Comparison of Thermal Characteristics and Fatty Acids Composition in Raw and Roasted Cocoa Beans from Peru (Criollo) and Ecuador (Forastero)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Cocoa Butter Extraction from Cocoa Beans
2.3. Proximate Analysis
2.4. Determination of Fatty Acid Composition
2.5. Sn-2 Positional Fatty Acid Analysis by Pancreatic Lipase
2.6. DSC Measurements
2.6.1. DSC Study of Cocoa Bean Powders
2.6.2. DSC Kinetics Parameters
2.6.3. Melting Characteristics
2.7. Thermogravimetry Analysis
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of Unroasted and Roasted Cocoa Beans Criollo and Forastero
3.2. Composition and Distribution of Fatty Acids of Cocoa Butter Extracted from Unroasted and Roasted Cocoa Beans Criollo and Forastero
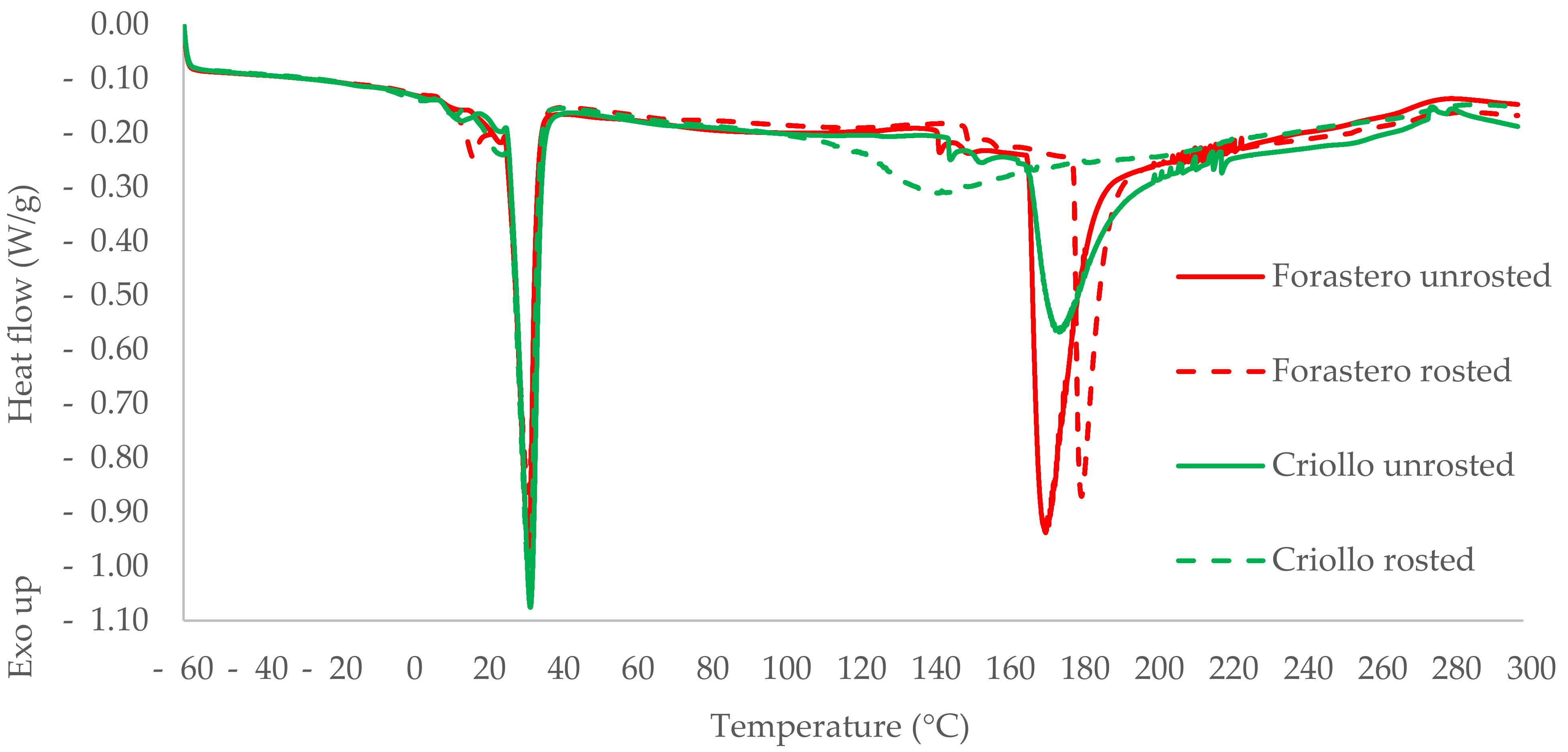
3.3. DSC Study of Beans and Cocoa Butter Extracted from Unroasted and Roasted Cocoa Beans Criollo and Forastero
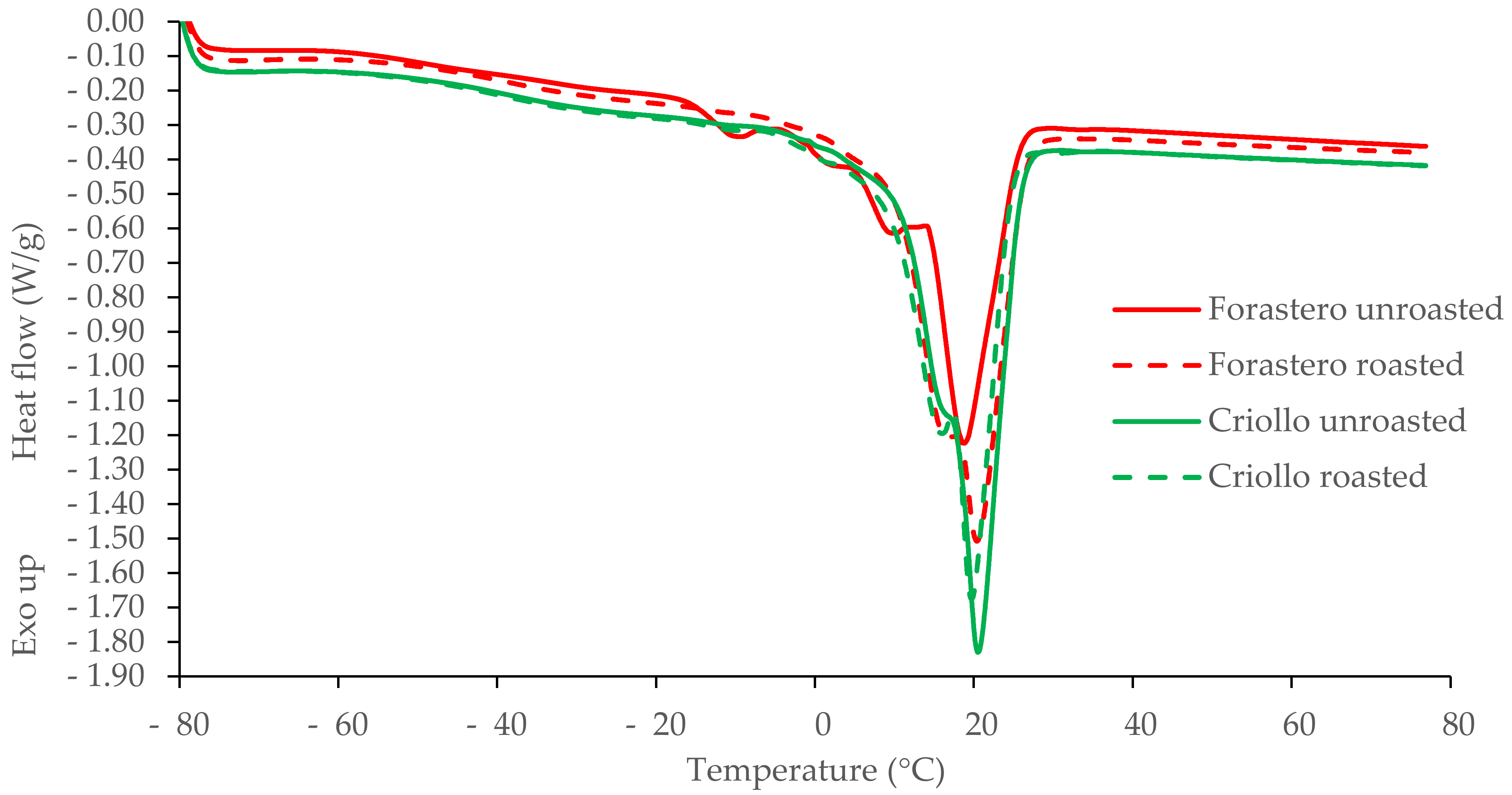
3.4. DSC Study of Cocoa Butter Extracted from Unroasted and Roasted Cocoa Beans Criollo and Forastero
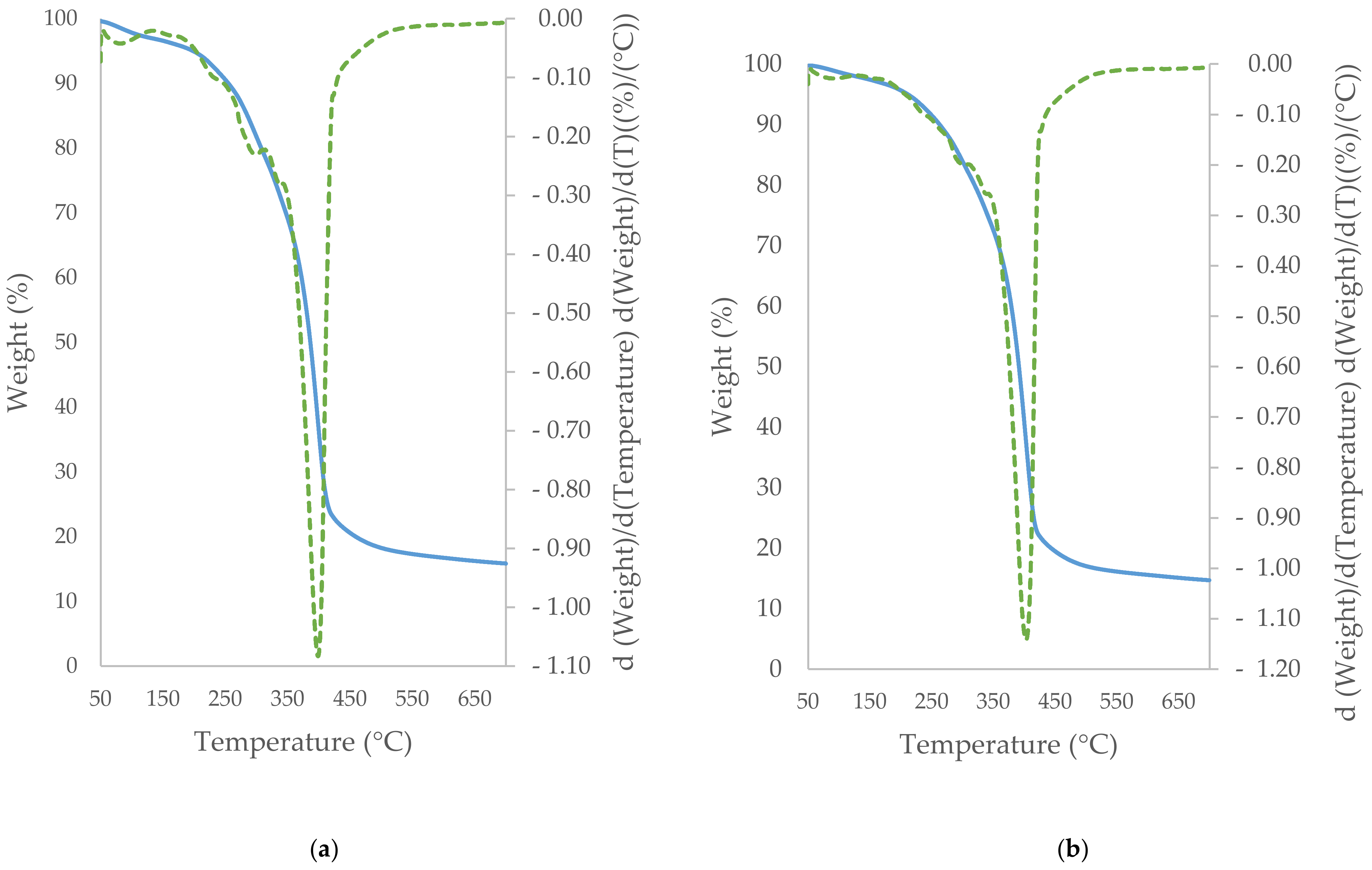
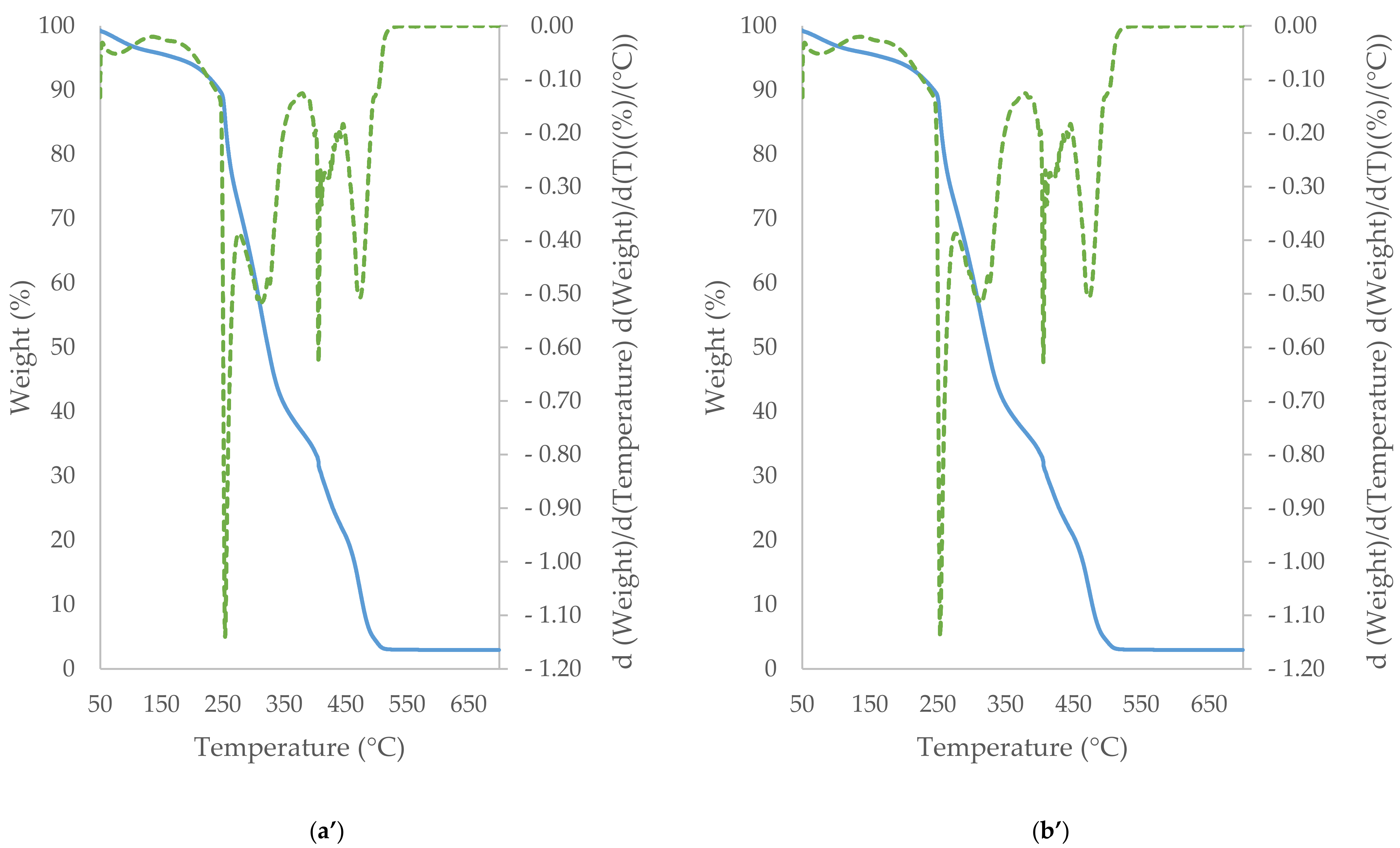
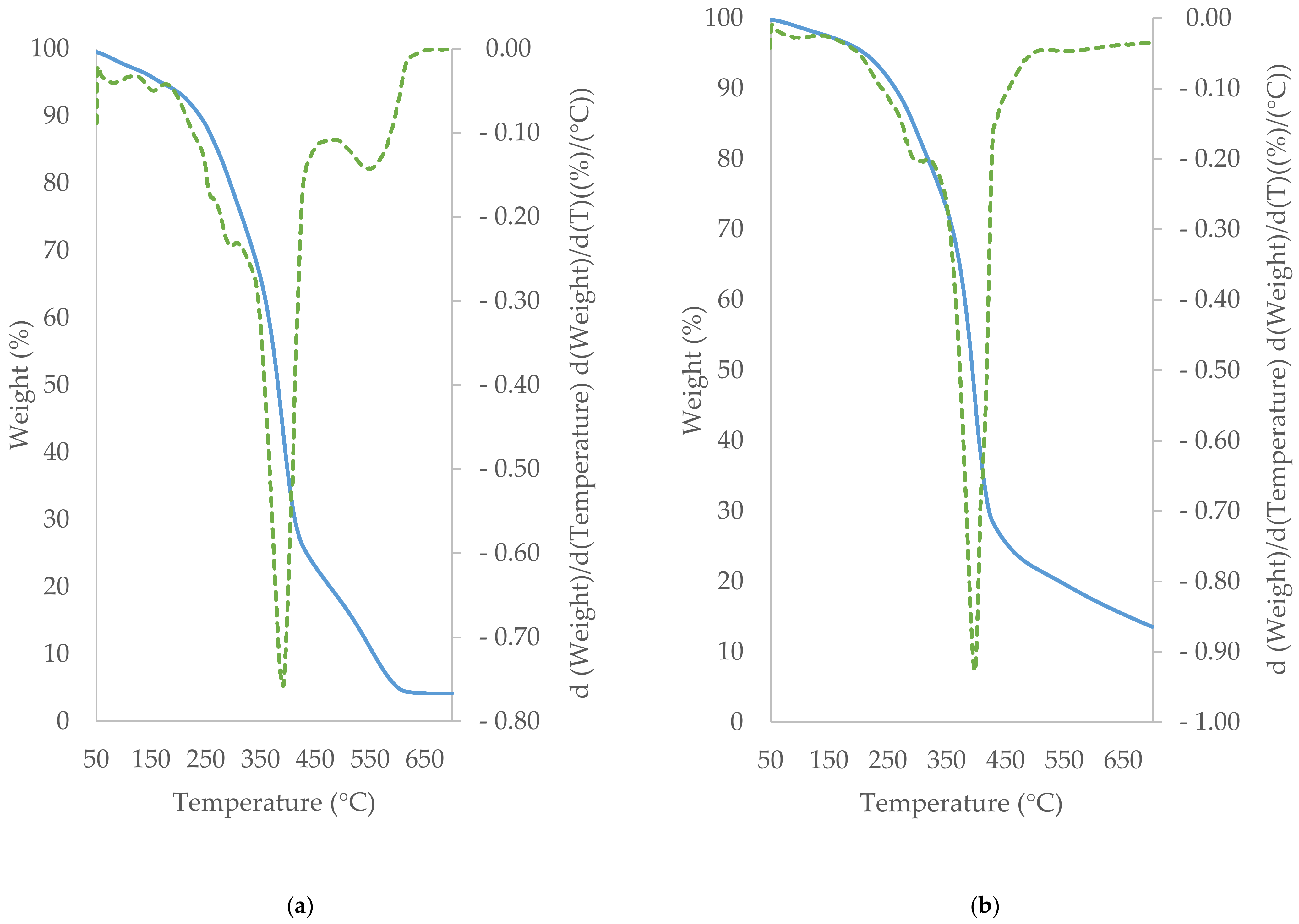
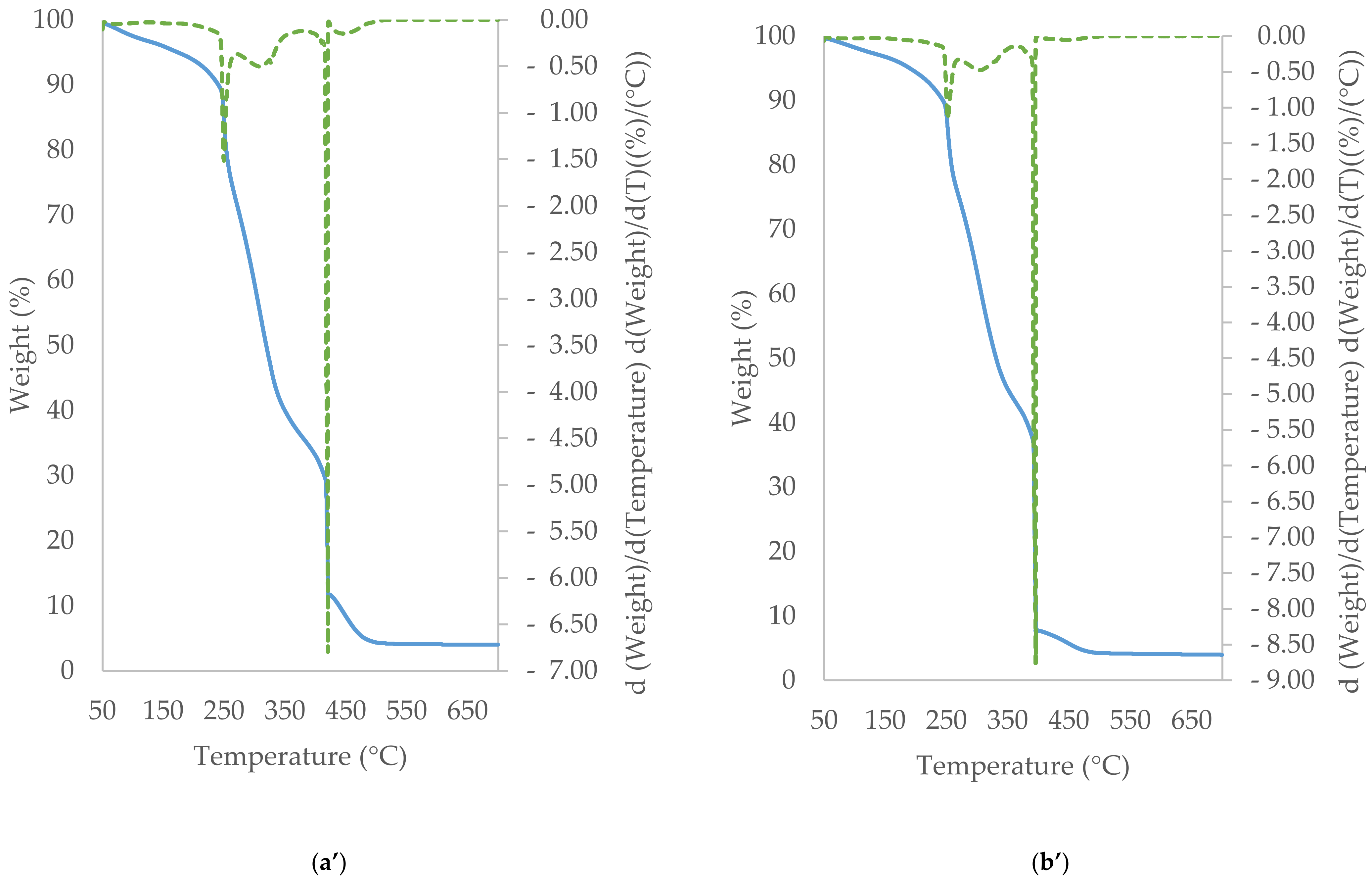
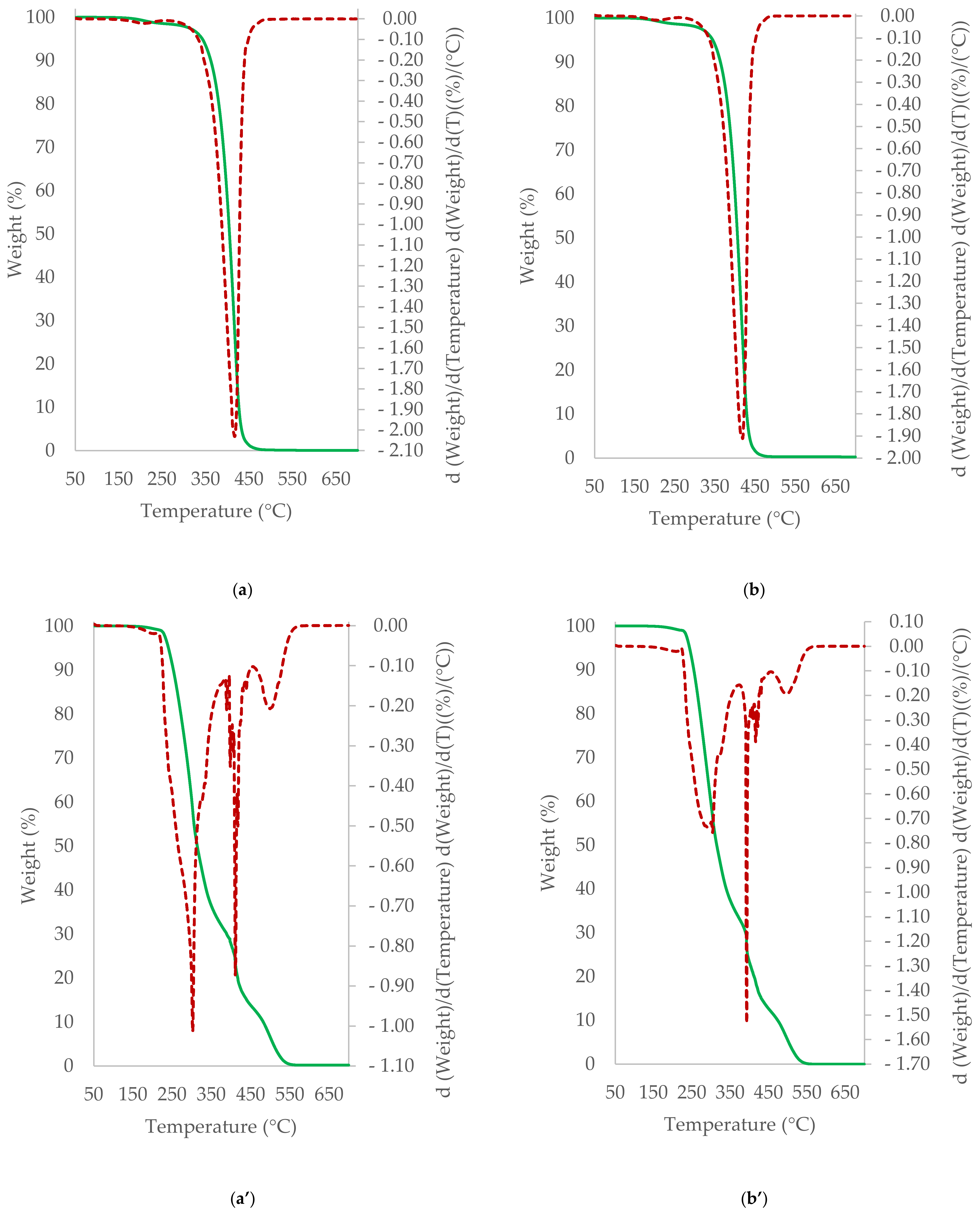
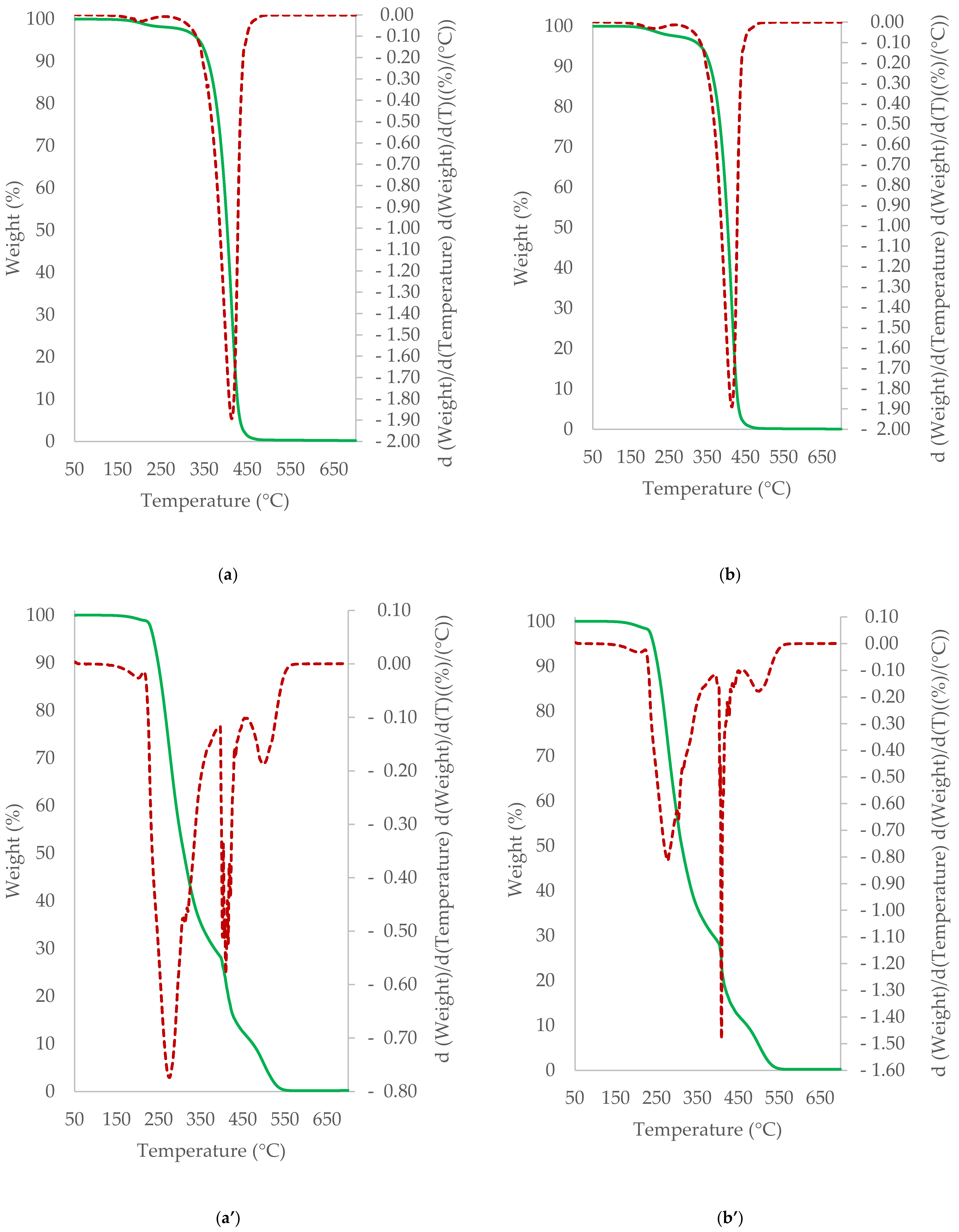
3.5. TGA/DTG Study of Cocoa Beans and Cocoa Butter Extracted from Unroasted and Roasted Cocoa Beans Criollo and Forastero
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipp, M.; Simoneau, C.; Ulberth, F.; Anklam, E.; Crews, C.; Brereton, P.; de Greyt, W.; Schwack, W.; Wiedmaier, C. Composition of genuine cocoa butter and cocoa butter equivalents. J. Food Compos. Anal. 2001, 14, 399–408. [Google Scholar] [CrossRef]
- Beckett, S.T. The Science of Chocolate, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Azir, M.; Abbasiliasi, S.; Azmi, T.; Ibrahim, T.; Noorzianna, Y.; Manaf, A.; Qurni Sazili, A.; Mustafa, S. Detection of Lard in Cocoa Butter—Its Fatty Acid Composition, Triacylglycerol Profiles, and Thermal Characteristics. Foods 2017, 6, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Ospina, J.; Di