Caulerpenyne Affects Bradykinin-Induced Intracellular Calcium Kinetics in LoVo Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Solutions
2.3. Treatment with Caulerpenyne
2.4. Measurement of Intracellular Free Calcium Concentration ([Ca2+]i)
2.5. Cytotoxicity of CYN
2.6. Statistical Analysis
3. Results and Discussion
3.1. Cytotoxicity Assay
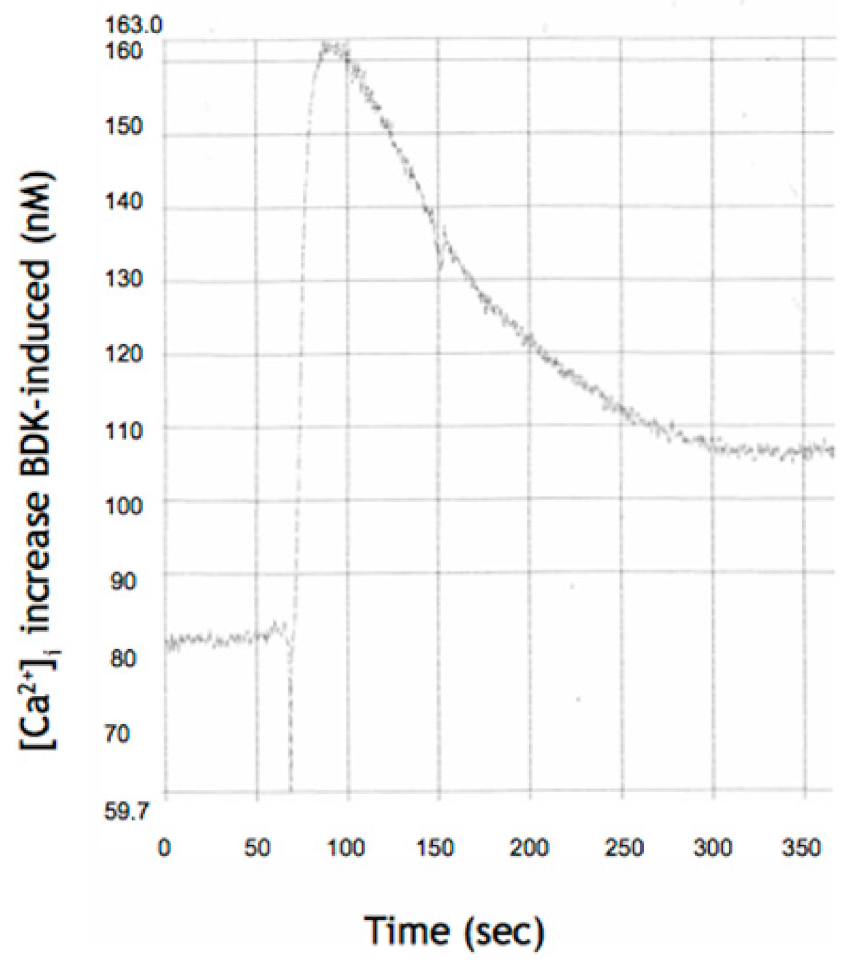
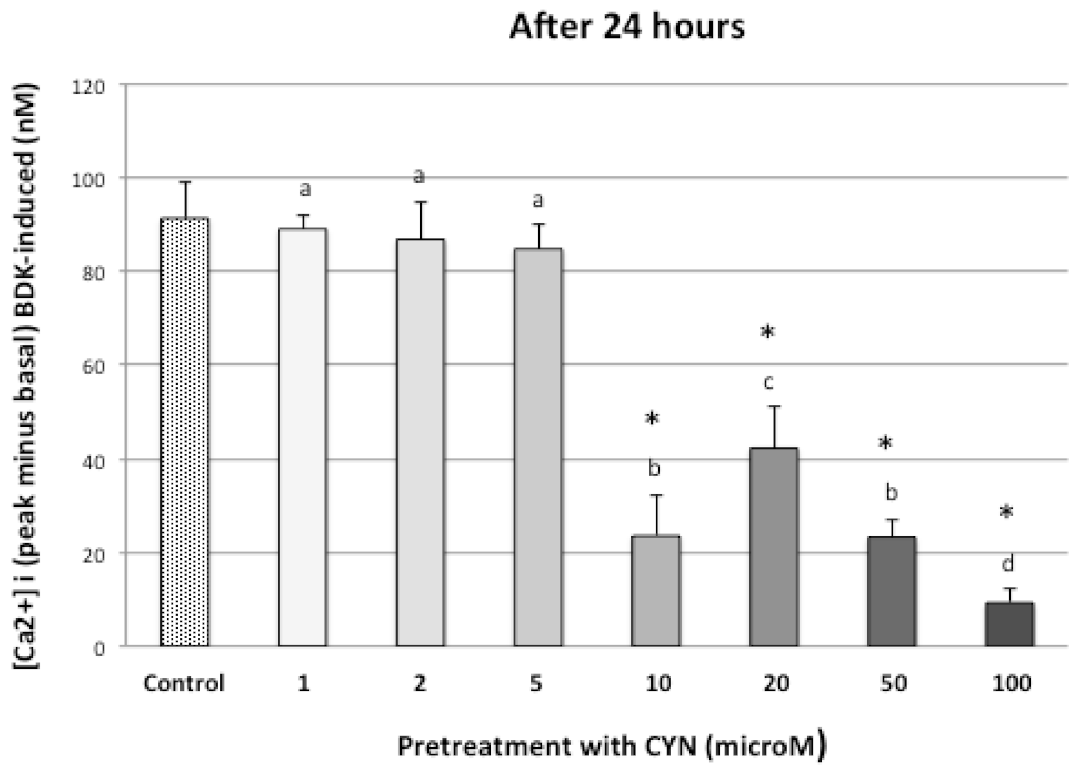
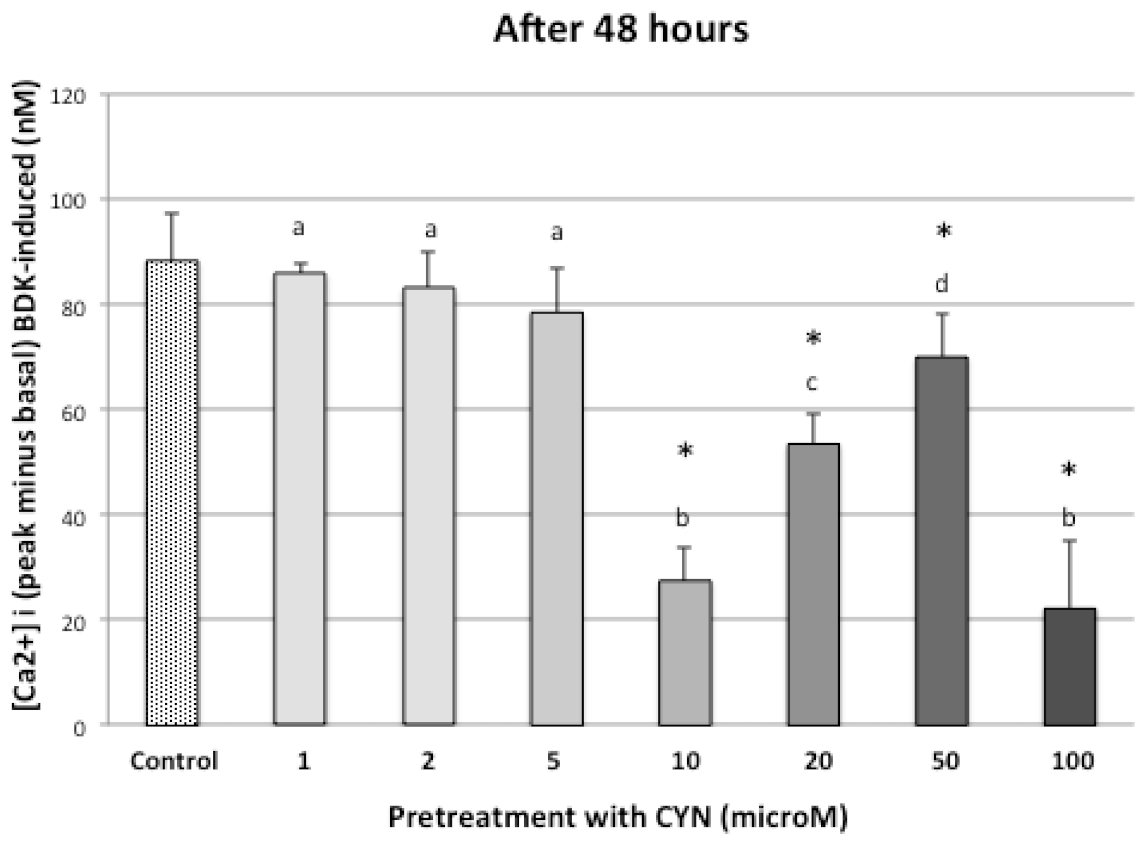
3.2. CYN on Intracellular Free Calcium Concentration, [Ca2+]i
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDK | bradykinin |
| CYN | caulerpenyne |
| [Ca2+]i | intracellular calcium concentration |
| IP3 | inositol 1,4,5-trisphosphate |
| IP3R | inositol 1,4,5-trisphosphate receptor |
| KRH | Krebs–Ringer–Hepes |
| PLC | phospholipase C |
References
- De Haro, L.; Treffot, M.J.; Jouglard, J.; Perringue, C. Trois cas d’intoxication de type ciguateresque après ingestion de sparidae de Mediterranée. Ictyol. Physiol. Acta 1993, 16, 133–146. [Google Scholar]
- Spanier, E.; Finkeltein, V.; Raikhlin-Eisehraft, B. Toxicity of the saupe, Sarpu salpu (Linnaeus, 1758), on the Mediterranean coast of Israel. J. Fish. Biol. 1989, 37, 503–504. [Google Scholar]
- Sureda, A.; Box, A.; Deudero, S.; Pons, A. Reciprocal effects of caulerpenyne and intense herbivorism on the antioxidant response of Bittium reticulatum and Caulerpa taxifolia. Ecotoxicol. Environ. Saf. 2009, 72, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Fischel, J.L.; Lemée, R.; Formento, P.; Caldani, C.; Moll, J.L.; Pesando, D.; Meinesz, A.; Grelier, P.; Pietra, P.; Guerriero, A.; et al. Cell growth inhibitory effects of caulerpenyne, a sesquiterpenoid from the marine algae Caulerpa taxifolia. Anticancer Res. 1995, 15, 2155–2160. [Google Scholar]
