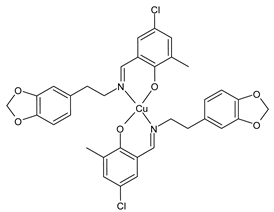
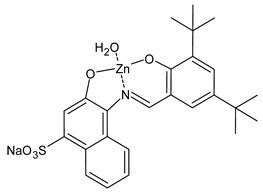
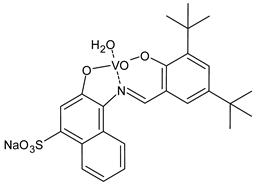
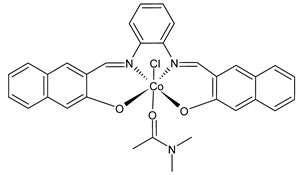
Abstract
Metal complexes play an essential role in pharmaceutical sciences for their multiple and important activities. Schiff bases are versatile pharmacophores able to form chelating complexes with several metals in different oxidation states. Complexes with Schiff bases are widely described in the literature for their multiple actions and numerous advantages, such as low cost and easy synthesis. They show multiple biological activities, including antimicrobial, antioxidant, antimalarial, antinflammatory and antitumor. Schiff bases may also form complexes with lanthanides and actinides acting as catalysts (e.g., in various synthetic processes) and antitumor agents. This review intends to extend on our previous paper regarding Schiff bases as antitumorals, highlighting the importance, in the field of the anticancer agents, of these tools as ligands of metal complexes.
1. Introduction
Schiff bases (R1R2C=NR3) are interesting organic compounds containing an azomethine (-CH=N-) or an imine (-C=N-) group generally formed by the condensation of active carbonyl groups and amino compounds, in which the nitrogen atom is bonded to an aryl or alkyl group [1]. These compounds form highly stable complexes with transition metal ions [2]. Metal complexes in which the metal is coordinated to different ligands, able to stabilize the metal and modify its chemical and pharmaceutical properties, have gained considerable importance in medicinal chemistry [3] as antitumor agents [4,5,6,7]. Complexes containing the transition metals platinum, gold and silver have attracted much attention due to their antitumor effects. Schiff bases, as well as diarylureas [8,9,10], have been defined as “privileged” ligands in organic synthesis, thanks to their affordability, easy synthesis and different biological activities and ability to form complexes with almost all metals [11,12,13]. Actually, they may form complexes with transition metals, platinum group metals (PGM) [14], lanthanides [15,16], and actinides [17,18] and many of them have been extensively described in the literature [19,20]. Metal complexes bearing in the structure a Schiff base are known for their numerous applications and biological activities including antimicrobial [21], antioxidant [22], antitumor [23], antinflammatory [24], and ureases inhibitors [25], and occupy a central role in the development of coordination chemistry [26,27,28]. Recently, the study of these compounds has attracted interest particularly for the development of new anticancer drugs. Among them, a pyrazolone Schiff base copper complex has been suggested as a potential drug candidate for the treatment of liver cancer, being endowed with high antiproliferative activity against hepatocellular carcinoma (HCC) [29]. Moreover, the use of low frequency electromagnetic fields and Mn(II) complex of a Schiff base derived from the pyridoxal has been described for the treatment of breast cancer [30]. Finally, a recent study reported that the introduction of Schiff bases in the N-phenylcarbazole/triphenylamine modified half-sandwiched iridium(III) compound led to an enhancement of the antitumor activity of about thirteen times that of cisplatin [31]. Interestingly, Schiff base-Fe2+ complexes have been incorporated into nanomicelles PAsc/Fe@Cy7QB, which represent chemodynamic theranostics able to specifically recognize and eradicate cancer cells in solid tumors [32]. Transition metal complexes with Schiff bases are also used as electrode modifiers in sensors to detect analytes of pharmaceutical, environmental and forensic interest, mainly to analyze illicit drugs [33]. Recently, the development of a coumarin Schiff base system has been described as a highly selective fluorescent/colorimetric probe for Cu2+ and as a tumor biomarker for glutathione detection [34]. Herein, we want to focus the attention on the antitumor activity of complexes with Schiff bases, reviewing firstly the data regarding the antiproliferative activities of Schiff base complexes with transition metals; then, we will dwell on platinum group metals (PGM), such as Pt(II) and Au(III). Finally, we will deepen complexed with lanthanides and discuss their antiproliferative activities [35,36]. Particularly, we want to highlight the importance of the antiproliferative activity of the metal complex-based Schiff bases, as a significant starting point for the development of new anticancer drugs, giving an update of recent reviews regarding this relevant topic in medicinal chemistry [37,38,39,40,41,42].
2. Antiproliferative Activity of Schiff Bases Complexed with Transition Metals
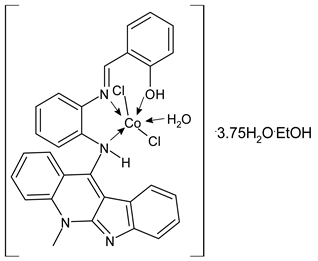
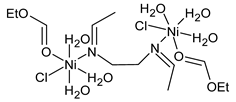
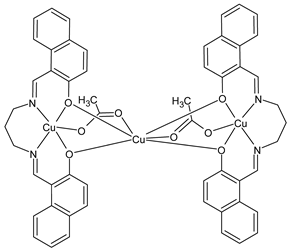
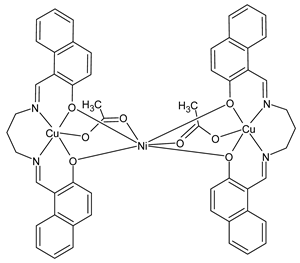
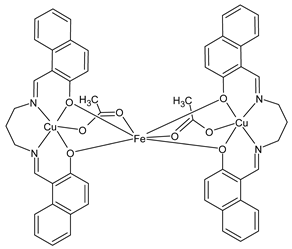
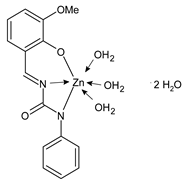
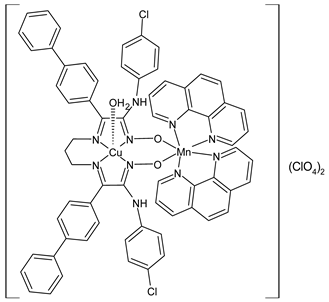
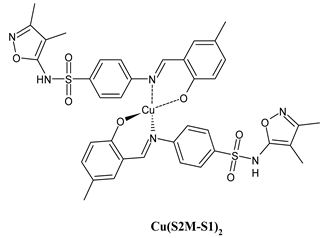
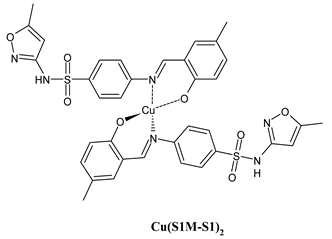
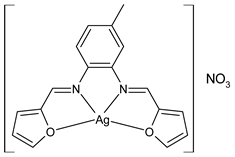
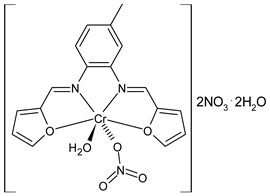
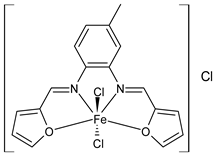
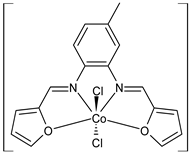
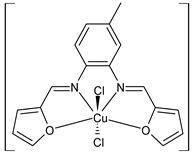
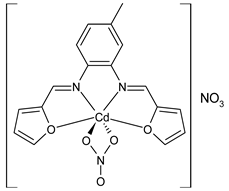
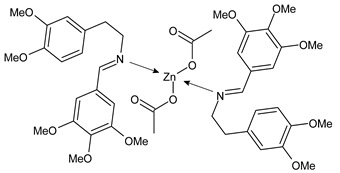
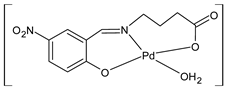