Mattia, C.D.; Paparella, A.; Mastrocola, D.; Martuscelli, M.; Chaves-Lopez, C. Effect of Fermentation, Drying and Roasting on Biogenic Amines and Other Biocompounds in Colombian Criollo Cocoa Beans and Shells. Foods 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krysiak, W.; Adamski, R.; Żyżelewicz, D. Factors affecting the color of roasted cocoa bean. J. Food Quality. 2013, 36, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Krysiak, W. Effects of convective and microwave roasting on the physicochemical properties of cocoa beans and cocoa butter extracted from this material. Grasas y Aceites 2011, 62, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Rocha, I.S.; de Santana, L.R.R.; Soares, S.E.; da Silva Bispo, E. Effect of the roasting temperature and time of cocoa beans on the sensory characteristics and acceptability of chocolate. Food Sci. Technol. Campinas. 2017, 37, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavour Formation and Character in Cocoa and Chocolate: A Critical Review. Crit. Rev. Food Sci. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, I. Instrumentation. In Industrial Chocolate Manufacture and Use, 3rd ed.; Beckett, S.T., Ed.; Blackwell Science: Oxford, UK, 1999; pp. 347–376. [Google Scholar]
- Simon, P.; Kolman, L. DSC Study of Oxidation Induction Periods. J. Therm. Anal. Calorim. 2001, 64, 2–813. [Google Scholar] [CrossRef]
- Gouveia, J.R.; de Lira Lixandrão, K.C.; Basílio Tavares, L.; Lixandrão Fernando, P.H.; Saltarelli Garcia, G.E.; dos Santos, D.J. Thermal Transitions of Cocoa Butter: A Novel Characterization Method by Temperature Modulation. Foods 2019, 8, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozawa, T.A. Modified method for kinetic analysis of thermoanalytical data. J. Therm. Anal. Calorim. 1976, 9, 369–373. [Google Scholar] [CrossRef]
- Martinez-Monteagudo, S.I.; Saldana, M.D.A.; Kennelly, J.J. Kinetics of non-isothermal oxidation of anhydrous milk fat rich in conjugated linoleic acid using differential scanning calorimetry. J. Therm. Anal. Calorim. 2012, 107, 973–981. [Google Scholar] [CrossRef]
