Virtual Reality Environment with Haptic Feedback Thimble for Post Spinal Cord Injury Upper-Limb Rehabilitation
Abstract
1. Introduction
2. Materials and Methods
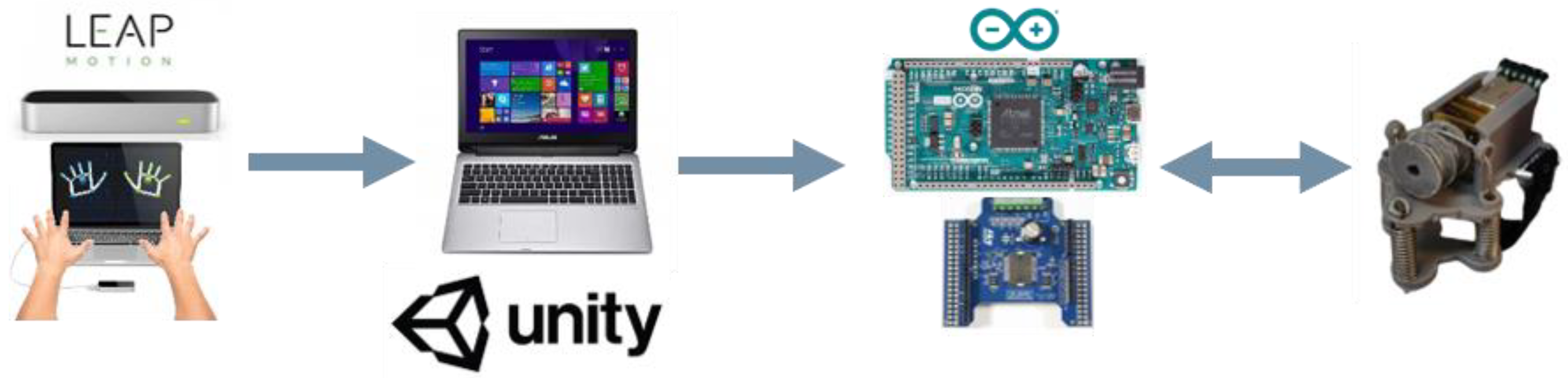
2.1. UL Rehabilitation Platform
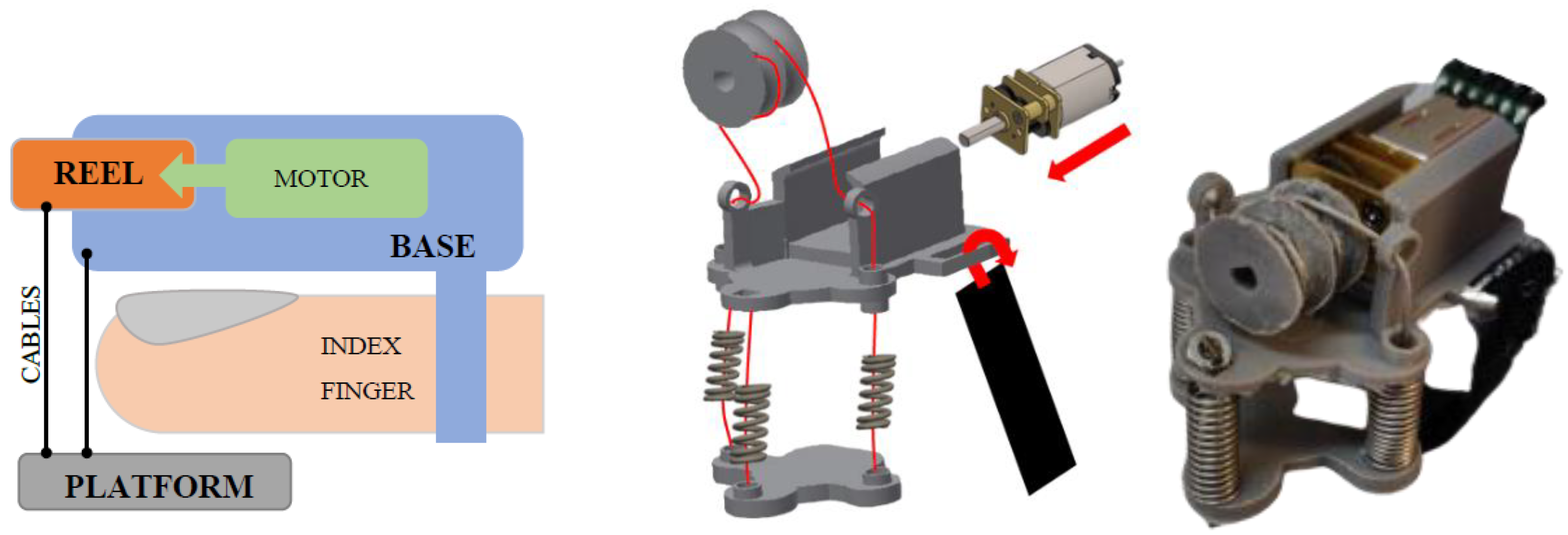
2.1.1. Haptic Thimble
2.1.2. Leap Motion
2.2. Serious Game Development
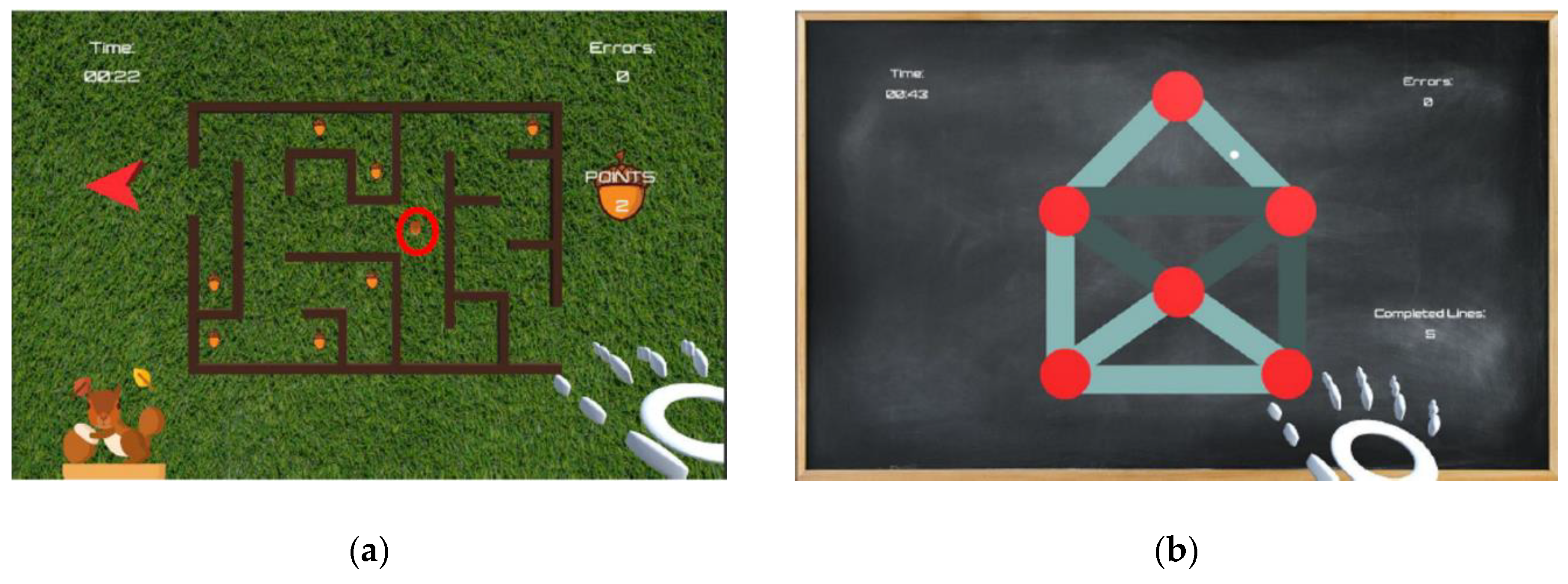
