1. Introduction
In recent decades, people have dramatically changed the way they cook food because of the changes in attitude towards food. In the last few years, with increasing lifestyles, the number of people choosing to embrace these convenient food products at the expense of taste and health has steadily increased. Consequently, the popularity of packaged food and instant meals has revolutionized the packaged food industry, with manufacturers seeking new methods to offer fresh and delicious food without inconvenience. Nowadays, home meal replacement (HMR) products that are simple meals to prepare and consume have become increasingly popular in the food and beverage industry. HMR products have the advantage of long shelf life while maintaining the nutrient content of the food. Globally, the demand for HMR products has increased due to increasing single-person households, women’s social activities, and the population of elderly people.
Increases in HMR products have encouraged the development of new high-quality seafood HMR products with a long shelf life; these contain the seasoned adductor muscle of the pen shell (AMPS) and common squid meat (CSM). Pen shell (
Atrina pectinata), a bivalve and common squid (
Todarodes pacificus), a cephalopod, are two popular and commercially important species. Globally, these organisms are distributed from south-east Africa to Malaysia and New Zealand, the Indo-western Pacific region, Japan, and Korea [
1,
2]. However, South Korea and Japan are the largest markets for raw and processed products of these two species [
3].
Khi-Jo-Gae and
O-Jing-Eo are popular names for
A. pectinate and
T. pacificus in Korea. Due to their high nutritional value, these species are often found on the seafood menu. For instance, the AMPS and CSM, the protein-rich, highly nutritional products of pen shell and common squid, have high consumption demand.
As the quality of seafood HMR products continues to improve, it is believed that applying advanced technologies to handle raw material, cook, and freeze during the manufacturing process is likely to result in excellent quality products [
4]. To this end, several technologies, including high-frequency defrosting (HFD), superheated steam cooking, and quick freezing techniques, are believed to enhance product quality. HFD, in which the amount of heat generated inside the product and the defrosting time are accurately controlled, can reduce thawing time, inhibit microbial activities, reduce drip loss, and maintain the quality of raw materials [
5,
6]. Furthermore, oxidation is minimized in superheated steaming due to the lack of oxygen during heating and roasting [
7], rendering superheated steam cooking an effective method in the food industry. In addition, superheated steam roasting reduces energy consumption during the roasting process [
8] and has been proven to reduce lipid oxidation and preserve food nutrient substances, color, and texture better than traditional cooking methods [
7,
9,
10,
11]. Quick freezing is a key technology for maintaining the quality and prolonging the shelf life of frozen food. This technology successfully inhibits changes in flavor, color, and texture due to oxidative, enzymatic, and microbial changes [
12,
13,
14].
Though these techniques have been explored in several food products, their applications in seafood-based HMR products are limited. Therefore, we hypothesized that the development of new high-quality fresh product-equivalent seafood HMR products, especially those containing the seasoned AMPS and CSM, could be achieved by employing advanced techniques. Moreover, the shelf life of packaged seafood products is an important factor determining the consumer acceptability and saleability of the product. Therefore, it is essential to decipher the ideal conditions that could prolong the shelf life of HMR products while improving their quality.
In this study, we aimed to elucidate the best method to produce good quality, highly nutritious HMR products with an improved shelf life prepared by mixing roasted AMPS and roasted CSM. Here, we produced the seasoning-mixed AMPS and CSM as test HMR products using a high-frequency defroster, superheated steam, and quick freezing techniques and evaluated its quality characteristics of test HMR products. The findings could unravel the potential of improved technologies, which could be used in the seafood industry to produce high-quality HMR products with little loss of nutrition and texture.
2. Materials and Methods
2.1. Materials
All chemicals used in this study, including potassium carbonate, sulfuric acid, boric acid, sodium hydroxide, trichloroacetic acid, phosphoric acid, N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide with 1% Butyldimethylchlorosilane (MTBSTFA with 1% t-BDMCS), methyl red solution, methylene blue solution, acetonitrile, 2-thiobarbituric acid, ethanol, and sodium bicarbonate, were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). The Difco plate count agar and EC medium used for microbiological analysis were purchased from BD Co. (Franklin Lakes, NJ, USA), whereas Sanita-Kun plates were obtained from JNC Corp. (Tokyo, Japan).