- Materazzi, S.; De Angelis Curtis, S.; Vecchio Ciprioti, S.; Risoluti, R.; Finamore, J. Thermogravimetric characterization of dark chocolate. J. Therm. Anal. Calorim. 2014, 116, 93–98. [Google Scholar] [CrossRef]
- Talbot, G. Chocolate temper. In Industrial Chocolate Manufacture and Use, 3rd ed.; Beckett, S.T., Ed.; Blackwell Science: Oxford, UK, 1999; pp. 261–275. [Google Scholar]
- Torres-Moreno, M.; Torrescasana, E.; Salas-Salvadó, J.; Blanch, C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015, 16, 125–132. [Google Scholar] [CrossRef]
- Ławrowski, P. Świat czekolady. Cukiernictwo i Piekarstwo 2018, 5, 60–66. (in Polish). [Google Scholar]
- Boselli, E.; Velazco, V.; Caboni, M.F.; Lercker, G. Pressurized liquid extraction of lipids for the determination of oxysterols in egg-containing food. J. Chromatogr. A 2001, 917, 239–244. [Google Scholar] [CrossRef]
- Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A.; Ostrowska-Ligęza, E. Effect of enzymatic interesterification on physicochemical and thermal properties of fat used in cookies. LWT Food Sci. Technol. 2016, 74, 99–105. [Google Scholar] [CrossRef]
- Pina-Rodriguez, A.M.; Akoh, C.C. Enrichment of amaranth oil with ethyl palmitate at the sn-2 position by chemical and enzymatic synthesis. J. Agr. Food Chem. 2009, 57, 4657–4662. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, A.; ŞahinYeşilçubuk, N. Enzymatic production of human milk fat analogues containing stearidonic acid and optimization by response surface methodology. LWT Food Sci. Technol. 2012, 46, 210–216. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Górska, A.; Wirkowska, M.; Koczoń, P. An assessment of various powdered baby formulas by conventional methods (DSC) or FT-IR spectroscopy. J. Therm. Anal. Calorim. 2012, 110, 465–471. [Google Scholar] [CrossRef]
- Wirkowska, M.; Ostrowska-Ligęza, E.; Górska, A.; Koczon, P. Thermal properties of fats extracted from powdered baby formulas. J. Therm. Anal. Calorim. 2012, 110, 137–143. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Górska, A.; Wirkowska-Wojdyła, M.; Bryś, J.; Dolatowska-Żebrowska, K.; Shamilowa, M.; Ratusz, K. Thermogravimetric characterization of dark and milk chocolates at different processing stages. J. Therm. Anal. Calorim. 