2.2.1. Maze
2.2.2. Path
2.2.3. Grab
2.3. Participants
2.4. Experimental Setup
2.5. Data Processing
3. Results
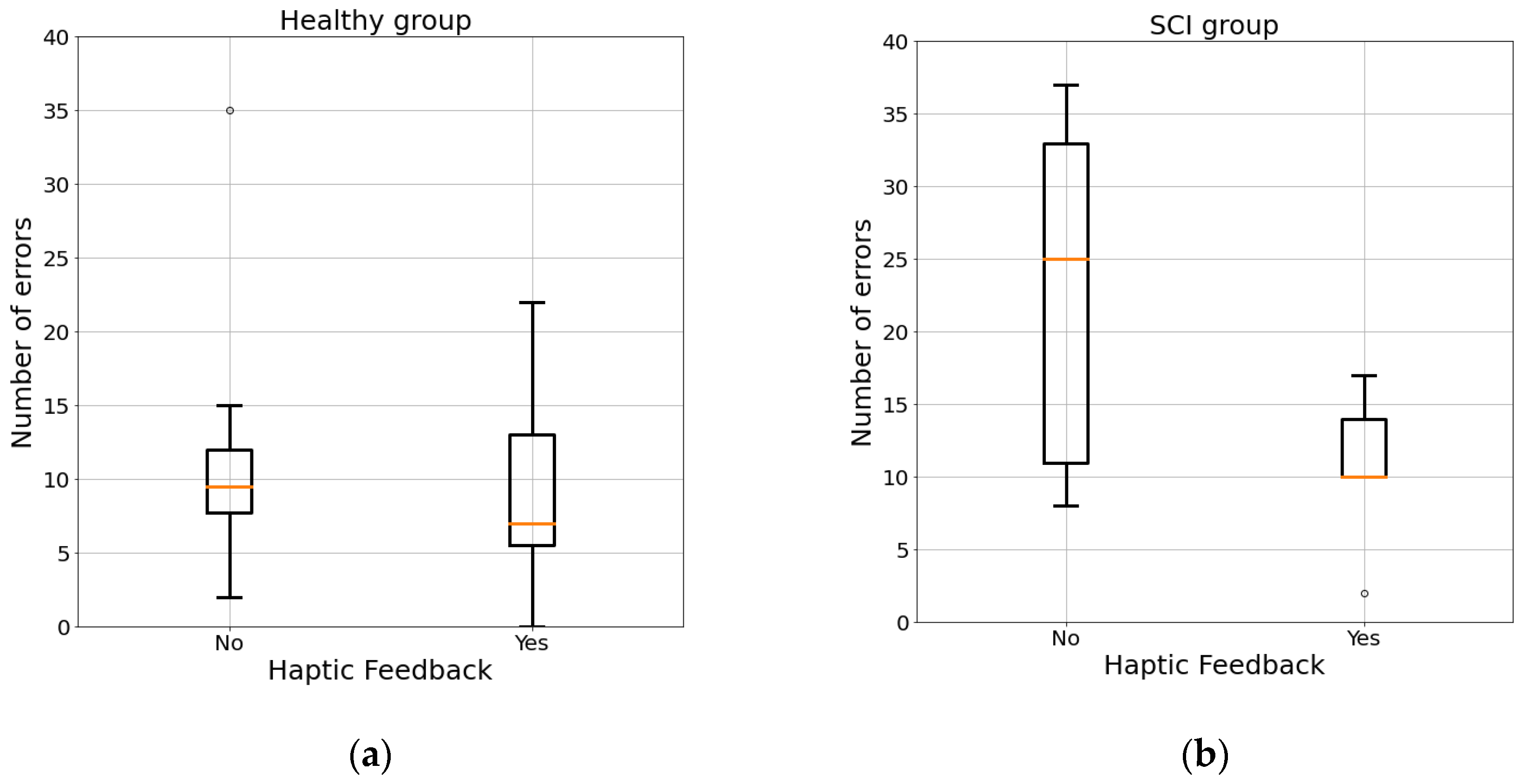
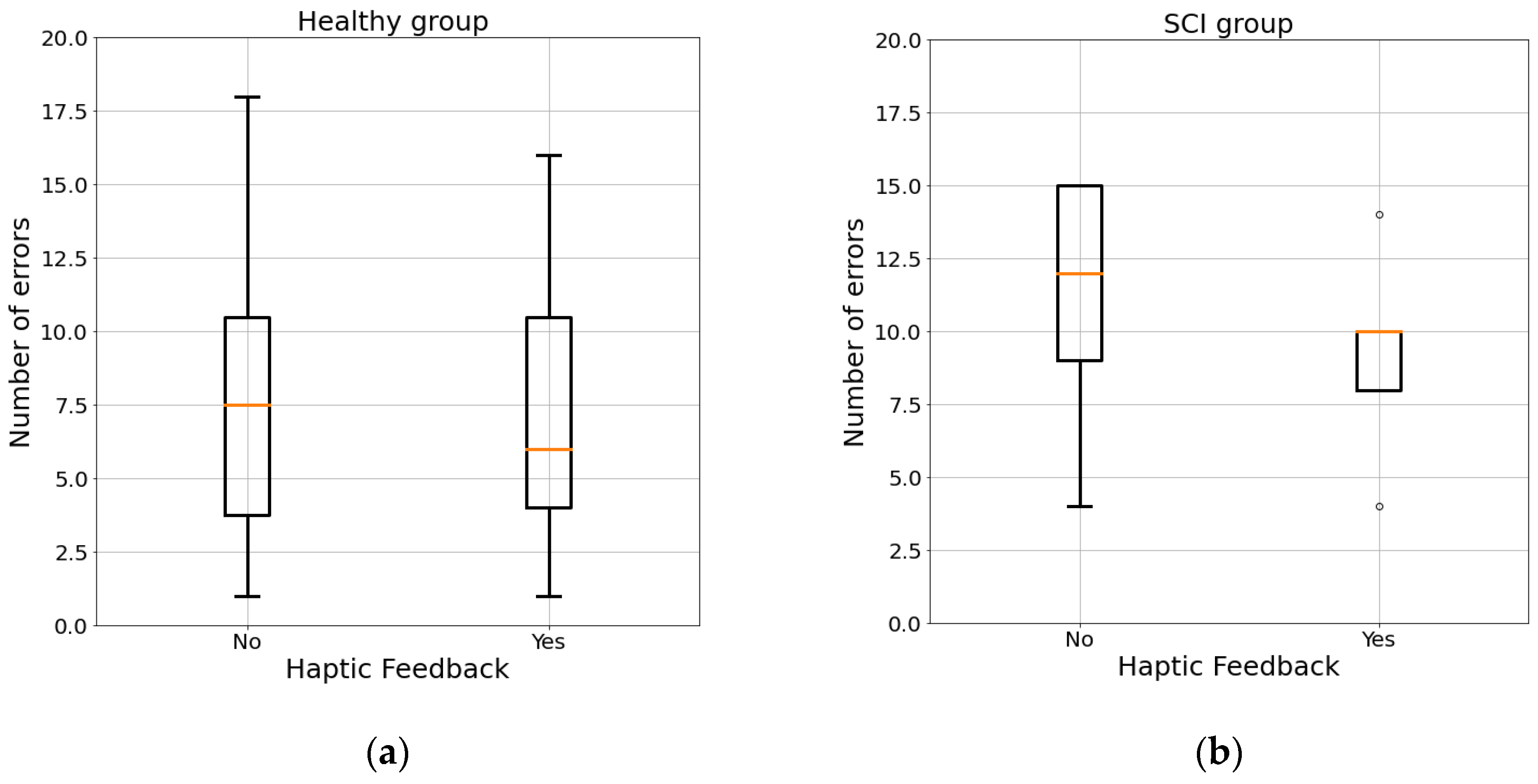
3.1. Maze
3.2. Path
3.3. Grab
4. Conclusions
- All the devices used to build the platform are low-cost and easily adaptable for different people, with a short preparation for the session.
- The configuration options used in this project allows different virtual scenarios and training situations personalized for different patients to be created.
- The final design of the thimble and of the electronic connections ensures good mobility and generates a good haptic response.
- The data storage makes the platform a useful tool for monitoring progress in rehabilitation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bickenbach, J.; Officer, A.; Shakespeare, T.; von Groote, P. International Perspectives on Spinal Cord Injury: Summary; ISCOS: Buckinghamshire, UK, 2013. [Google Scholar]
- National Institute of Neurological Disorders and Stroke. Spinal Cord Injury Information Page. Available online: https://www.ninds.nih.gov/Topic-Areas/spinal-cord-injury (accessed on 9 March 2021).