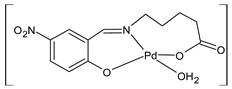
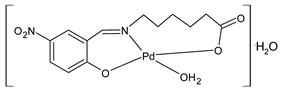
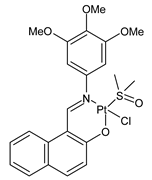
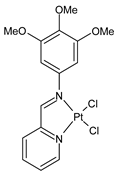
Complexes of Schiff bases with transition metals are summarized in Table 1. Several complexes of Schiff bases with Cu(II), Co(II) and Ni(II) were studied as antitumor agents against colorectal adenocarcinoma (HT29) cells by Emam et al. (2017) [43]. The Co(II) complex 1 showed higher anticancer activity than its corresponding ligand, particularly being able to inhibit cancer cells growth of about the 83.22% after 72 h, as determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Fetoh et al. (2019) [44] studied Schiff base complexes of Co(II) and Ni(II) ions with diethyl 3,3’-(ethane-1,2-diylbis(azaneylylidene))(3E,3′E)-dibutyrate (H2L1) and (3E,3′E)-3,3’-(ethane-1,2-diylbis (azanylylidene))bis(N-(pyridin-2-yl)butanamide) (H2L2) as ligands, and studied their antimicrobial and antiproliferative activity. [Ni2(L1)Cl2.6H2O].2H2O (2) and [Co2(H2L2)Cl4.2H2O].H2O (3) showed antiproliferative activity against the MCF-7 cell line (IC50 = 27.21 and 13.89 µM, respectively, versus IC50 = 4.17 µM of doxorubicin). Shi et al. (2020) [45] studied five transition metal complexes TM3L2(OAc)2 (TM = transition metal) with the N,N′-bis[(2-hydroxy-1-naphthalenyl)methylene]-propane-1,3-diamine, which is a bis-Schiff base, and determined the in vitro anticancer activities against seven human cancer cell lines (namely, gastric cancer MGC80-3, HeLa, human bladder T-24, HepG2, MDA, A549, human hepatocellular carcinoma Bel-7402 and human lung Wi-38 cancer cell lines), by the MTT assay. The complexes with Cu, Ni and Fe (4–6) showed higher antitumor activities than cisplatin and the highest inhibitory effects were found for complex 4 (IC50 lower than 0.5 μM for human bladder cancer cell line T-24) while showing nearly no toxicity to the normal cell HL-7702 as revealed by flow cytometry. The complex 4 induced tumor cell apoptosis by reducing the Bcl-2/Bax ratio, blocking the T-24 tumor cells at the G2/M phase, reducing the mitochondrial membrane potential, increasing the intracellular concentration of reactive oxygen species (ROS) and Ca2+, and changing protein expression. The apoptosis induced by complex 4 has been found to be triggered by Caspases 3/8 activation in T24 cells. Hassan et al. (2020) [46] synthesized the metal complexes of a tridentate Schiff base ligand (E)-1-(2-hydroxy-3-methoxybenzylidene)-3-phenylurea with Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and Zr(IV) (7–9 are shown in Table 1). The ligand and its metal complexes were screened for their cytotoxic activity against two cell lines, i.e., human colon carcinoma (HCT-116) and breast carcinoma cells (MCF-7), using cisplatin as standard drug and obtaining IC50 values of 7.22 μg/mL and 6.9 μg/mL against MCF-7 and HCT-116, respectively. All the complexes were more active than the free Schiff base ligand and, in particular, the higher cytotoxic activity was exerted by Cu, Co and Zn complexes (IC50 = 61.1, 90.4 and 61.3 µg/mL on MCF-7 cells, respectively; IC50 = 52.7, 60.6 and 51.9 µg/mL on HCT-116, respectively). Al-Serwi et al. (2020) [47] studied a Cu(II)–Mn(II) Schiff base tetradentate complex alone or in combination with cisplatin against squamous cell carcinoma cell line (SCC) in vitro, adopting the oral-derived gingival mesenchymal stem cells (GMSCs) as control. The IC50 values, obtained via MTT assay, were 1 µg/mL for cisplatin and 250 µM for the Schiff base complex [Cu(1-(biphenyl)-2-hydroxyimino-2-(4-chloroanilino)-1-ethanone)(H2O)Mn(phen)2](ClO4)2 (10) on both the cell lines. The treatment with the Schiff base complex led to an up-regulation of p53 and Bax gene expression and a down-regulation of Bcl2 gene expression in the SCC cell line, concomitantly with the increase of caspase-3 and Bax proteins expression and down-regulation of Bcl-2 protein. A higher apoptotic effect induced by a Schiff base complex was shown by annexin V-FITC apoptosis kit compared to the cisplatin-treated group. These effects were markedly enhanced on the combination of Schiff base and cisplatin. Alyar et al. (2021) [48] reported the synthesis and biological evaluation of new Schiff bases incorporating sulfisoxazole and sulfamethoxazole and their Cu(II) complexes. The anticancer activity was evaluated against three human cancer cell lines with the sulfonamide B test. Complexes of ligand S1M-S1 with Cu(II) named Cu(S2M-S1)2 (11) and Cu(S1M-S1)2 (12) (Table 1) exhibited promising cytotoxic activity against breast (MCF7) cells (IC50 = 40 μM versus 5-fluorouracil, 5-FU, IC50 = 0.10 μM). Moreover, docking studies were carried out on these compounds, suggesting that hydrogen bonds seem to play a pivotal role in the reversible interaction of small molecules and proteins. In this case, the oxygen and nitrogen atoms on the sulfisoxazole ring, the oxygen atom of sulfonyl groups and oxygen and nitrogen atoms attached to the benzene ring are thought to be involved in the hydrogen bonds’ formation. Ismail et al. (2021) [49] studied several complexes in which the N1,N2-bis(furan-2-ylmethylene)-4-methylbenzene-1,2-diamine (L) is complexed with several metals, namely Ag(I), Cr(III), Fe(III), Co(II), Cu(II) and Cd(II) (13–18), and studied their cytotoxicity against the HepG2 cell lines. The IC50 values were 12.9 μg/mL, 14.8 μg/mL, 7.31 μg/mL, 8.53 μg/mL, 17.1 μg/mL and 1.95 μg/mL, respectively. The