2.2. Experimental Sample
Frozen AMPS and CSM were obtained from EBADA Fishery Co. Ltd. (Busan, Korea). Sample weights were 46–60 g (average 53.7 g) for AMPS and 188–266 g (average 221.8 g) for CSM.
2.3. Drip Loss Analysis
Frozen AMPS and CSM were thawed under three different conditions: room temperature, running water, and HFD. For running water and room temperature thawing, samples were placed in plastic bags. For HFD thawing, the controller of the HFD machine (CHRFT-100, Chamco Co. Ltd., Busan, Korea) was set at 27 MHz for 10 min (with an input power of 11 kW). Drip loss was analyzed using the filter-paper wetness (FPW) method following Kauffman et al. [
15]. Quantitative filter paper No. 2 (55 mm; Advantech, Tokyo, Japan) was weighed (
y), placed on the samples during thawing, and then filter paper with absorbed fluid was weighed again (
x). The weight difference of filter paper was expressed as the weight of the absorbed exudate. Drip loss was quantified as a percentage following this formula:
2.4. Roasting Treatment
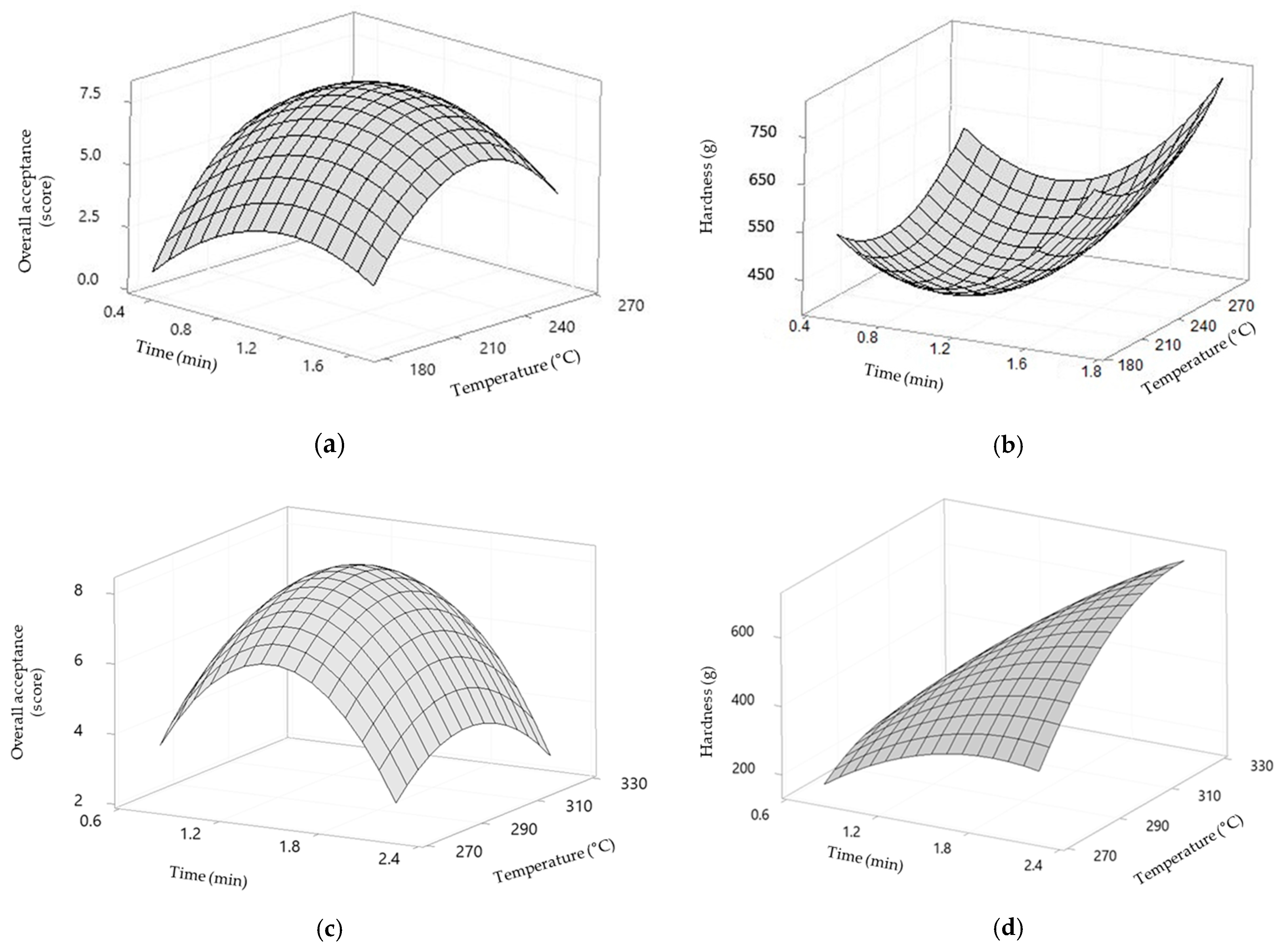
Thawed AMPS and CSM were cut into approximately 3.5 × 0.5 cm and 1 × 5 cm pieces, respectively. The inedible part of the CSM was removed, and the samples were then washed with tap water. Next, all samples were roasted using a superheated steam in an Aero Steam Oven (DFC-560A-2R/L, Naomoto Co., Osaka, Japan) at different temperatures and roasting times. For AMPS, the temperatures used were 192, 200, 220, 240, and 248 °C for 0.53, 0.67, 1.00, 1.33, and 1.47 min, respectively, while for CSM, the temperatures used were 272, 280, 300, 320, and 328 °C for 0.79, 1.00, 1.50, 2.00, and 2.21 min, respectively.