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Wyndaele, M.; Wyndaele, J.J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord 2006, 44, 523–529. [Google Scholar] [CrossRef] [PubMed]
- DeVivo, M.; Biering-Sorensen, F.; Charlifue, S.; Noonan, V.; Post, M.; Stripling, T.; Wing, P. International spinal cord injury core data set. Spinal Cord 2006, 44, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Merritt, C.H.; Taylor, M.A.; Yelton, C.J.; Ray, S.K. Economic impact of traumatic spinal cord injuries in the United States. Neuroimmunol. Neuroinflamm. 2019. [Google Scholar] [CrossRef]
- GSnoek, J.; Ijzerman, M.J.; Hermens, H.J.; Maxwell, D.; Biering-Sorensen, F. Survey of the needs of patients with spinal cord injury: Impact and priority for improvement in hand function in tetraplegics. Spinal Cord 2004. [Google Scholar] [CrossRef]
- Dimbwadyo-Terrer, I.; Trincado-Alonso, F.; de los Reyes-Guzmán, A.; Aznar, M.A.; Alcubilla, C.; Pérez-Nombela, S.; del Ama-Espinosa, A.; Polonio-López, B.; Gil-Agudo, Á. Upper limb rehabilitation after spinal cord injury: A treatment based on a data glove and an immersive virtual reality environment. Disabil. Rehabil. Assist. Technol. 2016. [Google Scholar] [CrossRef]
- Lo, C.; Tran, Y.; Anderson, K.; Craig, A.; Middleton, J. Functional priorities in persons with spinal cord injury: Using discrete choice experiments to determine preferences. J. Neurotrauma 2016. [Google Scholar] [CrossRef]
- Mekki, M.; Delgado, A.D.; Fry, A.; Putrino, D.; Huang, V. Robotic rehabilitation and spinal cord injury: A narrative review. Neurotherapeutics 2018. [Google Scholar] [CrossRef]
- Harvey, L.A.; Dunlop, S.A.; Churilov, L.; Galea, M.P. Early intensive hand rehabilitation is not more effective than usual care plus one-to-one hand therapy in people with sub-acute spinal cord injury (‘Hands On’): A randomised trial. J. Physiother. 2016, 62, 88–95. [Google Scholar] [CrossRef]
- Musselman, K.E.; Shah, M.; Zariffa, J. Rehabilitation technologies and interventions for individuals with spinal cord injury: Translational potential of current trends. J. Neuroeng. Rehabil. 2018. [Google Scholar] [CrossRef]
- Ding, Y.; Kastin, A.J.; Pan, W. Neural plasticity after spinal cord injury. Curr. Pharm. Des. 2005, 11, 1441–1450. [Google Scholar] [CrossRef]
- Dunlop, S.A. Activity-dependent plasticity: Implications for recovery after spinal cord injury. Trends Neurosci. 2008. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.T. Robotic rehabilitation of spinal cord injury individual. Ortop. Traumatol. Rehabil. 2013. [Google Scholar] [CrossRef][Green Version]
- Kadivar, Z.; Sullivan, J.L.; Eng, D.P.; Pehlivan, A.U.; O′Malley, M.K.; Yozbatiran, N.; Francisco, G.E. Robotic training and kinematic analysis of arm and hand after incomplete spinal cord injury: A case study. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar] [CrossRef]
- Singh, H.; Unger, J.; Zariffa, J.; Pakosh, M.; Jaglal, S.; Craven, B.C.; Musselman, K.E. Robot-assisted upper extremity rehabilitation for cervical spinal cord injuries: A systematic scoping review. Disabil. Rehabil. Assist. Technol. 2018, 13, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Yozbatiran, N.; Francisco, G.E. Robot-assisted therapy for the upper limb after cervical spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2019, 30, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Tieri, G.; Morone, G.; Paolucci, S.; Iosa, M. Virtual reality in cognitive and motor rehabilitation: Facts, fiction and fallacies. Expert Rev. Med. Devices 2018, 15, 107–117. [Google Scholar] [CrossRef]
- Prasad, S.; Aikat, R.; Labani, S.; Khanna, N. Efficacy of virtual reality in upper limb rehabilitation in patients with spinal cord injury: A pilot randomized controlled trial. Asian Spine J. 2018, 12, 927–934. [Google Scholar] [CrossRef]