cadmium(II) complex showed the highest cytotoxic activity, being more potent than vinblastine used as standard drug (IC50 = 2.93 μg/mL). Its activity was also higher with respect to that of the free ligand, suggesting an important role of the metal in the biological activity. Wongsuwan et al. (2021) [50] studied the in vitro anticancer activity of the Schiff base ligands (N-(8-quinolyl)-X-salicylaldimine) and their complexes with Fe(II) and Fe(III) against the A549 human lung adenocarcinoma cell line. The Fe(III) complex (compound 19, Table 1) showed an anticancer activity against the A549 cells (IC50 = 10 µM) higher than two well-known anticancer agents, i.e., etoposide and cisplatin (IC50 = 19 and 16 µM, respectively). Naureen et al. (2021) [51] analyzed two Schiff base ligands (L1, L2) and their complexes with Fe(III) and Zn(II) (20,21), studying their antitumor activity by means of potato disc tumor induction assay utilizing the Agrobacterium tumefaciens strains. In this assay, the complexes of the two Schiff bases with Zn showed a better activity than the corresponding Fe compounds: ((L1)2Zn(Ac)2 IC50 = 6.81 µg/mL and (L2)2Zn(Ac)2 IC50 = 7.58 µg/mL versus (L1)2FeCl3 IC50 = 13.91 µg/mL and (L2)2FeCl3 IC50 = 70.89 µg/mL). Vincristine was used as positive control with an IC50 value of 250 µg/mL. Additionally, the safety of these compounds was also evaluated, using the brine shrimp microwell cytotoxicity assay. The obtained results confirmed that Zn complexes showed a higher cytotoxicity than the corresponding Fe ones (IC50 > 134 µg/mL); moreover, in both the assays the ligands were more pharmacologically active, as well. Alkiş et al. (2021) [52] studied a Schiff base ligand and its two M(II) complexes formed with Co(II) and Ru(II). The former, [CoCl·L(H2O)2]·2H2O (22), is reported in Table 1 whereas the latter will be described below in Table 2. The compounds were studied for their in vitro anticancer activities against the human colon cancer cell line (Caco-2) by using the MTT assay and investigating the biocompatibility characteristics in the normal fibroblast cells line (L-929). Moreover, the effectiveness of electrochemotherapy (ECT) on cytotoxic activities of the compounds in Caco-2 cancer cell line was examined. Both the complexes showed interesting anticancer properties in the Caco-2 cells (IC50 = 610 µM for Co complex) while showing a low cytotoxic effect on L-929. Moreover, the authors demonstrated that the combined application of electroporation (EP)+complexes was much more effective than the application of complexes alone in the treatment of Caco-2 colon cancer cells. The Co(II) complex increased its cytotoxicity levels by 2.07 times in its combined applications with EP. Chen et al. (2021) [53] studied Zn(II) and Cu(II) complexes of three Schiff base ligands [Zn(La)2] (23), [Zn(Lb)2] (24), and [Cu(Lc)2] (25), as antiproliferative agents against the tumor cell lines T-24, HepG2 and SK-OV-3. The complexes 23–25 exhibited diverse cytotoxicity against the three cell lines, with IC50 values ranging between 9.00 and 46.72 µM versus 6.828‒7.719 µM of cisplatin, and they were not cytotoxic on normal liver cell line HL-7702. DNA binding studies supported by spectral analysis, DNA viscosity measurement and gel electrophoresis suggested that complexes 23–25 interacted with DNA mainly via weak intercalative binding interactions. The complex 24 was found to be able to arrest the HepG2 cells cycle at the S phase and induce apoptosis through the mitochondria-related apoptotic pathway. Abu-Dief et al. (2021) [54] studied two complexes in which the 1-((3,5-di-tert-butyl-2-hydroxybenzylidene)amino)naphthalen-2-ol-5-sodium sulfonate (DSHN) is complexed with Zn(II) and VO(II). The two complexes, DSHNZn (26) and DSHNVO (27), showed high antiproliferative activity against HCT-116, MCF-7, and HepG2 cancer cell lines (IC50 = 30.20, 13.90 and 21.10 µM for DSHNZn, respectively, and IC50 = 31.90, 15.30 and 22.80 µM for DSHNVO, respectively, versus IC50 = 13.30, 4.12 and 7.50 µM of vinblastine). The binding nature of the two complexes with calf thymus DNA (CTDNA) was also examined, indicating that their binding occurs just through the intercalation or replacement mode. Liao et al. (2021) [55] studied a Schiff base Co(III) complex [N,N’-bis(20-hydroxyphenylacetone)-o-ethanediamine]cobalt(III) (28). The cytotoxicity was evaluated against HeLa, human colon cancer cells (LoVo), A549 and the cisplatin-resistant cell line (A549/cis) by MTT assays using cisplatin as a positive reference. The cytotoxicity against the normal cell lines LO2 was also evaluated. Complex M3 effectively inhibited the growth of all the tested cancer cells in a dose-dependent manner during a 48 h treatment. The IC50 against HeLa was close to that of cisplatin (IC50 = 12.40 µM versus 9.74 <µM). The activity of the complex against the cisplatin-resistant cell line A549/cis was higher than the reference (IC50 = 18.03 µM versus 44.79 µM). Moreover, the complex 28 seems to be less toxic than cisplatin (IC50 = 6.27 µM versus 2.61 µM) and with a better selectivity index (SI). Furthermore, anticancer mechanistic studies showed that the complex 28 inhibited cell proliferation by blocking DNA synthesis and acting on nuclear division of HeLa cells over time.

Table 1.
Antiproliferative activity of Schiff base complexes with transition metals.

Table 2.
Antiproliferative activity of Schiff base complexes with platinum group metals (PGM).