2.5. Sample HMR Product Preparation
First, the stock of sauce was prepared by mixing the ingredients shown in
Table 1. A test seafood HMR product was then produced by mixing roasted AMPS, roasted CSM, and sauce at ratios (
w/
w) of 27.5, 27.5, and 45.0%, respectively. Afterward, 180 g of product was packaged in a polypropylene plastic bowl (New Ecopack Co. Ltd., Jeonju, Korea) and sealed with a plastic film using a tray sealing machine (TPS-TS3T, TPS Co. Ltd., Kyungki-do, Korea) at 180 °C for 5 s. Next, packaged products were frozen using a quick freezer (QF-700, Alpha Tech Co. Ltd., Incheon, Korea) at −35 °C for 10 min, and then stored at −13, −18, and −23 °C in a deep freezer (DF35035, IlShin BioBase Co. Ltd., Dongducheon, Korea) for shelf life estimation.
2.6. pH Measurement
Two grams of product were added to 3rd-distilled water at a ratio of 1:9 (w/v), and then homogenized using a homogenizer (SHG-15D, SciLab Co. Ltd., Seoul, Korea). Next, the pH of each sample was measured using a pH meter (ST 3100, Ohaus Co., Parsippany, NJ, USA).
2.7. Measurement of Volatile Basic Nitrogen (VBN)
VBN was performed using the Conway micro diffusion method. Five grams of product were diluted with 25 mL of 3rd-distilled water and homogenized using a vortex for 5 min. Filter paper No. 2 (55 mm; Advantech, Tokyo, Japan) was used to filter the mixture sample. Following filtration, potassium carbonate was added to the filtrate solution at a ratio of 1:1 (v/v) in the outer chamber, while 1 mL of 0.01 M H2SO4 was added to the inner chamber of the Conway unit. Conway cells were incubated at 37 °C for 90 min. Brunswick reagent (2–3 drops) was added to the inner chamber and titrated with 0.01 N NaOH.