2018, 134, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, F.; Bearden, M.; Keen, C. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet. Assoc. 2003, 103, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Pucciarelli, D.L. Cocoa and Heart Health: A Historical Review of the Science. Nutrients 2013, 5, 3854–3870. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.S. Cocoa Beans: From Tree to Factory. In Industrial Chocolate Manufacture and Use, 3rd ed.; Beckett, S.T., Ed.; Blackwell Science: Oxford, UK, 1999; pp. 8–35. [Google Scholar]
- Berbiye, I.Y. Raw Cocoa (Theobroma cacao L.) Quality Parameters—with Special Reference to West Africa. PhD Thesis, Biology Department, Faculty of Mathematics, Informatics and Natural Sciences, University of Hamburg, Hamburg, Germany, 2014. [Google Scholar]
- Oracz, J.; Nebesny, E. Effect of roasting parameters on the physicochemical characteristics of high-molecular-weight Maillard reaction products isolated from cocoa beans of different Theobroma cacao L. groups. Eur. Food Res. Technol. 2019, 245, 111–128. [Google Scholar] [CrossRef]
- Farah, D.M.H.; Zaibunnisa, A.H.; Misnawi. Optimization of cocoa beans roasting process using Response Surface Methodology based on concentration of pyrazine and acrylamide. Int. Food Res. J. 2012, 19, 1355–1359. [Google Scholar]
- Bongers, M.E.; de Lorijn, F.; Reitsma, J.B.; Groeneweg, M.; Taminiau, J.A.; Benninga, M.A. The clinical effect of a new infant formula in term infants with constipation: A double-blind, randomized croo-over trial. Nutr. J. 2007, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Lopez, A.; Castellote-Bargallo, A.I.; Campoy-Folgoso, C.; Rivero-Urgel, M.; Tormo-Carnice, R.; Infante-Pina, D.; Lopez-Sabater, M.C. The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newbornfeces. Early Hum. Dev. 2001, 65, 83–94. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Castellote-Bargallo, A.I.; Campoy-Folgoso, C.; Rivero-Urgel, M.; Lopez-Sabater, M.C. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and infant formulas. Eur. J. Clin. Nutr. 2002, 56, 1242–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agus, B.A.P.; Mohamad, N.N.; Hussain, N. Composition of unfermented, unroasted, roasted cocoa beans and cocoa shells from Peninsular Malaysia. J. Food Meas. Charact. 2018, 12, 2581–2589. [Google Scholar] [CrossRef]
