Synthesis and Physicochemical Properties of [(1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy]-thiophen-5-yl-substituted Tetrapyrazinoporphyrazine with Magnesium(II) Ion
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
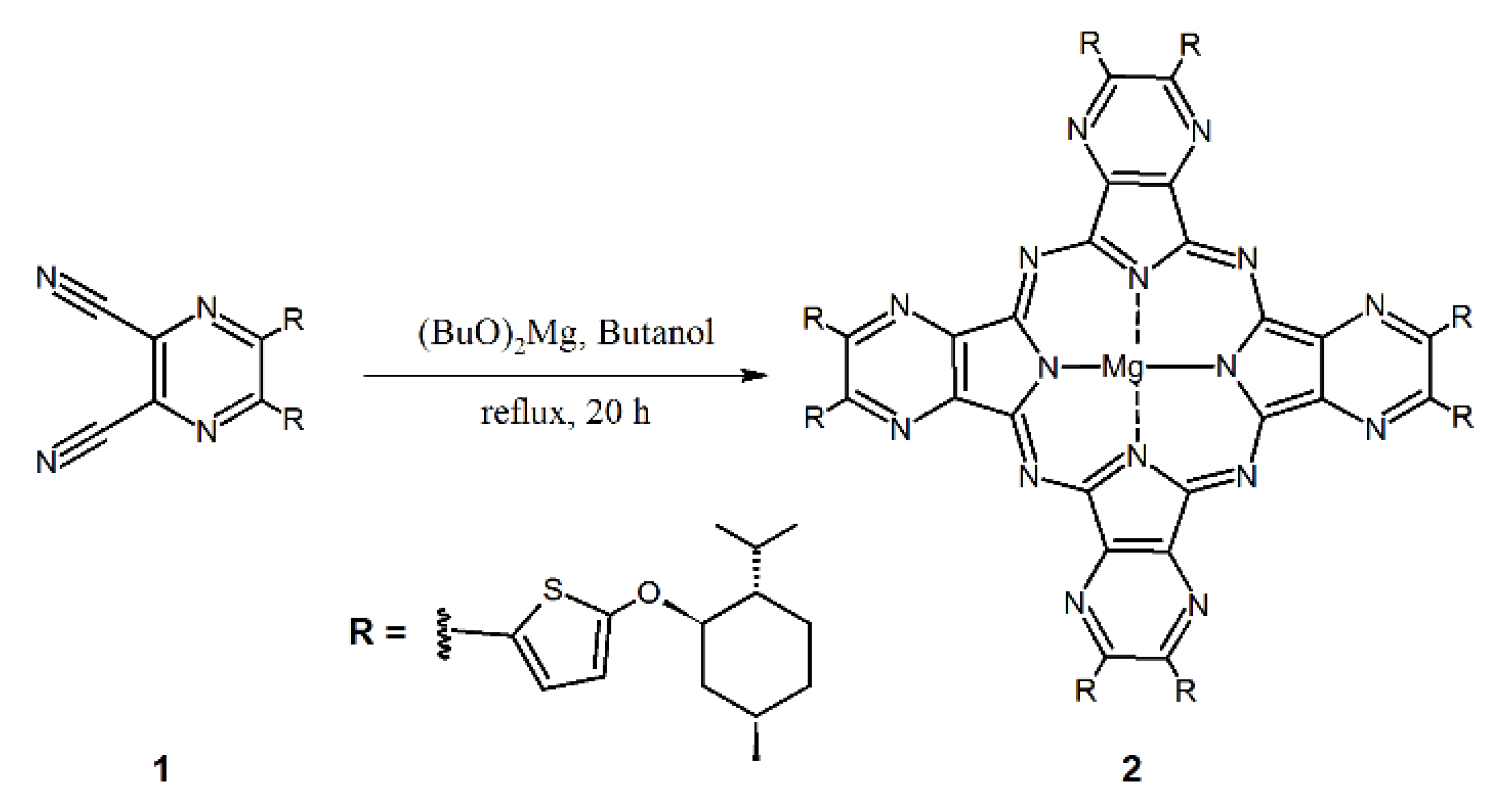
2.2. Synthetic Procedures
Magnesium(II) 2,3,9,10,16,17,23,24-octakis{2-[(1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy]-thiophen-5-yl}-1,4,8,11,15,18,22,25-(octaaza)phthalocyanine
2.3. Measurement of Singlet Oxygen Quantum Yield
2.4. Determination of Photostability Quantum Yield
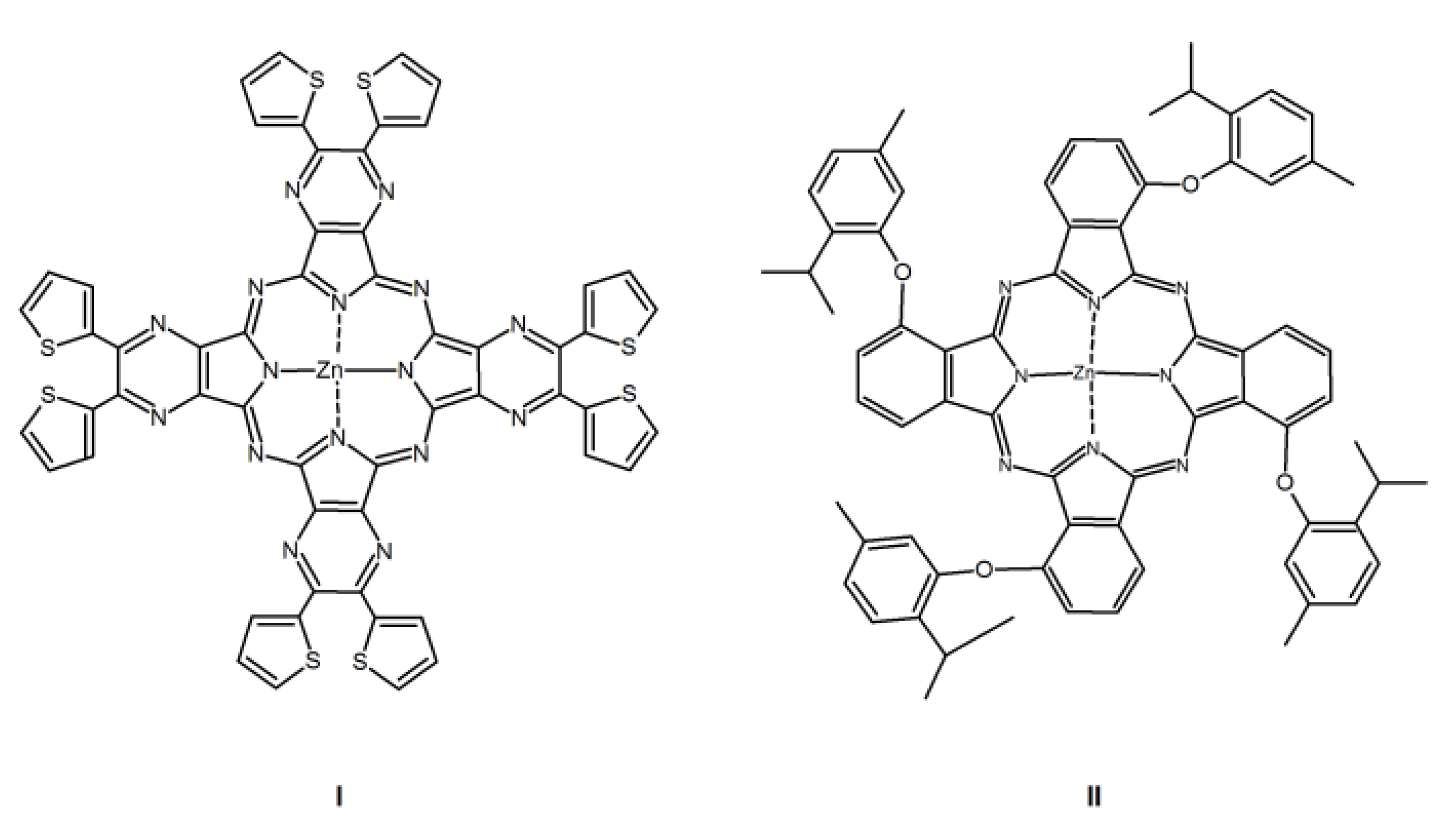
3. Results and Discussion
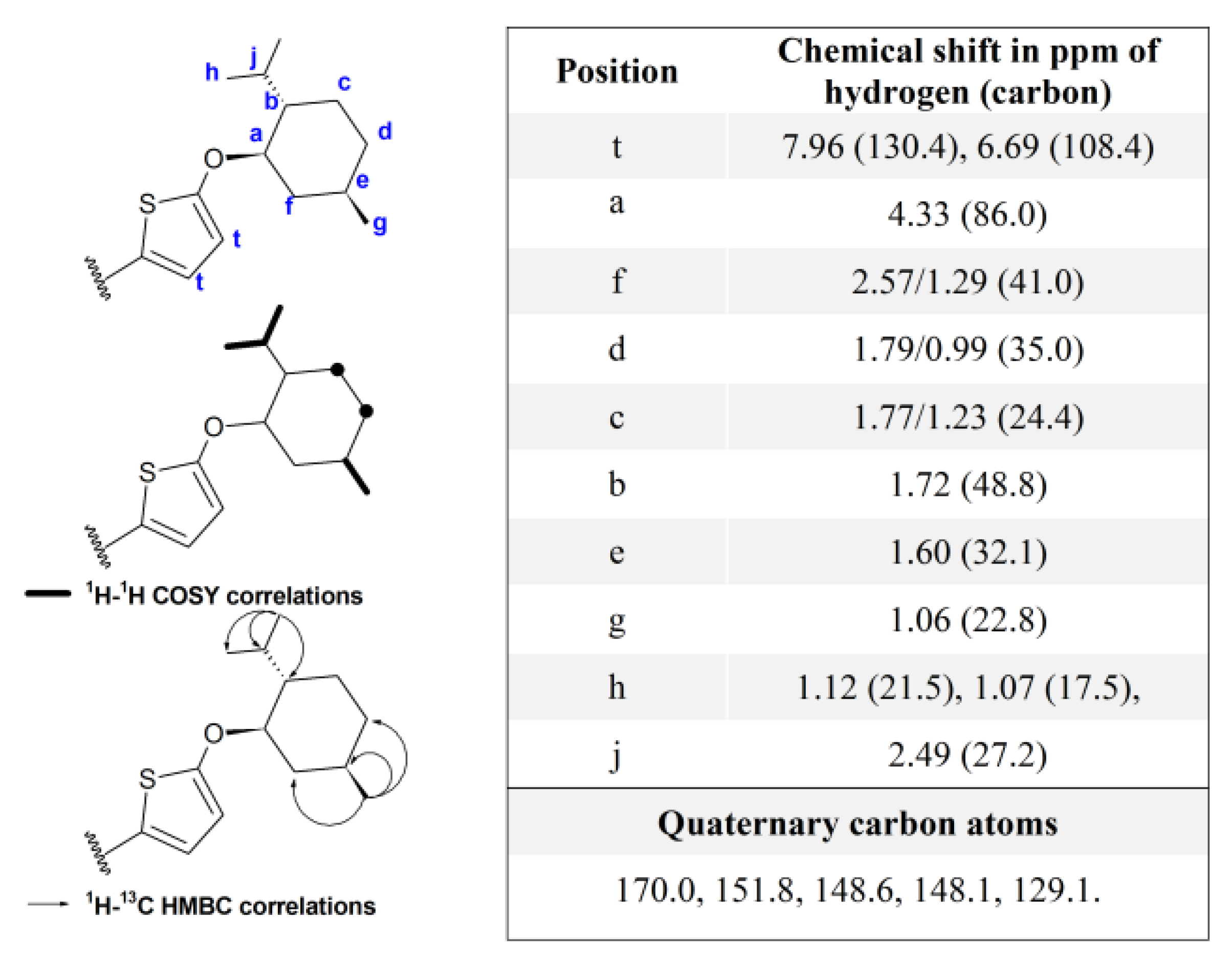
3.1. Synthesis and Characterization
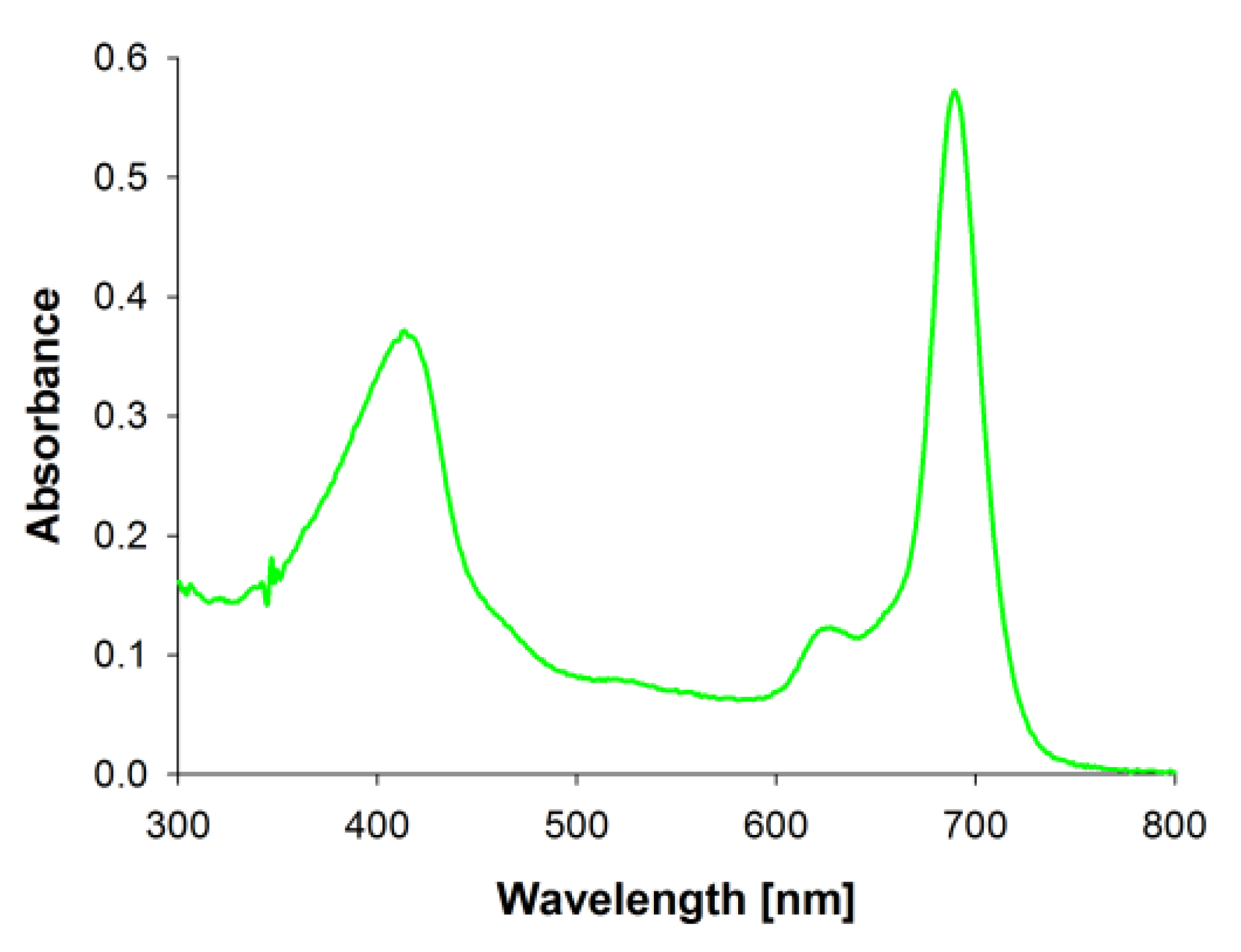
3.2. Photochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wöhrle, D.; Schnurpfeil, G.; Makarov, S.G.; Kazarin, A.; Suvorova, O.N. Practical Applications of Phthalocyanines–From Dyes and Pigments to Materials for Optical, Electronic and Photo-electronic Devices. Macroheterocycles 2012, 5, 191–202. [Google Scholar] [CrossRef]
- Huai, M.; Yin, Z.; Wei, F.; Wang, G.; Xiao, L.; Lu, J.; Zhuang, L. Electrochemical CO2 reduction on heterogeneous cobalt phthalocyanine catalysts with different carbon supports. Chem. Phys. Lett. 2020, 754, 7655. [Google Scholar] [CrossRef]
- Ma, D.D.; Han, S.G.; Cao, C.; Li, X.; Wu, X.T.; Zhu, Q.L. Remarkable electrocatalytic CO2 reduction with ultrahigh CO/H2 ratio over single-molecularly immobilized pyrrolidinonyl nickel phthalocyanine. Appl. Catal. B Environ. 2020, 264, 8530. [Google Scholar] [CrossRef]
- Mooste, M.; Tkesheliadze, T.; Kozlova, J.; Kikas, A.; Kisand, V.; Treshchalov, A.; Tamm, A.; Aruväli, J.; Zagal, J.H.; Kannan, A.M.; et al. Transition metal phthalocyanine-modified shungite-based cathode catalysts for alkaline membrane fuel cell. Int. J. Hydrogen Energy 2021, 46, 4365–4377. [Google Scholar] [CrossRef]