3. Antiproliferative Activity of Schiff Bases Complexed with Platinum Group Metals (PGM)
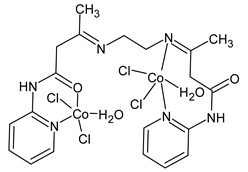
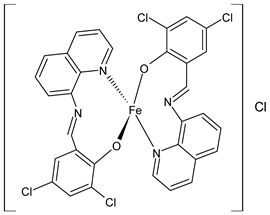
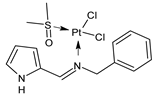
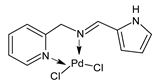
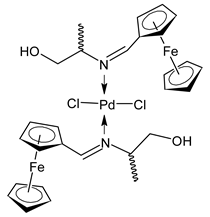
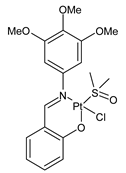
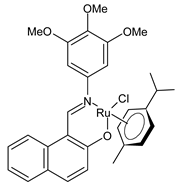
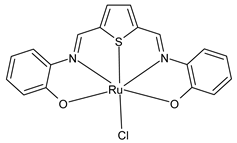
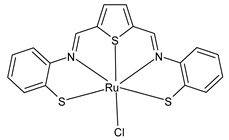
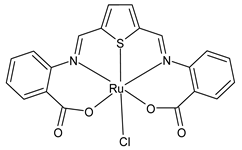
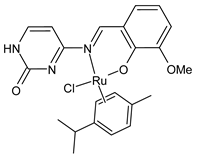
Platinum group metals are represented by Ru, Rh, Pd, Os, Ir and Pt and can be coordinated by Schiff base ligands as shown in Table 2. Mbugua et al. (2017) [56] reported a study on palladium(II) and platinum(II) complexes based on pyrrole Schiff bases and their anticancer activity against various human cancerous (Caco-2, HeLa, HepG2, MCF-7, and PC-3) and noncancerous (MCF-12A) cell lines using MTT and apoptosis assays, respectively. The 29 complex showed an enhanced and selective cytotoxicity particularly against the HepG2 (IC50 = 0.3 µg/mL) and Caco-2 (IC50 = 16.63 µg/mL) cells. It reduced the viability of the five cancerous cell lines, which included one breast cancer cell line, by more than 60%. The 30 complex reduced the cell viability, in all types, by more than 80%. The IC50 values were 13.0 and 15.81 µg/mL against HepG2 and Caco-2 cells, respectively. Moreover, it also demonstrated a strong intercalation binding affinity for the CTDNA (binding constant Kb = 8.049 × 104 M−1). Özdemir et al. (2020) [57] synthesized a series of palladium(II) complexes of N-(5-nitro-salicylidene)-Schiff bases (31–33, [Pd(L)(H2O)].xH2O) and studied the in vitro cytotoxicity against tumor cell lines HeLa and MCF-7, and the normal human cell line HEK-293. The complexes 31 and 32 showed a moderate antitumor activity against HeLa cell lines, while 33 was the most active, showing higher activity than the standard anticancer drug, doxorubicin. All three complexes were more active than doxorubicin against MCF-7 cancer lines, given that their killing percentage was found to be 100% at concentration of 1 µM. The complexes displayed an antiproliferative effect similar to that of doxorubicin at 1 µM dosage on HEK-293 cell lines and their cytotoxicity decreased with a decrease in the concentration. All the Pd(II) complexes caused a very noticeable modification in the morphology of MCF-7 cancer cells; indeed, the membrane blebbing induced by the apoptotic cell-death was present in cells treated with the complexes and the percentage of the blebbing cells reached to the maximum for all the complexes. Zhang et al. (2021) [58] studied a Schiff base ferrocene-palladium(II) complex (34) and studied its antiproliferative activity on human liver cancer cells (Sk-Hep-1 e MHCC97-L), human breast cancer cells (MDA-MB-231), human non-small cell lung cancer cells (A549), human non-small cell lung cancer cells resistant to paclitaxel (A549/TAXOL), and mouse embryonic fibroblasts (NIH3T3). It suppressed the viability of different tumor cell lines excellently with a potency up to 20-fold better than cisplatin as well as possessing lower toxicity in normal cells. The highest activity was observed against Sk-Hep-1 (IC50 = 0.88 μM versus 18.1 μM of cisplatin), MHCC97-L (IC50 = 8.28 μM versus 38.7 μM of cisplatin) and NIH3T3 (IC50 = 8.35 μM versus > 100 μM of cisplatin). The mechanism underlying the antiproliferative activities was suggested to proceed through the induction of the caspase 3-dependent apoptosis in vitro. Additionally, the cellular target of compound 34 was predicted by molecular docking studies, suggesting that it possesses a high affinity to specifically bind the BIR3 domain of XIAP. Acharya et al. (2021) [59] studied Pt- and Ru-based complexes, which are two of the most successful chemotherapeutic agents having a significant role in cancer chemotherapy despite their side effects. Pt(II) and Ru(II) complexes (35–38) containing Schiff bases displayed an in vitro antiproliferative activity against different aggressive cancer cells, viz., human pancreatic carcinoma (MIAPaCa-2), hepatocellular carcinoma (HepG2), triple-negative human metastatic breast adenocarcinoma (MDA-MB-231), human embryonic kidney (HEK-293), and human foreskin fibroblast (HFF-1) compared to the clinical drug oxaliplatin, used as reference (IC50 = 5.7, 9.8, 19.2, 2.1 and 7.0 µM, respectively). The most active complex (37) is 10–15 times more toxic than oxaliplatin against the MDA-MB-231 cells. Complexes 35–37 initiate the disruption of the microtubule network in MDA-MB-231 cells in a dose-dependent manner within 6 h of incubation, thus affecting the cytoskeleton function, by inhibiting tyrosine phosphorylation of vascular endothelial growth factor receptor 2 (VEGFR2), a key step in angiogenesis. This finally leads to the arrest of the cell cycle in the G2/M phase and apoptosis. Moubeen et al. (2021) [60] studied mononuclear octahedral Ru(III) complexes 39–41 with Schiff bases. The antiproliferative activities of the complexes on human cervical cancer cells (HeLa) and human breast cancer cells (MCF-7) were evaluated by MTT assay. All the complexes showed higher growth inhibition against the HeLa and MCF-7 cell lines, Ru(III) complex (41) being the most active, showing an IC50 against HeLa cells very close to that of cisplatin. Studies of the interaction with the CTDNA supported that these complexes bind DNA as demonstrated by gel electrophoresis studies. Interestingly, it was found that all the Ru(III) complexes cleave the super coiled (SC) pUC19 plasmid DNA efficiently. The study of Alkiş et al. (2021) [52] has been already described above with regard to the Co(II) complex. The other complex, with Ru(II) [RuCl(p-cymene)L] (42), showed an in vitro higher activity than the Co(II) analogue complex (22), showing an IC50 value of 510 µM against the human colon cancer cell line (Caco-2) and low cytotoxic effects on L-929, as determined by MTT assay. Furthermore, as well this time, Ru(II) complexes increased the cytotoxicity levels by 2.12 times in its combined applications with EP.