- Youssef, M.M.; Taiseer, M.; Bakr, A. Effect of Roasting on Physicochemical Properties of Cocoa Beans: An Overview. Alex. J. Food Sci. Technol. 2019, 16, 2. [Google Scholar]
- Redgwell, R.J.; Trovato, V.; Curti, D. Cocoa bean carbohydrates: Roasting-induced changes and polymer interactions. Food Chem. 2003, 80, 511–516. [Google Scholar] [CrossRef]
- Ostrowska-Ligąza, E.; Bekas, W.; Kowalska, D.; Łobacz, M.; Wroniak, M.; Kowalski, B. Kinetics of commercial olive oil oxidation: Dynamic differential scanning calorimetry and Rancimat studies. Eur. J. Lipid Sci. Technol. 2010, 112, 268–274. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Ostrowska-Ligęza, E.; Krygier, K. Impact of Selected Chemical Characteristics of Cold-Pressed Oils on their Oxidative Stability Determined Using the Rancimat and Pressure Differential Scanning Calorimetry Method. Food Anal. Method. 2018, 11, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.N.; Sarwar, A.; Wahab, M.F. Chemometric assessment of thermal oxidation of some edible oils. J. Therm. Anal. Calorim. 2010, 102, 369–374. [Google Scholar] [CrossRef]
- Cifti, O.N.; Kowalski, B.; Gogus, F.; Fadiloglu, S. Effect of the addition of a cocoa butter-like fat enzymatically produced from olive pomace oil on the oxidative stability of cocoa butter. J. Food Sci. 2009, 4, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Bryś, J.; Vaz Flores, I.F.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC methods to characterize human milk fat substitutes obtained from lard and milk thistle oil mixtures. J. Therm. Anal. Calorim. 2017, 130, 319–327. [Google Scholar] [CrossRef]
- Wąsowicz, E.; Gramza, A.; Hęś, M.; Jeleń, H.H.; Korczak, J.; Małecka, M.; Mildner-Szkudlarz, S.; Rudzińska, M.; Samotyja, U.; Zawirska-Wojtasiak, R. Oxidation of lipids in food. Pol. J. Food Nutr. Sci. 2004, 54, 87–100. [Google Scholar]