- Cavas, L.; Baskin, Y.; Yurdakoc, K.; Olgun, N. Antiproliferative and newly attributed apoptotic activities from an invasive marine alga: Caulerpa racemosa var. cylindracea. J. Exp. Mar. Biol. Ecol. 2006, 339, 111–119. [Google Scholar] [CrossRef]
- Nicoletti, E.; Della Pietà, F.; Calderone, V.; Bandecchi, P.; Pistello, M.; Morelli, I.; Cinelli, F. Antiviral properties of a crude extract from a green alga Caulerpa taxifolia (Vahl) C Agardh. Phytother. Res. 1999, 13, 245–247. [Google Scholar] [CrossRef]
- Lemée, R.; Pesando, D.; Durand-Clement, M.; Dubreuil, A.; Meinesz, A.; Guerriero, A.; Pietra, F. Preliminary survey of toxicity of the green alga Caulerpa taxifolia introduced into the Mediterranean. J. Appl. Phycol. 1993, 5, 485–493. [Google Scholar] [CrossRef]
- Galgani, I.; Pesando, D.; Porthe-Nibelle, J.; Fossat, B.; Girard, J.P. Effect of caulerpenyne, a toxin extracted from Caulerpa taxifolia on mechanisms regulating intracellular pH in sea urchin eggs and sea bream hepatocytes. J. Biochem. Toxicol. 1996, 11, 243–250. [Google Scholar] [CrossRef]
- Barbier, P.; Guise, S.; Huitorel, P.; Amade, P.; Pesando, D.; Briand, C.; Peyrot, V. Caulerpenyne from Caulerpa taxifolia has an antiproliferative activity on tumor cell line SK-N-SH and modifies the microtubule network. Life Sci. 2001, 70, 415–429. [Google Scholar] [CrossRef]
- Parent-Massin, D.; Fournier, V.; Amade, P.; Lemée, R.; Durand-Clément, M.; Delescluse, C.; Pesando, D. Evaluation of the toxicological risk to humans of caulerpenyne using human hematopoietic progenitors, melanocytes, and keratinocytes in culture. J. Toxicol. Environ. Health 1996, 47, 47–59. [Google Scholar] [CrossRef]
- Teixeira, V.L.; Rocha, F.D.; Houghton, P.J.; Kaplan, M.A.C.; Pereira, R.C. α-Amylase inhibitors from Brazilian seaweeds and their hypoglycemic potential. Fitoterapia 2007, 78, 35–36. [Google Scholar] [CrossRef]