4. Antiproliferative Activity of Schiff Base Complexed with Lanthanides
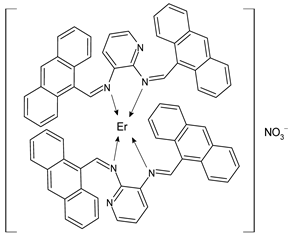
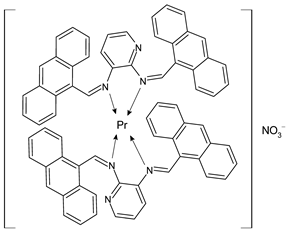
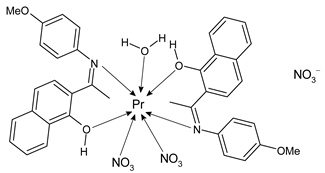
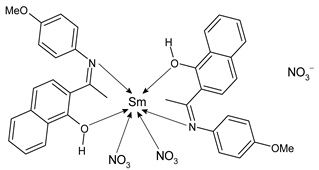
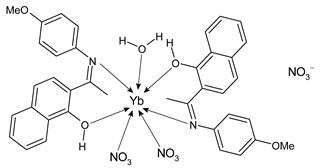
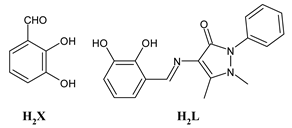
Lanthanide-based complexes with Schiff bases are depicted in Table 3. Andiappan et al. (2018) [61] studied a series of lanthanides (i.e., praseodymium (Pr), erbium (Er), and ytterbium (Yb)) based with a Schiff base ligand (SBL), the N2,N3-bis(anthracen-9-ylmethylene) pyridine-2,3-diamine and studied their cytotoxicity activity against Vero, human breast cancer (MCF7), and cervical (HeLa) anticancer cell lines by MTT assay. The SBLPr (43) and SBLEr (44) complexes exhibited anticancer activity against the above-mentioned cancer cell lines, reducing their viability by about 50% at a concentration of 25 µg/mL). Both the complexes were shown to induce the apoptosis of MCF7 and HeLa cells as observed by the acridine orange (AO)/ethidium bromide (EB) staining assay. a further confirmation was obtained by the propidium iodide (PI) staining assay that showed that both the complexes induced DNA fragmentation. Sathiyanarayanan et al. (2020) [62] described a series of lanthanum(III) complexes with a Schiff base, particularly praseodymium (Pr), samarium (Sm), and ytterbium (Yb) (45, 46 and 47, respectively). In vitro antiproliferative activity studies by MTT assay showed that the compounds were active against HeLa tumor cells, the compound 46 being the most active with an IC50 equal to 34 µg/mL. Hoechst 33,258 staining and AO/EB dual staining suggested that the cells underwent the apoptosis mechanism in a dose-dependent manner and the flow cytometric analysis evidenced a block at the G1 phase, where there was no cell replication. Moreover, the docking studies suggested that the anti-cancer effect of complex 46 may occur via the caspase 3-specific substrate activation (polyADP-ribose polymerase, PARP, cleavage). The complex 46 binds DNA via intercalation producing an arrest at the S-phase of HeLa cells. Hua et al. (2021) [63] studied a hexanuclear Nd(III) complex {[Nd6(HL)2L2×2(NO3)8(EtOH)6](EtOH)(H2O)3} (48) (given the complexity of the structure it has not been reported herein and only the H2L and H2X ligands are depicted in Table 3) and studied its antitumor activity against the human hepatocellular carcinoma SMMC-7721 cells by MTT assay. It showed a higher activity than the reference compound (IC50 = 4.42 µM versus 10.11 µM of gemcitabine).

Table 3.
Antiproliferative activity of lanthanide-based complexes with Schiff bases.
5. Summary
The complexes of Schiff base are attracting much attention for their biological activities, including antibacterial, antifungal, anti-inflammatory, antioxidant and anticancer. The main aim of this review was to explore and summarize the available recent research studies regarding the Schiff bases’ metal complexes as antitumorals. We considered complexes with transition metals, with PGM and lanthanides, focusing on the latest studies conduced by different authors in recent years. Some of them showed interesting antiproliferative activity against a large panel of cancer cell lines (MCF7, HeLa, HepG2, HCT-116, and so on) even higher than the adopted reference drugs. Most of their anticancer activities have been investigated using well-known and validated viability assays, revealing IC50 values equal or even lower than the clinical used drugs, used as references, principally the cisplatin. In many cases, a better cytotoxic profile has been recorded, the viability of the normal cells being not or little affected. In all cases, the authors prove that these complexes are able to induce cancer cell death by apoptosis, regulating the principal actors playing a specific role in initiating/sustaining the apoptotic mechanism. Moreover, in the majority of cases DNA represents the main target, as demonstrated by in vitro studies using the CTDNA as simple, but effective, assay to determine DNA intercalation properties. Some of these studies have been supported by in silico proofs, in which the ability of the specific groups of these complexes to bind DNA has been supposed. Finally, interesting evidence about a role in regulating the cell cytoskeleton targeting the actin have been reported. This makes us confident that many Schiff bases complexed with metals could represent promising molecules to develop new and suitable therapies for the treatment of numerous kinds of cancers, some of which with high incidence rates.
Author Contributions
Conceptualization, M.S.S. and A.C.; writing—original draft preparation, A.C. and C.S.; studies on complexes with transition metals, A.M.; studies on complexes with PGM, J.C.; studies on complexes with lanthanides, E.S.; writing—review and editing, D.I. and C.R.; supervision, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
The work of C.R. has been partially supported by a grant from the Italian Ministry of Health (Ricerca Corrente).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
A549 = human non-small cell lung cancer cells; A549/cis = cisplatin-resistance cell line; A549/TAXOL = human non-small cell lung cancer cells resistant to paclitaxel); AO = acridine orange; Bel-7402 = human hepatocellular carcinoma cells; Caco-2 = human colon cancer cell line; CTDNA = calf thymus DNA; EB = ethidium bromide; ECT = electrochemotherapy; EP = electroporation; 5-FU = 5-fluorouracil; GMSCs = gingival mesenchymal stem cells; HCC = hepatocellular carcinoma; HCT-116 = human colon cancer cells lines; HeLa = human cervical cancer cells; HepG2 = human hepatoma cell lines; HL-7702 = human normal liver cell lines; IC50 = concentration which kills or inhibits cell viability by 50%; L-929 = normal fibroblast cells lines; LoVo = human colon cancer cells; MCF-7 = human breast adenocarcinoma cell lines; MCF-12A = non-tumorigenic breast epithelial cells; MDA-MB-231 = human breast cancer cells; MGC80-3 = gastric cancer cell lines; MHCC97-L = human liver cancer cells; MIAPaCa-2 = pancreatic carcinoma cell lines; NIH3T3 = mouse embryonic fibroblasts; PARP = polyADP-ribose polymerase; PC-3 = prostate cancer cells; PI = propidium iodide; SC = super coiled; SCC = oral squamous cell carcinoma cell lines; Sk-Hep-1 = human liver cancer cells; SMMC-7721 = human hepatocellular carcinoma cell lines; T-24 = human bladder cancer cell line; TM = transition metal; VEGFR2 = vascular endothelial growth factor receptor 2; Wi-38 = human lung cell lines.