- Ostrowska-Ligęza, E.; Marzec, A.; Gorska, A.; Wirkowska-Wojdyła, M.; Bryś, J.; Rejch, A.; Czarkowska, K. A comparative study of thermal and textural properties of milk, white and dark chocolates. Thermochim. Acta. 2019, 671, 60–69. [Google Scholar] [CrossRef]
- De Souza, G.A.; Santos, O.J.C.; Conceição, M.M.; Dantas Silva, M.C.; Prasad, S. A thermoanalytic and kinetic study of sunflower oil. Braz. J. Chem. Eng. 2004, 21, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Szabo, M.; Chambre, D.; Iditoiu, C. TG/DTG/DTA for the oxidation behaviour characterization of vegetable and animal fats. J. Therm. Anal. Calorim. 2012, 110, 281–285. [Google Scholar] [CrossRef]
- Górska, A.; Brzezińska, R.; Wirkowska-Wojdyła, M.; Bryś, J.; Domian, E.; Ostrowska-Ligęza, E. Application of Thermal Methods to Analyze the Properties of Coffee Silverskin and Oil Extracted from the Studied Roasting By-Product. Appl. Sci. 2020, 10, 8790. [Google Scholar] [CrossRef]
- Fontanari, G.G.; Kobelnik, M.; Marques, M.R.; Arêas, J.A.G.; Franzin, B.T.; Pastre, I.A.; Fertonani, F.L. Thermal and kinetic studies of white lupin (Lupinus albus) oil. J. Therm. Anal. Calorim. 2018, 131, 775–782. [Google Scholar] [CrossRef]
- Wille, R.; Lutton, E. Polymorphism of cocoa butter. J. Am. Oil Chem. Soc. 1966, 43, 491–496. [Google Scholar] [CrossRef] [PubMed]
| Ecuador Cocoa Beans Forastero Type | |||
|---|---|---|---|
| Unroasted | Roasted | ||
| Proximates % (g/100 g) | Moisture Ash Total protein Carbohydrate and fibre (by difference) Total fat | 5.70 ± 0.34 *,a 3.96 ± 0.24 a 14.10 ± 0.71 a 29.54 ± 6.50 a 46.70 ± 3.27 a | 3.1 ± 0.19 b 4.06 ± 0.24 a 14.60 ± 0.73 a 32.74 ± 7.20 a 45.50 ± 3.19 a |
| Minerals | Calcium, Ca (mg/kg) Magnesium, Mg (mg/kg) Potassium, K (%) Phosphorus, P (mg/kg) Iron, Fe (mg/kg) | 1598 ± 320 a 3629 ± 726 a 1.26 ± 0.25 a 5247 ± 1050 a 53.6 ± 10.7 a | 1479 ± 296 a 3547 ± 709 a 1.30 ± 0.26 a 5150 ± 1030 a 135 ± 27.0 b |
| Peru Cocoa Beans Criollo Type | |||
| Unroasted | Roasted | ||
| Proximates % (g/100 g) | Moisture Ash Total protein Carbohydrate and fibre (by difference) Total fat | 5.50 ± 0.33 *,a 3.53 ± 0.21 a 14.62 ± 0.73 a 35.35 ± 7.78 a 41.00 ± 2.87 a | 4.90 ± 0.29 a 3.38 ± 0.20 a 14.80 ± 0.74 a 35.02 ± 7.70 a 41.90 ± 2.93 a |