- Gounden, D.; Nombona, N.; Van Zyl, W.E. Recent advances in phthalocyanines for chemical sensor, non-linear optics (NLO) and energy storage applications. Coord. Chem. Rev. 2020, 420, 3359. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.I.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Sobotta, L.; Mrugalska, S.P.; Mielcarek, J.; Goslinski, T.; Balzarini, J. Photosensitizers Mediated Photodynamic Inactivation against Virus Particles. Mini-Rev. Med. Chem. 2015, 15, 503–521. [Google Scholar] [CrossRef]
- Sobotta, L.; Mrugalska, S.P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef] [PubMed]
- Donzello, M.P.; Ercolani, C.; Novakova, V.; Zimcik, P.; Stuzhin, P.A. Tetrapyrazinoporphyrazines and their metal derivatives. Part I: Synthesis and basic structural information. Coord. Chem. Rev. 2016, 309, 107–179. [Google Scholar] [CrossRef]
- Novakova, V.; Donzello, M.P.; Ercolani, C.; Zimcik, P.; Stuzhin, P.A. Tetrapyrazinoporphyrazines and their metal derivatives. Part II: Electronic structure, electrochemical, spectral, photophysical and other application related properties. Coord. Chem. Rev. 2018, 361, 1–73. [Google Scholar] [CrossRef]
- Donzello, M.P.; Viola, E.; Bergami, C.; Dini, D.; Ercolani, C.; Giustini, M.; Kadish, K.M.; Meneghetti, M.; Monacelli, F.; Rosa, A.; et al. Tetra-2,3-pyrazinoporphyrazines with Externally Appended Pyridine Rings. Chemical and Redox Properties and Highly Effective Photosensitizing Activity for Singlet Oxygen Production of Penta- and Monopalladated Complexes in Dimethylformamide Solution. Inorg. Chem. 2008, 47, 8757–8766. [Google Scholar] [CrossRef]
- Donzello, M.P.; Viola, E.; Cai, X.; Mannina, L.; Ercolani, C.; Kadish, K.M. Tetra-2,3-pyrazinoporphyrazines with Externally Appended Pyridine Rings. Central (ZnII, CuII, MgII(H2O), CdII) and Exocyclic (PdII) Metal Ion Binding in Heteropentametallic Complexes from Tetrakis-2,3-[5,6-di(2-pyridyl)pyrazino]porphyrazine. Inorg. Chem. 2010, 49, 2447–2456. [Google Scholar] [CrossRef]