- Demain, S.; Metcalf, C.; Merrett, G.; Zheng, D.; Cunningham, S. A narrative review on haptic devices: Relating the physiology and psychophysical properties of the hand to devices for rehabilitation in central nervous system disorders. Disabil. Rehabil. Assist. Technol. 2012, 8. [Google Scholar] [CrossRef]
- Piggott, L.; Wagner, S.; Ziat, M. Haptic neurorehabilitation and virtual reality for upper limb paralysis: A review. Crit. Rev. Biomed. Eng. 2016, 44, 1–32. [Google Scholar] [CrossRef]
- Viau, A.; Feldman, A.G.; McFadyen, B.J.; Levin, M.F. Reaching in reality and virtual reality: A comparison of movement kinematics in healthy subjects and in adults with hemiparesis. J. Neuroeng. Rehabil. 2004, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Connelly, L.; Jia, Y.; Toro, M.L.; Stoykov, M.E.; Kenyon, R.V.; Kamper, D.G. A pneumatic glove and immersive virtual reality environment for hand rehabilitative training after stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 551–559. [Google Scholar] [CrossRef]
- Cappello, L.; Meyer, J.T.; Galloway, K.C.; Peisner, J.D.; Granberry, R.; Wagner, D.A.; Engelhardt, S.; Paganoni, S.; Walsh, C.J. Assisting hand function after spinal cord injury with a fabric-based soft robotic glove. J. Neuroeng. Rehabil. 2018, 15, 59. [Google Scholar] [CrossRef]
- Gutiérrez, Á.; Sepúlveda-Muñoz, D.; Gil-Agudo, Á.; de los Reyes Guzmán, A. Serious game platform with haptic feedback and emg monitoring for upper limb rehabilitation and smoothness quantification on spinal cord injury patients. Appl. Sci. 2020, 10, 963. [Google Scholar] [CrossRef]
- Yeh, S.-C.; Lee, S.-H.; Chan, R.-C.; Wu, Y.; Zheng, L.-R.; Flynn, S. The efficacy of a haptic-enhanced virtual reality system for precision grasp acquisition in stroke rehabilitation. J. Healthc. Eng. 2017, 2017, 9840273. [Google Scholar] [CrossRef] [PubMed]
- Yem, V.; Kajimoto, H. Wearable tactile device using mechanical and electrical stimulation for fingertip interaction with virtual world. In Proceedings of the 2017 IEEE Virtual Reality (VR), Los Angeles, CA, USA, 18–22 March 2017; pp. 99–104. [Google Scholar] [CrossRef]
- Pacchierotti, C.; Tirmizi, A.; Prattichizzo, D. Improving transparency in teleoperation by means of cutaneous tactile force feedback. ACM Trans. Appl. Percept. 2014, 11. [Google Scholar] [CrossRef]
- Chinello, F.; Malvezzi, M.; Pacchierotti, C.; Prattichizzo, D. Design and development of a 3RRS wearable fingertip cutaneous device. In Proceedings of the 2015 IEEE International Conference on Advanced Intelligent Mechatronics (AIM), Busan, Korea, 7–11 July 2015; pp. 293–298. [Google Scholar] [CrossRef]
- Pessina, L.-A. Artificial Skin Could Help Rehabilitation and Enhance Virtual Reality. 2019. Available online: https://actu.epfl.ch/news/artificial-skin-could-help-rehabilitation-and-enha/ (accessed on 9 March 2021).
- Meli, L.; Scheggi, S.; Pacchierotti, C.; Prattichizzo, D. Using the Leap Motion Controller for Hand Tracking and Wearable Haptic Devices for Contact Rendering. In Proceedings of the World Haptics, Chicago, IL, USA, 22–26 June 2015. [Google Scholar]
- Saggini, R.; Carmignano, S.M.; Palermo, T.; Bellomo, R.G. Mechanical Vibration in Rehabilitation: State of the Art. J. Nov. Physiother. 2016, 6, 314. [Google Scholar] [CrossRef]
- Sofronia, R.E.; Savii, G.; Davidescu, A. Haptic devices in engineering and medicine. In Proceedings of the 2010 International Joint Conference on Computational Cybernetics and Technical Informatics, Timisoara, Romania, 27–29 May 2010; pp. 373–378. [Google Scholar]
- Colgan, A. How Does the Leap Motion Controller Work? 2014. Available online: http://blog.leapmotion.com/hardware-to-software-how-does-the-leap-motion-controller-work/ (accessed on 9 March 2021).