References
- Schiff, H. Mittheilungen aus dem universitätslaboratorium in Pisa: Eine neue reihe organischer basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.; Dey, S.K.; Ara, M.H.; Karim, K.; Islam, A.B.M. A review on synthesis and versatile applications of some selected Schiff bases with their transition metal complexes. Egypt. J. Chem. 2020, 63, 5–6. [Google Scholar] [CrossRef]
- Hameed, A.; al-Rashida, M.; Uroos, M.; Ali, S.A.; Khan, K.M. Schiff bases in medicinal chemistry: A patent review (2010–2015). Expert Opin. Ther. Pat. 2017, 27, 63–79. [Google Scholar] [CrossRef]
- Chimento, A.; Saturnino, C.; Iacopetta, D.; Mazzotta, R.; Caruso, A.; Plutino, M.R.; Mariconda, A.; Ramunno, A.; Sinicropi, M.S.; Pezzi, V.; et al. Inhibition of human topoisomerase I and II and anti-proliferative effects on MCF-7 cells by new titanocene complexes. Bioorg. Med. Chem. 2015, 23, 7302–7312. [Google Scholar] [CrossRef]
- Sirignano, E.; Saturnino, C.; Botta, A.; Sinicropi, M.S.; Caruso, A.; Pisano, A.; Lappano, R.; Maggiolini, M.; Longo, P. Synthesis, characterization and cytotoxic activity on breast cancer cells of new half-titanocene derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 3458–3462. [Google Scholar] [CrossRef]
- Ceramella, J.; Mariconda, A.; Iacopetta, D.; Saturnino, C.; Barbarossa, A.; Caruso, A.; Rosano, C.; Sinicropi, M.S.; Longo, P. From coins to cancer therapy: Gold, silver and copper complexes targeting human topoisomerases. Bioorg Med. Chem. Lett. 2020, 30, 126905. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Rosano, C.; Sirignano, M.; Mariconda, A.; Ceramella, J.; Ponassi, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Is the way to fight cancer paved with gold? metal-based carbene complexes with multiple and fascinating biological features. Pharmaceuticals 2020, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as antitumor agents. Appl. Sci. 2021, 11, 374. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Pellegrino, M.; Aquaro, S.; Franchini, C.; Sinicropi, M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff bases: A versatile pharmacophore. J. Catal. 2013. [Google Scholar] [CrossRef] [Green Version]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Bonomo, M.G.; Franchini, C.; Sinicropi, M.S. Schiff bases: Interesting scaffolds with promising antitumoral properties. Appl. Sci. 2021, 11, 1877. [Google Scholar] [CrossRef]
- Kaya, S.; Erkan, S.; Karakaş, D. Computational investigation of molecular structures, spectroscopic properties and antitumor-antibacterial activities of some Schiff bases. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 244, 118829. [Google Scholar] [CrossRef]
- Kordestani, N.; Rudbari, H.A.; Bruno, G.; Rosario, S.; Braun, J.D.; Herbert, D.E.; Blacque, O.; Correia, I.; Zaman, M.A.; Bindu, M.M.; et al. Solid-state to solution helicity inversion of pseudotetrahedral chiral copper (II) complexes with 2, 4-dihalo-salicylaldiminate ligands. Dalton Transact. 2020, 49, 8247–8264. [Google Scholar]
- Miroslaw, B. Homo-and Hetero-Oligonuclear Complexes of Platinum Group Metals (PGM) Coordinated by Imine Schiff Base Ligands. Int. J. Mol. Sci. 2020, 21, 3493. [Google Scholar] [CrossRef]
- Pilichos, E.; Font-Bardia, M.; Escuer, A.; Mayans, J. Structural and magnetic studies of mononuclear lanthanide complexes derived from N-rich chiral Schiff bases. Dalton Transact. 2021, 50, 1746–1753. [Google Scholar] [CrossRef]
- Paswan, S.; Jaiswal, N.; Modanawal, V.K.; Patel, M.K.; Singh, R.K.P. An experimental and theoretical investigation of lanthanide complexes [Ln = Nd, Yb, Eu, Dy and tb] with 4-((2-hydroxy-naphthalen-1-yl) methylene amino) benzenesulfonamide ligand. Inorg. Chim. Acta 2020, 513, 119955. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Tzimopoulos, D.I.; Holynska, M.; Perlepes, S.P. Oligonuclear Actinoid Complexes with Schiff Bases as Ligands—Older Achievements and Recent Progress. Int. J. Mol. Sci. 2020, 21, 555. [Google Scholar] [CrossRef] [Green Version]
- Omar, Z.T.; Jadhav, S.; Mohsin, M.; Faizaa, A.S.; Rai, M. Complexation study of synthesized pharmacological organic ligands with samarium. Rus. J. Inorg. Chem. 2020, 65, 2046–2052. [Google Scholar] [CrossRef]
- Rodríguez, M.R.; Balsa, L.M.; Piro, O.E.; Etcheverría, G.A.; García-Tojal, J.; Pis-Diez, R.; León, I.E.; Parajón-Costa, B.P.; González-Baró, A.C. Synthesis, crystal structure, spectroscopic characterization, DFT calculations and cytotoxicity assays of a new Cu(II) complex with an acylhydrazone ligand derived from thiophene. Inorganics 2021, 9, 9. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Bian, M.; Yang, Z.; Ma, X.; Liu, W. Pt (II) and Au (III) complexes containing Schiff-base ligands: A promising source for antitumor treatment. Eur. J. Med. Chem. 2021, 211, 113098. [Google Scholar] [CrossRef] [PubMed]
- Horozić, E.; Suljagić, J.; Suljkanovic, M. Synthesis, characterization, antioxidant and antimicrobial activity of Copper (II) complex with Schiff base derived from 2,2-dihydroxyindane-1, 3-dione and Tryptophan. Am. J. Org. Chem. 2019, 9, 9–13. [Google Scholar]
- Ejidike, I. Cu(II) Complexes of 4-[(1E)-N-{2-[(Z)-Benzylidene-amino]ethyl}ethanimidoyl]benzene-1,3-diol Schiff base: Synthesis, spectroscopic, in-vitro antioxidant, antifungal and antibacterial studies. Molecules 2018, 23, 1581. [Google Scholar] [CrossRef] [Green Version]
- Eltayeb, N.E.; Lasri, J.; Soliman, S.M.; Mavromatis, C.; Hajjar, D.; Elsilk, S.E.; Babgi, B.A.; Hussien, M.A. Crystal structure, DFT, antimicrobial, anticancer and molecular docking of (4E)-4-((aryl)methyleneamino)-1,2-dihydro-2,3-dimethyl-1-phenylpyrazol-5-one. J. Mol. Struct. 2020, 1213, 128185. [Google Scholar] [CrossRef]