- Park, J.M.; Song, C.J.; Yao, W.; Jung, C.Y.; Hyun, I.H.; Seong, D.H.; Jaung, J.Y. Synthesis of carbohydrate-conjugated azaphthalocyanine complexes for PDT. Tetrahedron Lett. 2015, 56, 4967–4970. [Google Scholar] [CrossRef]
- Tomachynskyi, S.; Korobko, S.; Tomachynski, L.; Pavlenko, V. Synthesis and spectral properties of new tetrakis-2,3-{5,7-bis[(E)-2-(4-methylphenyl)vinyl]pyrazino}porphyrazine metal complexes. Opt. Mater. 2011, 33, 1553–1556. [Google Scholar] [CrossRef]
- Lee, B.H.; Jaung, J.Y.; Jang, S.C.; Yi, S.C. Synthesis and optical properties of push–pull type tetrapyrazinoporphyrazines. Dye. Pigment. 2005, 65, 159–167. [Google Scholar] [CrossRef]
- Kopecky, K.; Šatínský, D.; Novakova, V.; Miletin, M.; Svoboda, A.; Zimcik, P. Synthesis of mono-, di-, tri- and tetracarboxy azaphthalocyanines as potential dark quenchers. Dye. Pigment. 2011, 91, 112–119. [Google Scholar] [CrossRef]
- Kostka, M.; Zimcik, P.; Miletin, M.; Klemera, P.; Kopecky, K.; Musil, Z. Comparison of aggregation properties and photodynamic activity of phthalocyanines and azaphthalocyanines. J. Photochem. Photobiol. A Chem. 2006, 178, 16–25. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Gao, R.; Zhang, R.; Zhao, J. Improved performance of Li/SOCl2 batteries using binuclear metal azaphthalocyanines as electrocatalysts. Electrochim. Acta 2016, 222, 203–211. [Google Scholar] [CrossRef]
- Alejandro, V.C.; Mónica, F.P.; Xelha, A.P.; Mario, R.; Gabriel, R.O.; Norberto, F.; Eva, R.-G. Brominated BODIPYs as potential photosensitizers for photodynamic therapy using a low irradiance excitation. Polyhedron 2020, 176, 8. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.A.; Yang, J.; Gai, L.; Tian, J.; Sui, X.; Lu, H. NIR halogenated thieno[3, 2-b]thiophene fused BODIPYs with photodynamic therapy properties in HeLa cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 9027. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, L.; Qian, Y. A near-infrared and lysosomal targeting thiophene-BODIPY photosensitizer: Synthesis and its imaging guided photodynamic therapy of cancer cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 9512. [Google Scholar] [CrossRef]