2.8. Measurement of Thiobarbituric Acid-Reactive Substances (TBARS)
TBARS were measured following the method by Peiretti et al. [
16] with modifications. Briefly, 5 g of each sample was homogenized (SHG-15D, SciLab Co. Ltd., Seoul, Korea) in 12.5 mL of TCA solution containing 20% trichloroacetic acid in 2 M phosphoric acid and adjusted to 25 mL with 3rd-distilled water. Next, homogenate samples were centrifuged at 1500 rpm for 10 min, and the upper layer was collected and filtered using filter paper No. 2 (55 mm; Advantech, Tokyo, Japan). The supernatant was then mixed with a 0.005 M thiobarbituric acid solution at a ratio of 1:1 (
v/
v) and incubated at 95 °C for 30 min in a water bath (JSWB-22TL, JS Research Inc., Gongju City, Korea). The samples were then left to cool to room temperature. Samples (200 μL) and the blank group (3rd-distilled water) were placed on a 96-well plate and measured at 530 nm using a SPECTROstar Nano Microplate Reader (S/N601-0618, BMG Labtech Ltd., Ortenberg, Germany). Malondialdehyde (MDA) bis (dimethyl acetal) was used as the standard.
2.9. Proximate Analysis
Proximate analysis was performed using AOAC standard methods. Moisture was measured following the methods AOAC 952.08 [
17] using an oven at 105 °C for 24 h. Ash was determined following the AOAC 938.08 method [
18] in a furnace at 550 °C. Sodium was measured following AOAC 971.27 method [
19]. Crude protein was determined following AOAC 960.48 method [
20]. N content from the test product was then multiplied with 6.26 to obtain the value of crude protein. Calories, carbohydrates, sugars, dietary fiber, crude fat content, cholesterol, vitamin D, potassium iron, and calcium were measured following AOAC 971.10 [
21], AOAC 998.18 [
22], AOAC 985.29 [
23], AOAC 948.15 [
24], AOAC 994.10 [
25], AOAC 936.14 [
26], AOAC 2011.11 [
27], AOAC 990.05 [
28], and AOAC 984.27 [
29], respectively.
2.10. Amino Acid Analysis
Prior to hydrolysis, the protein was extracted following the Kjeldahl method, and the amino acid analysis was performed using the AOAC 994.12b method [
30]. Briefly, a 50 μL aliquot of a solution containing a mixture of 91 μg/mL L-amino acids in 0.1 N HCl was dried. Subsequently, 100 μL of neat MTBSTFA, followed by 100 μL of acetonitrile, were added. The mixture was then heated to 100 °C for 4 h. Next, the sample was neutralized with sodium bicarbonate and subjected to gas chromatography-mass spectrometry (GC-MS) analysis using the GCMS-QP2020 system (Shimadzu Corp., Kyoto, Japan). An injection volume of 0.5 µL/min was used for the separations performed on an SLB™-5ms Capillary GC Column (20 m × 0.18 mm I.D., 0.18 μm; Sigma-Aldrich, Inc., St. Louis, MO, USA) using helium as the carrier gas. During the separation, the oven temperature was programmed as follows—initially, it was set at 100 °C for 1 min, then increased to 290 °C at 35 °C/min and held for 3 min, and finally, it was raised to 360 °C at a rate of 40 °C/min and held for 2 min. The temperature for the inlet was set at 250 °C, while the temperature for the mass storage device (MSD) interface was set at 325 °C.
2.11. Fatty Acid Composition Analysis
The fatty acid composition analysis was performed following a hydrolytic method described by Sutikno et al. [
11]. Briefly, ether was used to extract fatty acids and methylate them into fatty acid methyl esters (FAMEs). FAMEs were then analyzed using gas chromatography (GCMS-QP2020) fitted with a DB-wax capillary column (30 m × 0.25 mm i.d., 0.25 mm film thickness, Agilent). Helium at a constant linear velocity of 30 cm/s was used as the carrier gas. The split ratio was set at 1/10. During the separation, the column oven was programmed as follows: initial column oven temperature was set at 100 °C; held for 1 min, and increased to at 25 °C/min to 100 °C and after 1 min, and finally, it was raised to 240 °C at 5 °C/min; held for 2 min. The temperature for the inlet was set at 250 °C, while the temperature for Flame Ionization Detector (FID) was set at 270 °C. FAME standard mixture (EN 14078, Paragon Scientific Ltd., Wirral, UK) was used to identify the peak and calculate the response factor. The results were expressed as g/100 g of dry matter.