- Al Zoubi, W. Biological activities of Schiff bases and their complexes: A review of recent works. Int. J. Org. Chem. 2013, 3, 73. [Google Scholar] [CrossRef] [Green Version]
- de Fátima, Â.; de P. Pereira, C.; Olímpio, C.R.S.D.G.; de Freitas Oliveira, B.G.; Franco, L.L.; da Silva, P.H.C. Schiff bases and their metal complexes as urease inhibitors—A brief review. J. Adv. Res. 2018, 13, 113–126. [Google Scholar]
- Shah, S.S.; Shah, D.; Khan, I.; Ahmad, S.; Ali, U.; ur Rahman, A. Synthesis and antioxidant activities of schiff bases and their complexes: An updated review. Biointerf. Res. Appl. Chem. 2020, 10, 6936–6963. [Google Scholar]
- Ibrahim, M.M.; Fathy, A.M.; Al-Harbi, S.A.; Sallam, S.A.; Al-Juaid, S.; Ramadan, A.E.M.M. Palladium(II) based imines; synthesis, characterization, X-ray structural analysis; DFT and catalytic hydrogenation study. J. Organometall. Chem. 2021, 939, 121764. [Google Scholar] [CrossRef]
- Chen, S.L.; Liu, X.Y.; Li, S.C.; Wu, C.; Li, Z.Y.; Li, T.T. Synthesis and crystal structure of a new Zn (II) complex with anti-leukemia activity. Inorg. Nano-Met. Chem. 2021, 51, 224–229. [Google Scholar] [CrossRef]
- Nurmamat, M.; Yan, H.; Wang, R.; Zhao, H.; Li, Y.; Wang, X.; Nurmaimaiti, K.; Kurmanjiang, T.; Luo, D.; Baodi, J.; et al. Novel copper(II) complex with a 4-acylpyrazolone derivative and coligand induce apoptosis in liver cancer cells. ACS Med. Chem. Lett. 2021, 12, 467–476. [Google Scholar] [CrossRef]
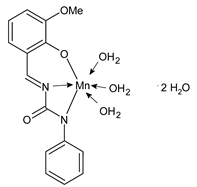
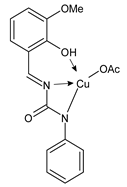
- Yadamani, S.; Neamati, A.; Homayouni-Tabrizi, M.; Beyramabadi, S.A.; Yadamani, S.; Gharib, A.; Morsali, A.; Khashi, M. Treatment of the breast cancer by using low frequency electromagnetic fields and Mn(II) complex of a Schiff base derived from the pyridoxal. Breast 2018, 41, 107–112. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Ge, X.; Wang, Q.; Xie, Y.; Hao, Y.; Zhang, Y.; Zhang, L.; Shang, W.; Liu, Z. Lysosome-targeted iridium(III) compounds with pyridine-triphenylamine Schiff base ligands: Syntheses, antitumor applications and mechanisms. Inorg. Chem. Front. 2020, 7, 91–100. [Google Scholar] [CrossRef]
- Wang, N.; Zeng, Q.; Zhang, R.; Xing, D.; Zhang, T. Eradication of solid tumors by chemodynamic theranostics with H2O2-catalyzed hydroxyl radical burst. Theranostics 2021, 11, 2334. [Google Scholar] [CrossRef] [PubMed]
- Oiye, É.N.; Ribeiro, M.F.M.; Katayama, J.M.T.; Tadini, M.C.; Balbino, M.A.; Eleotério, I.C.; Magalhães, J.; Castro, A.S.; Silva, R.S.M.; da Cruz Júnior, J.W.; et al. Electrochemical Sensors Containing Schiff Bases and their Transition Metal Complexes to Detect Analytes of Forensic, Pharmaceutical and Environmental Interest. A Review. Crit. Rev. Anal. Chem. 2019, 49, 488–509. [Google Scholar] [CrossRef]
- Wang, Z.G.; Ding, X.J.; Huang, Y.Y.; Yan, X.J.; Ding, B.; Li, Q.Z.; Xie, C.Z.; Xu, J.Y. The development of coumarin Schiff base system applied as highly selective fluorescent/colorimetric probes for Cu2+ and tumor biomarker glutathione detection. Dyes Pigments 2020, 175, 108156. [Google Scholar] [CrossRef]
- Song, X.-Q.; Wang, Z.-G.; Wang, Y.; Huang, Y.-Y.; Sun, Y.-X.; Ouyang, Y.; Xie, C.-Z.; Xu, J.-Y. Syntheses, characterization, DNA/HSA binding ability and antitumor activities of a family of isostructural binuclear lanthanide complexes containing hydrazine Schiff base. J. Biomol. Struct. Dyn. 2020, 38, 733–743. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- Malik, M.A.; Dar, O.A.; Gull, P.; Wani, M.Y.; Hashmi, A.A. Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. MedChemComm 2018, 9, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Tadele, K.T.; Tsega, T.W. Schiff bases and their metal complexes as potential anticancer candidates: A review of recent works. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2019, 19, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, A.; Jeyasubramanian, K.; Thangagiri, B.; Raja, J.D. Recent advances in Schiff base metal complexes derived from 4-amoniantipyrine derivatives and their potential applications. J. Mol. Struct. 2020, 1222, 128885. [Google Scholar] [CrossRef]
- Matela, G. Schiff bases and complexes: A review on anti-cancer activity. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2020, 20, 1908–1917. [Google Scholar] [CrossRef]
- Chaudhary, N.K.; Guragain, B.; Chaudhary, S.K.; Mishra, P. Schiff base metal complex as a potential therapeutic drug in medical science: A critical review. BIBECHANA 2021, 18, 214–230. [Google Scholar] [CrossRef]
- Islam, R.; Uddin, E.; Bitu, N.A.; Asraf, A.; Hossen, F.; Haque, M.; Mannan, A. Recent Advances in Biological and Catalytic Activities of Schiff base containing Acetylacetone and their Metal Complexes-A Short Overview. Asian J. Res. Chem. 2020, 13, 395–406. [Google Scholar] [CrossRef]
- Emam, S.; El Sayed, I.; Ayad, M.; Hathout, H. Synthesis, characterization and anticancer activity of new Schiff bases bearing neocryptolepine. J. Mol. Struct. 2017, 1146, 600–619. [Google Scholar] [CrossRef]
- Fetoh, A.; Asla, K.A.; El-Sherif, A.A.; El-Didamony, H.; El-Reash, G.M.A. Synthesis, structural characterization, thermogravimetric, molecular modelling and biological studies of Co(II) and Ni(II) Schiff bases complexes. J. Mol. Struct. 2019, 1178, 524–537. [Google Scholar] [CrossRef]