- Önal, H.T. Photo Induced Anti-Inflammatory Activities of a Thiophene Substituted Sub-phthalocyanine Derivative. Photodiagn. Photodyn. Ther. 2020, 30, 1701. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, X.; Li, Z.; Niu, L.; Yi, J.; Zhang, F. The third-order optical nonlinearities of thiophene-bearing phthalocyanines studied by Z-scan technique. J. Photochem. Photobiol. A Chem. 2011, 218, 64–68. [Google Scholar] [CrossRef]
- Sehlotho, N.; Nyokong, T. Electrocatalytic oxidation of thiocyanate, l-cysteine and 2-mercaptoethanol by self-assembled monolayer of cobalt tetraethoxy thiophene phthalocyanine. Electrochim. Acta 2006, 51, 4463–4470. [Google Scholar] [CrossRef]
- Donzello, M.P.; Ercolani, C.; Stuzhin, P.A. Novel families of phthalocyanine-like macrocycles—Porphyrazines with annulated strongly electron-withdrawing 1,2,5-thia/selenodiazole rings. Coord. Chem. Rev. 2006, 250, 1530–1561. [Google Scholar] [CrossRef]
- Luo, Q.; Cheng, S.; Tian, H. Synthesis and photochromism of a new binuclear porphyrazinato magnesium(II). Tetrahedron Lett. 2004, 45, 7737–7740. [Google Scholar] [CrossRef]
- Goslinski, T.; White, A.J. Synthesis, characterization and spectroscopic properties of novel periphery–Functionalized unsymmetrical porphyrazines containing mixed dithienylpyrrolyl and dimethylamino groups. Polyhedron 2009, 28, 2579–2584. [Google Scholar] [CrossRef]
- Mørkved, E.H.; Andreassen, T.; Novakova, V.; Zimcik, P. Zinc azaphthalocyanines with thiophen-2-yl, 5-methylthiophen-2-yl and pyridin-3-yl peripheral substituents: Additive substituent contributions to singlet oxygen production. Dye. Pigment. 2009, 82, 276–285. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Kasprzak, S.K.; Olender, D.; Zaprutko, L. Molecular Consortia—Various Structural and Synthetic Concepts for More Effective Therapeutics Synthesis. Int. J. Mol. Sci. 2018, 19, 1104. [Google Scholar] [CrossRef] [Green Version]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 3024. [Google Scholar] [CrossRef]