- Bitou, N.; Ninomiya, M.; Tsujita, T.; Okuda, H. Screening of lipase inhibitors from marine algae. Lipids 1999, 34, 441–444. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Paul, V.J.; Fenical, W.; Norris, J.N.; Carvalho, M.S.; Jacobs, R.S. Phospholipase A2 inhibitors from marine algae. Hydrobiologia 1993, 260, 521–529. [Google Scholar] [CrossRef]
- Cengiz, S.; Cavas, L.; Yurdakoc, K. Alpha-amylase inhibition kinetics by caulerpenyne. Mediterr. Mar. Sci. 2010, 11, 93–103. [Google Scholar] [CrossRef]
- Brunelli, M.; Garcia-Gil, M.; Mozzachiodi, R.; Roberto, M.; Scuri, R.; Traina, G.; Zaccardi, M.L. Neurotoxic effects of caulerpenyne. Prog. Neuro-Psychopharmacol. Biol. Psychiat. 2000, 24, 939–954. [Google Scholar] [CrossRef]
- Mozzachiodi, R.; Scuri, R.; Roberto, M.; Brunelli, M. Caulerpenyne, a toxin from the seaweed Caulerpa Taxifolia, depresses afterhyperpolaryzation in invertebrate neurons. Neuroscience 2001, 107, 519–526. [Google Scholar] [CrossRef]
- Pesando, D.; Lemkealb, R.; Ferrua, C.; Amade, P.; Girard, J.-P. Effects of of caulerpenyne, the major toxin from Caulerpa taxifolia on mechanisms related to sea urchin egg cleavage. Aquat. Toxicol. 1996, 35, 139–l55. [Google Scholar] [CrossRef]
- Taylor, C.W.; Tovey, S.C. IP(3) receptors: Toward understanding their activation. Cold Spring Harbor Perspect. Biol. 2010, 2, a004010. [Google Scholar] [CrossRef] [PubMed]
- Tippmer, S.; Quitterer, U.; Kolm, V.; Faussner, A.; Roscher, A.; Mosthaf, L.; Muller-Esterl, W.; Haring, H. Bradykinin induces translocation of the protein kinase C isoforms alpha, epsilon, and zeta. Eur. J. Biochem. 1994, 225, 297–304. [Google Scholar] [CrossRef]