| Minerals | Calcium, Ca (mg/kg) Magnesium, Mg (mg/kg) Potassium, K (%) Phosphorus, P (mg/kg) Iron, Fe (mg/kg) | 1002 ± 200 a 3648 ± 729 a 1.06 ± 0.21 a 5933 ± 1190 a 38.9 ± 7.8 a | 1008 ± 202 a 3524 ± 705 a 1.10 ± 0.22 a 5579 ± 1116 a 55.0 ± 11.0 b |
| Samples | Fatty Acid | Fatty Acid Composition in TAG | Fatty Acid Composition (%) in Positions | The Share of the Fatty Acid in sn-2 Position (%) | |
|---|---|---|---|---|---|
| sn-2 | sn-1,3 | ||||
| Ecuador (Forastero) | C16:0 | 27.84 ± 0.63 | 21.91 ± 0.44 | 30.81 | 23.71 |
| Roasted | C18:0 | 34.46 ± 0.45 | 25.83 ± 0.18 | 38.78 | 22.21 |
| C18:1n-9c | 32.09 ± 0.13 | 47.40 ± 0.78 | 24.44 | 64.66 | |
| C18:2n-6c | 3.43 ± 0.03 | 4.87 ± 0.53 | 2.71 | 59.78 | |
| C20:0 | 1.31 ± 0.08 | - | 1.97 | - | |
| Ecuador (Forastero) | C16:0 | 30.02 ± 1.46 | 10.57 ± 0.13 | 39.75 | 11.74 |
| Unroasted | C18:0 | 33.54 ± 1.63 | 12.99 ± 0.04 | 43.82 | 12.91 |
| C18:1n-9c | 32.76 ± 0.68 | 69.90 ± 0.18 | 14.19 | 71.12 | |
| C18:2n-6c | 2.50 ± 0.02 | 5.63 ± 0.01 | 0.94 | 75.07 | |
| C20:0 | 1.04 ± 0.02 | - | 1.56 | - | |
| Peru (Criollo) | C16:0 | 27.11 ± 0.87 | 3.03 ± 0.01 | 39.14 | 3.73 |
| Roasted | C18:0 | 33.12 ± 0.46 | 2.76 ± 0.01 | 48.29 | 2.78 |
| C18:1n-9c | 33.48 ± 0.33 | 81.35 ± 0.08 | 9.54 | 81.00 | |
| C18:2n-6c | 3.54 ± 0.04 | 9.55 ± 0.14 | 0.53 | 90.05 | |
| C20:0 | 1.30 ± 0.07 | - | 1.95 | - | |
| Peru (Criollo) | C16:0 | 28.03 ± 1.55 | 3.52 ± 0.08 | 40.28 | 4.18 |
| Unroasted | C18:0 | 33.44 ± 0.91 | 3.28 ± 0.01 | 48.51 | 3.27 |
| C18:1n-9c | 32.44 ± 0.46 | 80.51 ± 0.08 | 8.40 | 82.73 | |
| C18:2n-6c | 3.28 ± 0.06 | 8.70 ± 0.06 | 0.56 | 88.55 | |
| C20:0 | 1.38 ± 0.11 | - | 2.07 | - | |
| Heating Rate, β/°C | Oxidation Onset Temperatures Ton/°C | |||
|---|---|---|---|---|
| Forastero Unroasted | Forastero Roasted | Criollo Unroasted | Criollo Roasted | |
| 2.5 | 188.08 ± 1.90 | 185.44 ± 2.08 | 185.61 ± 0.04 | 195.82 ± 0.11 |
| 4 | 195.35 ± 0.93 | 189.85 ± 1.70 | 192.38 ± 0.01 | 201.53 ± 0.49 |
| 5 | 196.64 ± 0.34 | 194.13 ± 1.27 | 195.75 ± 0.96 | 205.36 ± 0.45 |
| 7.5 | 203.35 ± 1.61 | 199.32 ± 1.63 | 202.92 ± 0.40 | 210.46 ± 0.84 |