- Rizo, F.J.O.; Esnal, I.; Martínez, O.C.A.; Durán, G.C.F.A.; Banuelos, J.; Arbeloa, I.L.; Pannell, K.H.; Magaña, M.A.J.; Cabrera, P.E. 8-Alkoxy- and 8-Aryloxy-BODIPYs: Straightforward Fluorescent Tagging of Alcohols and Phenols. J. Org. Chem. 2013, 78, 5867–5877. [Google Scholar] [CrossRef]
- Lv, W.; Duan, J.; Ai, S. “Pinwheel-like” phthalocyanines with four non-peripheral chiral menthol units: Synthesis, spectroscopy, and electrochemistry. Chin. Chem. Lett. 2019, 30, 389–391. [Google Scholar] [CrossRef]
- Romero, M.P.; Gobo, N.R.; De Oliveira, K.T.; Iamamoto, Y.; Serra, O.A.; Louro, S.R. Photophysical properties and photodynamic activity of a novel menthol–zinc phthalocyanine conjugate incorporated in micelles. J. Photochem. Photobiol. A Chem. 2013, 253, 22–29. [Google Scholar] [CrossRef]
- De Oliveira, K.T.; De Assis, F.F.; Ribeiro, A.O.; Neri, C.R.; Fernandes, A.U.; Baptista, M.S.; Lopes, N.P.; Serra, O.A.; Iamamoto, Y. Synthesis of Phthalocyanines−ALA Conjugates: Water-Soluble Compounds with Low Aggregation. J. Org. Chem. 2009, 74, 7962–7965. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Lijewski, S.; Dlugaszewska, J.; Nowicka, J.; Mielcarek, J.; Goslinski, T. Photodynamic inactivation of Enterococcus faecalis by conjugates of zinc(II) phthalocyanines with thymol and carvacrol loaded into lipid vesicles. Inorg. Chim. Acta 2019, 489, 180–190. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; El Nebrisi, E.G.; Yang, K.H.S.; Howarth, F.C.; Al Kury, L.T. Cellular and Molecular Targets of Menthol Actions. Front. Pharmacol. 2017, 8, 472. [Google Scholar] [CrossRef] [Green Version]