- Saggini, R.; Bellomo, R.G.; Cosenza, L. Vibration in neurorehabilitation: A narrative review. Med. Res. Arch. 2017, 5, 1–10. [Google Scholar]



| Game | Exercise Type | Feedback | Therapeutic Objective |
|---|---|---|---|
| Maze | Path guidance | Vibration | Arm movement, accuracy |
| Path | Path guidance | Pressure | Arm movement, accuracy |
| Grab | Reaching objects, grasping and releasing | Pressure | Coordination, hand-grasp improvement |
| Patient | Age | Gender | Injury Level | AIS * |
|---|---|---|---|---|
| 1 | 40 | F | C4 | D |
| 2 | 33 | M | C7 | C |
| 3 | 43 | M | C8 | A |
| 4 | 40 | M | C5 | B |
| 5 | 43 | M | C6 | A |
| Healthy Subjects (n = 8) | SCI Patients (n = 5) | |||
|---|---|---|---|---|
| Average | Standard Deviation | Average | Standard Deviation | |
| Maze | ||||
| ROM X (mm) | 362.86 | 14.93 | 369.33 | 23.52 |
| ROM Y (mm) | 231.79 | 18.10 | 277.34 | 40.84 |
| Duration (s) | 36.62 | 9.44 | 49.50 | 14.47 |
| Points | 8.50 | 0.76 | 6.90 | 1.82 |
| Errors | 10.69 | 7.33 | 16.70 | 8.58 |
| Error no feedback | 12.12 | 7.61 | 22.80 | 12.93 |
| Error feedback | 9.25 | 7.09 | 10.60 | 5.64 |
| Path | ||||
| ROM X (mm) | 288.65 | 22.61 | 282.86 | 9.72 |
| ROM Y (mm) | 330.04 | 17.52 | 319.98 | 10.94 |
| Duration (s) | 38.62 | 11.39 | 54.40 | 6.57 |
| Points | 13.94 | 2.76 | 14.1 | 3.38 |
| Max value sent | 114.31 | 58.86 | 90.3 | 36.34 |
| Errors | 7.75 | 4.77 | 10.10 | 4.05 |
| E. NO feedback | 7.85 | 5.54 | 11.00 | 4.64 |
| E. feedback | 7.62 | 5.40 | 9.20 | 3.63 |
| Grab | ||||
| ROM (mm) | 95.92 | 12.10 | 106.80 | 13.08 |
| Points | 10.88 | 3.09 | 6.40 | 2.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, Á.; Farella, N.; Gil-Agudo, Á.; de los Reyes Guzmán, A. Virtual Reality Environment with Haptic Feedback Thimble for Post Spinal Cord Injury Upper-Limb Rehabilitation. Appl. Sci. 2021, 11, 2476. https://doi.org/10.3390/app11062476
Gutiérrez Á, Farella N, Gil-Agudo Á, de los Reyes Guzmán A. Virtual Reality Environment with Haptic Feedback Thimble for Post Spinal Cord Injury Upper-Limb Rehabilitation. Applied Sciences. 2021; 11(6):2476. https://doi.org/10.3390/app11062476
Chicago/Turabian StyleGutiérrez, Álvaro, Nicola Farella, Ángel Gil-Agudo, and Ana de los Reyes Guzmán. 2021. "Virtual Reality Environment with Haptic Feedback Thimble for Post Spinal Cord Injury Upper-Limb Rehabilitation" Applied Sciences 11, no. 6: 2476. https://doi.org/10.3390/app11062476
APA StyleGutiérrez, Á., Farella, N., Gil-Agudo, Á., & de los Reyes Guzmán, A. (2021). Virtual Reality Environment with Haptic Feedback Thimble for Post Spinal Cord Injury Upper-Limb Rehabilitation. Applied Sciences, 11(6), 2476. https://doi.org/10.3390/app11062476