2.12. Total Bacterial Count (TBC) and Total Coliform Count
TBC was determined according to the instructions of Chen et al. [
31]. The sample was mixed with sterile saline at a ratio of 1:9 (
w/
v) in sterilized bags, and homogenized using a Stomacher 400 Circulator (Seward Ltd., West Sussex, UK) for 3 min. Three serial dilutions of an aliquot of the homogenate were plated onto a specific medium. For TBC, Difco plate count agar (BD Co., Franklin Lakes, NJ, USA) was used and incubated at 37 ± 1 °C for 48 h. For the coliform count, EC medium (BD Co., Franklin Lakes, NJ, USA) was used and incubated at 37 ± 1 °C for 24 h; if no gas was observed, results were recorded as negative (-). For
Salmonella sp. and
Staphylococcus sp. counts, Sanita-Kun plates (JNC Corp., Tokyo, Japan) were used and incubated at 35 ± 1 °C for 48 h.
2.13. Texture Analysis
A texture analyzer (CT3 4500, Brookfield Engineering Laboratories Inc., Middleboro, MA, USA) operated by TexturePRO CT software (Middleboro, MA, USA) was used to measure hardness. The texture analysis was performed by compressing the sample to 50% of its height using a cylinder glass probe (12.7 mm in diameter and 35 mm in length). The test speed was 0.5 mm/s. The textural analysis was performed at room temperature with triplicate measurements of each sample.
2.14. Sensory Evaluation
Sensory analysis, including color, flavor, aroma, texture, and overall acceptability, were evaluated. Test seafood HMR was reheated using a microwave (RE-M50, Samsung Electronics Co. Ltd., Seoul, Korea) for 1 min 30 s (700 W). Twenty-one trained and certified sensory evaluation panelists (aged 25–40 years) were employed in this test—all of them were researchers at the Industry Academic Cooperation Foundation, Silla University (Busan, Korea). The sensory test was approved by the Silla University Institutional Review Board (Approval No. 1041449-202006-HR-007; 4 October 2020). Each panelist was asked to evaluate and numerically rate all samples. A hedonic scale of 1 to 9 points was used—1 indicating ‘remarkably dislike’ and 9 indicating ‘extreme like.’ Five was considered the threshold value; any sample less than a score of 5 was considered unacceptable [
32].
2.15. Shelf Life Estimation
The expiration date was established following the Ministry of Food and Drug Safety guidelines, the Republic of Korea. The accelerated experiment to define the expiration date and evaluate the shelf life qualities of the test-HMR products was conducted for 90 days. Test HMRs were stored at −18 °C (distribution temperature) as a control, −13 °C, and −23 °C. Samples were collected seven times over 90 days, and microbiological and physical tests were performed. The number of common bacteria, such as
E. coli, Staphylococcus aureus, and
Salmonella spp., and the sensory evaluation scores were used as indicators of quality shelf life. Program simulation (
https://www.foodsafetykorea.go.kr, accessed on 17 December 2020) was used to estimate the product’s shelf life.
2.16. Statistical Analysis
All experiments were performed in triplicate (n = 3); the data are displayed as mean ± standard deviation (SD). Drip loss, TBC, and sensory properties were analyzed via a one-way analysis of variance (ANOVA) at a 95% level of probability (p < 0.05) using IBM SPSS v 23.0 (IBM, Corp., Armonk, NY, USA) software. Response surface methodology (RSM) was analyzed using Minitab v 14.0 (Minitab Inc., Birmingham, UK). Temperature and heating time were set as independent variables, while overall acceptance and hardness were set as dependent variables.