- Pergolizzi, J.V.; Taylor, R.; LeQuang, J.A.; Raffa, R.B.; The NEMA Research Group. The role and mechanism of action of menthol in topical analgesic products. J. Clin. Pharm. Ther. 2018, 43, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Freires, I.A.; Denny, C.; Benso, B.; De Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Kifer, D.; Mužinić, V.; Klarić, M.Š. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 2016, 69, 689–696. [Google Scholar] [CrossRef]
- Hloušková, Z.; Tydlitát, J.; Kong, M.; Pytela, O.; Mikysek, T.; Klikar, M.; Almonasy, N.; Dvořák, M.; Jiang, Z.; Růžička, A.; et al. Structure-Catalytic Activity in a Series of Push-Pull Dicyanopyrazine/Dicyanoimidazole Photoredox Catalysts. Chemics 2018, 3, 4262–4270. [Google Scholar] [CrossRef]
- Sobotta, L.; Fita, P.; Szczolko, W.; Wrotynski, M.; Wierzchowski, M.; Goslinski, T.; Mielcarek, J. Functional singlet oxygen generators based on porphyrazines with peripheral 2,5-dimethylpyrrol-1-yl and dimethylamino groups. J. Photochem. Photobiol. A Chem. 2013, 269, 9–16. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Durmuş, M.; Atilla, D.; Gürek, A.G.; Ahsen, V.; Nyokong, T. Synthesis, photophysical and photochemical studies on long chain zinc phthalocyanine derivatives. Synth. Met. 2008, 158, 839–847. [Google Scholar] [CrossRef]
- Seotsanyana-Mokhosi, I.; Kuznetsova, N.A.; Nyokong, T. Photochemical studies of tetra-2,3-pyridinoporphyrazines. J. Photochem. Photobiol. A Chem. 2001, 140, 215–222. [Google Scholar] [CrossRef]
- Tillo, A.; Stolarska, M.; Kryjewski, M.; Popenda, L.; Sobotta, L.; Jurga, S.; Mielcarek, J.; Goslinski, T. Phthalocyanines with bulky substituents at non-peripheral positionsSynthesis and physico-chemical properties. Dye. Pigment. 2016, 127, 110–115. [Google Scholar] [CrossRef]
- Güzel, E.; Arslan, B.S.; Atmaca, G.Y.; Nebioğlu, M.; Erdoğmuş, A. High Photosensitized Singlet Oxygen Generating Zinc and Chloroindium Phthalocyanines Bearing (4-isopropylbenzyl)oxy Groups as Potential Agents for Photophysicochemical Applications. Chemics 2019, 4, 515–520. [Google Scholar] [CrossRef]