- Shi, S.; Yu, S.; Quan, L.; Mansoor, M.; Chen, Z.; Hu, H.; Liu, D.; Liang, Y.; Liang, F. Synthesis and antitumor activities of transition metal complexes of a bis-Schiff base of 2-hydroxy-1-naphthalenecarboxaldehyde. J. Inorg. Biochem. 2020, 210, 111173. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Heakal, B.H.; Younis, A.; Abdelmoaz, M.A.; Abdrabou, M.M. Conventional and Microwave-Assisted Synthesis, Antimicrobial and Antitumor Studies of Tridentate Schiff Base Derived from O-vanillin and Phenyl Urea and its Complexes. Adv. J. Chem. Sect. A 2020, 3, 621–638. [Google Scholar]
- Al-Serwi, R.H.; Othman, G.; Attia, M.A.; Enan, E.T.; El-Sherbiny, M.; Mahmoud, S.; Elsherbiny, N. Enhancement of cisplatin cytotoxicity by Cu(II)–Mn(II) schiff base tetradentate complex in human oral squamous cell carcinoma. Molecules 2020, 25, 4688. [Google Scholar] [CrossRef]
- Alyar, S.; Özmen, Ü.Ö.; Adem, Ş.; Alyar, H.; Bilen, E.; Kaya, K. Synthesis, spectroscopic characterizations, carbonic anhydrase II inhibitory activity, anticancer activity and docking studies of new Schiff bases of sulfa drugs. J. Mol. Sci. 2021, 1223, 128911. [Google Scholar] [CrossRef]
- Ismail, B.A.; Nassar, D.A.; Abd El–Wahab, Z.H.; Ali, O.A. Synthesis, characterization, thermal, DFT computational studies and anticancer activity of furfural-type Schiff base complexes. J. Mol. Struct. 2021, 1227, 129393. [Google Scholar] [CrossRef]
- Wongsuwan, S.; Chatwichien, J.; Pinchaipat, B.; Kumphune, S.; Harding, D.J.; Harding, P.; Boonmak, J.; Youngme, S.; Chotima, R. Synthesis, characterization and anticancer activity of Fe (II) and Fe(III) complexes containing N-(8-quinolyl) salicylaldimine Schiff base ligands. JBIC J. Biol. Inorg. Chem. 2021, 1–13. [Google Scholar]
- Naureen, B.; Miana, G.A.; Shahid, K.; Asghar, M.; Tanveer, S.; Sarwar, A. Iron(III) and zinc(II) monodentate Schiff base metal complexes: Synthesis, characterisation and biological activities. J. Mol. Struct. 2021, 1231, 129946. [Google Scholar] [CrossRef]
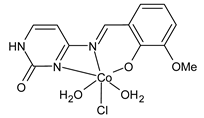
- Alkış, M.E.; Keleştemür, Ü.; Alan, Y.; Turan, N.; Buldurun, K. Cobalt and ruthenium complexes with pyrimidine based Schiff base: Synthesis, characterization, anticancer activities and electrochemotherapy efficiency. J. Mol. Struct. 2021, 1226, 129402. [Google Scholar] [CrossRef]
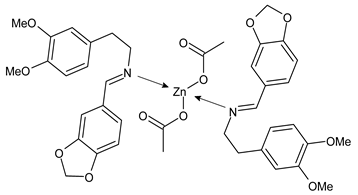
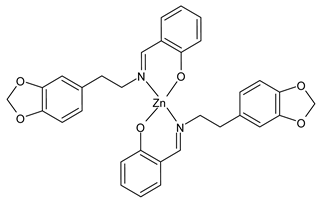
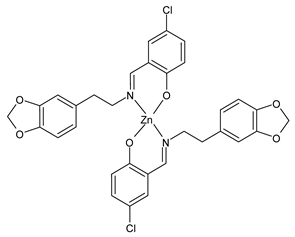
- Chen, S.Y.; Jiang, X.H.; Liu, R.X.; Huang, Y.; Shen, W.Y.; Jiang, Y.H.; Huamg, K.B.; Liu, Y.C. New cytotoxic zinc(II) and copper(II) complexes of Schiff base ligands derived from homopiperonylamine and halogenated salicylaldehyde. Inorg. Chim. Acta 2021, 516, 120171. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Bawazeer, A.M.; Shaaban, S.; Adam, M.S.S. Targeting ctDNA binding and elaborated in-vitro assessments concerning novel Schiff base complexes: Synthesis, characterization, DFT and detailed in-silico confirmation. J. Mol. Liq. 2021, 322, 114977. [Google Scholar] [CrossRef]
- Liao, W.H.; Song, X.Q.; Kong, Y.J.; Bao, R.D.; Li, F.F.; Zhou, J.; Zhao, Q.H.; Xu, J.Y.; Xie, N.; Xie, M.J. A novel Schiff base cobalt(III) complex induces a synergistic effect on cervical cancer cells by arresting early apoptosis stage. BioMetals 2021, 34, 277–289. [Google Scholar] [CrossRef]
- Mbugua, S.N.; Sibuyi, N.R.; Njenga, L.W.; Odhiambo, R.A.; Wandiga, S.O.; Meyer, M.; Lancelette, R.A.; Onani, M.O. New palladium(II) and platinum(II) complexes based on pyrrole Schiff bases: Synthesis, characterization, X-ray structure, and anticancer activity. ACS Omega 2020, 5, 14942–14954. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Gürkan, P.; Demir, Y.D.Ş.; Ark, M. Novel palladium (II) complexes of N-(5-nitro-salicylidene)-Schiff bases: Synthesis, spectroscopic characterization and cytotoxicity investigation. J. Mol. Struct. 2020, 1207, 127852. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, G.; Gong, G.; Sheng, Y.; Lu, X.; Cai, W.; Wang, F.; Zhao, G. A novel ferrocene-palladium metal complex: Synthesis, single crystal structure, in vitro cytotoxicity study and molecular docking. J. Mol. Struct. 2021, 1232, 130021. [Google Scholar] [CrossRef]
- Acharya, S.; Maji, M.; Chakraborty, M.P.; Bhattacharya, I.; Das, R.; Gupta, A.; Mukherjee, A. Disruption of the microtubule network and inhibition of VEGFR2 phosphorylation by cytotoxic N, O-coordinated Pt(II) and Ru(II) complexes of trimethoxy aniline-based Schiff bases. Inorg. Chem. 2021, 60, 3418–3430. [Google Scholar] [CrossRef]
- Moubeen, S.; El-Shahat, M.; Aziz, A.; Attia, A. Synthesis, characterization and biological evaluation of novel octahedral Ru (III) complexes containing pentadentate Schiff base ligands. Curr. Chem. Lett. 2021, 10, 17–32. [Google Scholar] [CrossRef]
- Andiappan, K.; Sanmugam, A.; Deivanayagam, E.; Karuppasamy, K.; Kim, H.-S.; Vikraman, D. In vitro cytotoxicity activity of novel Schiff base ligand–lanthanide complexes. Sci. Rep. 2018, 8, 3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathiyanarayanan, V.; Prasath, P.V.; Sekhar, P.C.; Ravichandran, K.; Easwaramoorthy, D.; Mohammad, F.; Al-Lohedan, H.A.; Oh, W.C.; Sagadevan, S. Docking and in vitro molecular biology studies of p-anisidine-appended 1-hydroxy-2-acetonapthanone Schiff base lanthanum(III) complexes. RSC Adv. 2020, 10, 16457–16472. [Google Scholar] [CrossRef]
- Hua, L.; Li, W.; Chen, Y.; Liang, K.; Cai, H.; Wang, J.; Wang, S.; Yin, T.; Liang, L. A hexanuclear Nd (III) complex derived from a Schiff base with significant antitumor and antifungal activity. Appl. Organometall. Chem. 2021, 35, e6081. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).