| 10 | 208.44 ± 1.36 | 203.97 ± 2.24 | 207.77 ± 0.02 | 217.20 ± 0.06 |
| 12.5 | 210.88 ± 1.08 | 209.11 ± 0.25 | 212.88 ± 0.53 | 220.05 ± 0.03 |
| 15 | 213.43 ± 0.54 | 210.83 ± 0.39 | 213.99 ± 0.33 | 222.34 ± 0.73 |
| Parameter | Forastero Unroasted | Forastero Roasted | Criollo Unroasted | Criollo Roasted |
|---|---|---|---|---|
| a | 7023.8 | 6466.8 | 5863.6 | 6544.0 |
| b | 15.608 | 14.538 | 13.191 | 14.374 |
| R2 | 0.994 | 0.994 | 0.996 | 0.988 |
| Ea/kJ mol−1 | 127.87 | 117.73 | 106.75 | 119.14 |
| log Z | 13.74 | 12.70 | 11.40 | 12.53 |
| Z/min−1 | 5.45 × 1013 | 5.03 × 1012 | 2.50 × 1011 | 3.41 × 1012 |
| Melting point, T1/°C | 9.12 ± 0.01 A | 15.90 ± 0.03 B | 15.58 ± 0.16 a | 15.92 ± 0.09 a |
| Melting point, T2/°C | 18.84 ± 0.06 C | 20.66 ± 0.19 C | 20.66 ± 0.01 b | 19.67 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowska-Ligęza, E.; Dolatowska-Żebrowska, K.; Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A. Comparison of Thermal Characteristics and Fatty Acids Composition in Raw and Roasted Cocoa Beans from Peru (Criollo) and Ecuador (Forastero). Appl. Sci. 2021, 11, 2698. https://doi.org/10.3390/app11062698
Ostrowska-Ligęza E, Dolatowska-Żebrowska K, Wirkowska-Wojdyła M, Bryś J, Górska A. Comparison of Thermal Characteristics and Fatty Acids Composition in Raw and Roasted Cocoa Beans from Peru (Criollo) and Ecuador (Forastero). Applied Sciences. 2021; 11(6):2698. https://doi.org/10.3390/app11062698
Chicago/Turabian StyleOstrowska-Ligęza, Ewa, Karolina Dolatowska-Żebrowska, Magdalena Wirkowska-Wojdyła, Joanna Bryś, and Agata Górska. 2021. "Comparison of Thermal Characteristics and Fatty Acids Composition in Raw and Roasted Cocoa Beans from Peru (Criollo) and Ecuador (Forastero)" Applied Sciences 11, no. 6: 2698. https://doi.org/10.3390/app11062698
APA StyleOstrowska-Ligęza, E., Dolatowska-Żebrowska, K., Wirkowska-Wojdyła, M., Bryś, J., & Górska, A. (2021). Comparison of Thermal Characteristics and Fatty Acids Composition in Raw and Roasted Cocoa Beans from Peru (Criollo) and Ecuador (Forastero). Applied Sciences, 11(6), 2698. https://doi.org/10.3390/app11062698