- Sobotta, L.; Sniechowska, J.; Ziental, D.; Dlugaszewska, J.; Potrzebowski, M.J. Chlorins with (trifluoromethyl)phenyl substituents–Synthesis, lipid formulation and photodynamic activity against bacteria. Dye. Pigment. 2019, 160, 292–300. [Google Scholar] [CrossRef]
- Sobotta, L.; Dlugaszewska, J.; Kasprzycki, P.; Lijewski, S.; Teubert, A.; Mielcarek, J.; Gdaniec, M.; Goslinski, T.; Fita, P.; Tykarska, E. In vitro photodynamic activity of lipid vesicles with zinc phthalocyanine derivative against Enterococcus faecalis. J. Photochem. Photobiol. B Biol. 2018, 183, 111–118. [Google Scholar] [CrossRef]
- Sobotta, L.; Dlugaszewska, J.; Gierszewski, M.; Tillo, A.; Sikorski, M.; Tykarska, E.; Mielcarek, J.; Goslinski, T. Photodynamic inactivation of Enterococcus faecalis by non-peripherally substituted magnesium phthalocyanines entrapped in lipid vesicles. J. Photochem. Photobiol. B Biol. 2018, 188, 100–106. [Google Scholar] [CrossRef]
- Härtner, J.; Reinscheid, U.M. Conformational analysis of menthol diastereomers by NMR and DFT computation. J. Mol. Struct. 2008, 872, 145–149. [Google Scholar] [CrossRef]
- Karplus, M. Vicinal Proton Coupling in Nuclear Magnetic Resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, J.; Lin, Y.; Chen, Z. Boosting resolution in NMR spectroscopy by chemical shift upscaling. Anal. Chim. Acta 2020, 1110, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K. Functional singlet oxygen generators based on phthalocyanines. Coord. Chem. Rev. 2012, 256, 1556–1568. [Google Scholar] [CrossRef]
- Stolarska, M.; Sobotta, G.A.; Mlynarczyk, D.T.; Dlugaszewska, J.; Goslinski, T.; Mielcarek, J.; Sobotta, L. Photodynamic Activity of Tribenzoporphyrazines with Bulky Periphery against Wound Bacteria. Int. J. Mol. Sci. 2020, 21, 6145. [Google Scholar] [CrossRef]
