** |
|---|---|---|---|---|---|---|
| Beauveria bassiana | ||||||
| SE101 | 14.0 ± 5.1 e | 9.143 | 0.705 | 7.762 | 10.524 | 0.40 ± 0.008 bc |
| SE103 | 52.0 ± 5.8 cd | 6.462 | 0.455 | 5.570 | 7.353 | 0.37 ± 0.005 bc |
| SE104 | 94.0 ± 4.0 a | 5.362 | 0.186 | 4.997 | 5.726 | 0.47 ± 0.03 b |
| SE106 | 80.0 ± 7.1 abc | 5.900 | 0.263 | 5.385 | 6.415 | 0.42 ± 0.02 bc |
| SE107 | 92.0 ± 5.8 ab | 5.312 | 0.149 | 5.020 | 5.605 | 0.46 ± 0.01 b |
| SE109 | 92.0 ± 3.7 ab | 5.239 | 0.179 | 4.888 | 5.590 | 0.52 ± 0.04 b |
| SE110 | 64.0 ± 7.5 bc | 6.188 | 0.382 | 5.438 | 6.937 | 0.41 ± 0.04 bc |
| SE111 | 92.0 ± 3.7 ab | 5.674 | 0.247 | 5.190 | 6.158 | 0.47 ± 0.05 b |
| Psi | 98.0 ± 2.0 a | 6.320 | 0.264 | 5.802 | 6.838 | 0.50 ± 0.06 b |
| 447 | 54.0 ± 6.8 cd | 7.519 | 0.553 | 6.434 | 8.603 | 0.48 ± 0.02 b |
| 1213 | 28.0 ± 7.3 de | 5.286 | 0.714 | 3.886 | 6.686 | 0.47 ± 0.02 b |
| 1260 | 69.1 ± 9.5 abc | 4.636 | 0.199 | 4.247 | 5.026 | 0.42 ± 0.03 bc |
| 1258 | 92.0 ± 3.7 ab | 5.261 | 0.248 | 4.776 | 5.746 | 0.52 ± 0.03 b |
| Metarhizium anisopliae | ||||||
| SE201 | 78.0 ± 8.0 abc | 8.590 | 0.505 | 7.601 | 9.579 | 0.86 ± 0.03 a |
| SE202 | 86.2 ± 2.5 ab | 4.864 | 0.177 | 4.517 | 5.210 | 0.46 ± 0.01 b |
| Isaria fumosorosea | ||||||
| SE301 | 10.0 ± 3.2 e | 4.200 | 1.828 | 0.618 | 7.782 | 0.28 ± 0.01 c |
| Fungi | Pathogenicity/Virulence |
|---|---|
| B. bassiana | Insect mortality: 22–100% [18]; LT50: 2 days [20] |
| M. anisopliae | Insect mortality: 82–100% [18]; LT50: 2 days [20] |
| Isaria | Insect mortality: 95% [19] |
| Fungi/Isolate | Diameter (nm) * | Polydispersity Index |
|---|---|---|
| Beauveria bassiana | ||
| SE101 | 127.8 ± 0.63 | 0.262 ± 0.01 |
| SE103 | 77.08 ± 1.31 | 0.255 ± 0.01 |
| SE104 | 173.63 ± 16.11 | 0.635 ± 0.12 |
| SE106 | 111.16 ± 5.73 | 0.282 ± 0.01 |
| SE107 | 136.63 ± 9.41 | 0.627 ± 0.02 |
| SE109 | 61.14 ± 2.00 | 0.474 ± 0.02 |
| SE110 | 90.29 ± 5.50 | 0.250 ± 0.01 |
| SE111 | 148.7 ± 7.19 | 0.482 ± 0.04 |
| Psi | 289.13 ± 7.85 | 0.598 ± 0.01 |
| 447 | 40.14 ± 0.61 | 0.467 ± 0.004 |
| 1213 | 69.36 ± 1.57 | 0.342 ± 0.05 |
| 1260 | 77.56 ± 2.14 | 0.306 ± 0.05 |
| 1258 | 126.06 ± 0.57 | 0.165 ± 0.01 |
| Metarhizium anisopliae | ||
| SE202 | 132.3 ± 10.86 | 0.279 ± 0.04 |
| Isaria fumosorosea | ||
| SE301 | 131.3 ± 12.39 | 0.551 ± 0.11 |
| Pathogenicity | LT50 | Biomass Production | Synthesis of AgNPs | |
|---|---|---|---|---|
| Pathogenicity | 1 | −0.218 | 0.428 | −0.087 |
| LT50 | 1 | 0.450 | −0.504 * | |
| Biomass Production | 1 | −0.864 ** | ||
| Synthesis of AgNPs | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, T.S.; Passos, E.M.d.; Seabra, M.G.d.J.; Souto, E.B.; Severino, P.; Mendonça, M.d.C. Entomopathogenic Fungi Biomass Production and Extracellular Biosynthesis of Silver Nanoparticles for Bioinsecticide Action. Appl. Sci. 2021, 11, 2465. https://doi.org/10.3390/app11062465
Santos TS, Passos EMd, Seabra MGdJ, Souto EB, Severino P, Mendonça MdC. Entomopathogenic Fungi Biomass Production and Extracellular Biosynthesis of Silver Nanoparticles for Bioinsecticide Action. Applied Sciences. 2021; 11(6):2465. https://doi.org/10.3390/app11062465
Chicago/Turabian StyleSantos, Tárcio S., Eliana M. dos Passos, Matheus G. de Jesus Seabra, Eliana B. Souto, Patrícia Severino, and Marcelo da Costa Mendonça. 2021. "Entomopathogenic Fungi Biomass Production and Extracellular Biosynthesis of Silver Nanoparticles for Bioinsecticide Action" Applied Sciences 11, no. 6: 2465. https://doi.org/10.3390/app11062465
APA StyleSantos, T. S., Passos, E. M. d., Seabra, M. G. d. J., Souto, E. B., Severino, P., & Mendonça, M. d. C. (2021). Entomopathogenic Fungi Biomass Production and Extracellular Biosynthesis of Silver Nanoparticles for Bioinsecticide Action. Applied Sciences, 11(6), 2465. https://doi.org/10.3390/app11062465