- Traina, G.; Cannistraro, S.; Bagnoli, P. Effects of somatostatin on intracellular calcium concentration in PC12 cells. J. Neurochem. 1996, 66, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators260, with greatly improvement fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Traina, G.; Bagnoli, P. Mechanisms mediating somatostatin-induced reduction of cytosolic free calcium in PC12 cells. Neurosci. Lett. 1999, 265, 123–126. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnóczky, G. Intracellular Ca2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef]
- Kass, G.E.; Orrenius, S. Calcium signaling and cytotoxicity. Environ. Health Perspect. 1999, 107 (Suppl. 1), 25–35. [Google Scholar] [CrossRef] [PubMed]
- Reber, B.F.; Stucki, J.W.; Reuter, H. Unidirectional interaction between two intracellular calcium stores in rat phaeochromocytoma (PC12) cells. J. Physiol. 1993, 468, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, A.M.; Cheng, E.H.; Boucher, R.C. Effect of bradykinin on intracellular calcium regulation in human ciliated airway epitelium. Am. J. Physiol. 1991, 261, L63–L69. [Google Scholar]
- Calabrese, E.J.; Baldwin, L.A. The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol. Sci. 2003, 71, 246–250. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traina, G. Caulerpenyne Affects Bradykinin-Induced Intracellular Calcium Kinetics in LoVo Cells. Appl. Sci. 2021, 11, 2697. https://doi.org/10.3390/app11062697
Traina G. Caulerpenyne Affects Bradykinin-Induced Intracellular Calcium Kinetics in LoVo Cells. Applied Sciences. 2021; 11(6):2697. https://doi.org/10.3390/app11062697
Chicago/Turabian StyleTraina, Giovanna. 2021. "Caulerpenyne Affects Bradykinin-Induced Intracellular Calcium Kinetics in LoVo Cells" Applied Sciences 11, no. 6: 2697. https://doi.org/10.3390/app11062697
APA StyleTraina, G. (2021). Caulerpenyne Affects Bradykinin-Induced Intracellular Calcium Kinetics in LoVo Cells. Applied Sciences, 11(6), 2697. https://doi.org/10.3390/app11062697