- Dilber, G.; Altunparmak, H.; Nas, A.; Kantekin, H.; Durmuş, M. The peripheral and non-peripheral 2H-benzotriazole substituted phthalocyanines: Synthesis, characterization, photophysical and photochemical studies of zinc derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.K.; Zheng, G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem. Rev. 2015, 115, 11012–11042. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Licsandru, D.; Boscencu, R.; Socoteanu, R.; Nacea, V.; Ferreira, L.F.V. A Singlet Oxygen Photogeneration and Luminescence Study of Unsymmetrically Substituted Mesoporphyrinic Compounds. Int. J. Photoenergy 2009, 2009, 1–10. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lijewski, S.; Tydlitát, J.; Czarczynska-Goslinska, B.; Klikar, M.; Mielcarek, J.; Goslinski, T.; Sobotta, L. Synthesis and Physicochemical Properties of [(1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy]-thiophen-5-yl-substituted Tetrapyrazinoporphyrazine with Magnesium(II) Ion. Appl. Sci. 2021, 11, 2576. https://doi.org/10.3390/app11062576
Lijewski S, Tydlitát J, Czarczynska-Goslinska B, Klikar M, Mielcarek J, Goslinski T, Sobotta L. Synthesis and Physicochemical Properties of [(1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy]-thiophen-5-yl-substituted Tetrapyrazinoporphyrazine with Magnesium(II) Ion. Applied Sciences. 2021; 11(6):2576. https://doi.org/10.3390/app11062576
Chicago/Turabian StyleLijewski, Sebastian, Jiří Tydlitát, Beata Czarczynska-Goslinska, Milan Klikar, Jadwiga Mielcarek, Tomasz Goslinski, and Lukasz Sobotta. 2021. "Synthesis and Physicochemical Properties of [(1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy]-thiophen-5-yl-substituted Tetrapyrazinoporphyrazine with Magnesium(II) Ion" Applied Sciences 11, no. 6: 2576. https://doi.org/10.3390/app11062576
APA StyleLijewski, S., Tydlitát, J., Czarczynska-Goslinska, B., Klikar, M., Mielcarek, J., Goslinski, T., & Sobotta, L. (2021). Synthesis and Physicochemical Properties of [(1R,2S,5R)-2-isopropyl-5-methylcyclohexyloxy]-thiophen-5-yl-substituted Tetrapyrazinoporphyrazine with Magnesium(II) Ion. Applied Sciences, 11(6), 2576. https://doi.org/10.3390/app11062576







