The Effect of Biogas Origin on the Electricity Production by Solid Oxide Fuel Cells
Abstract
:Featured Application
Abstract
1. Introduction
2. Theory
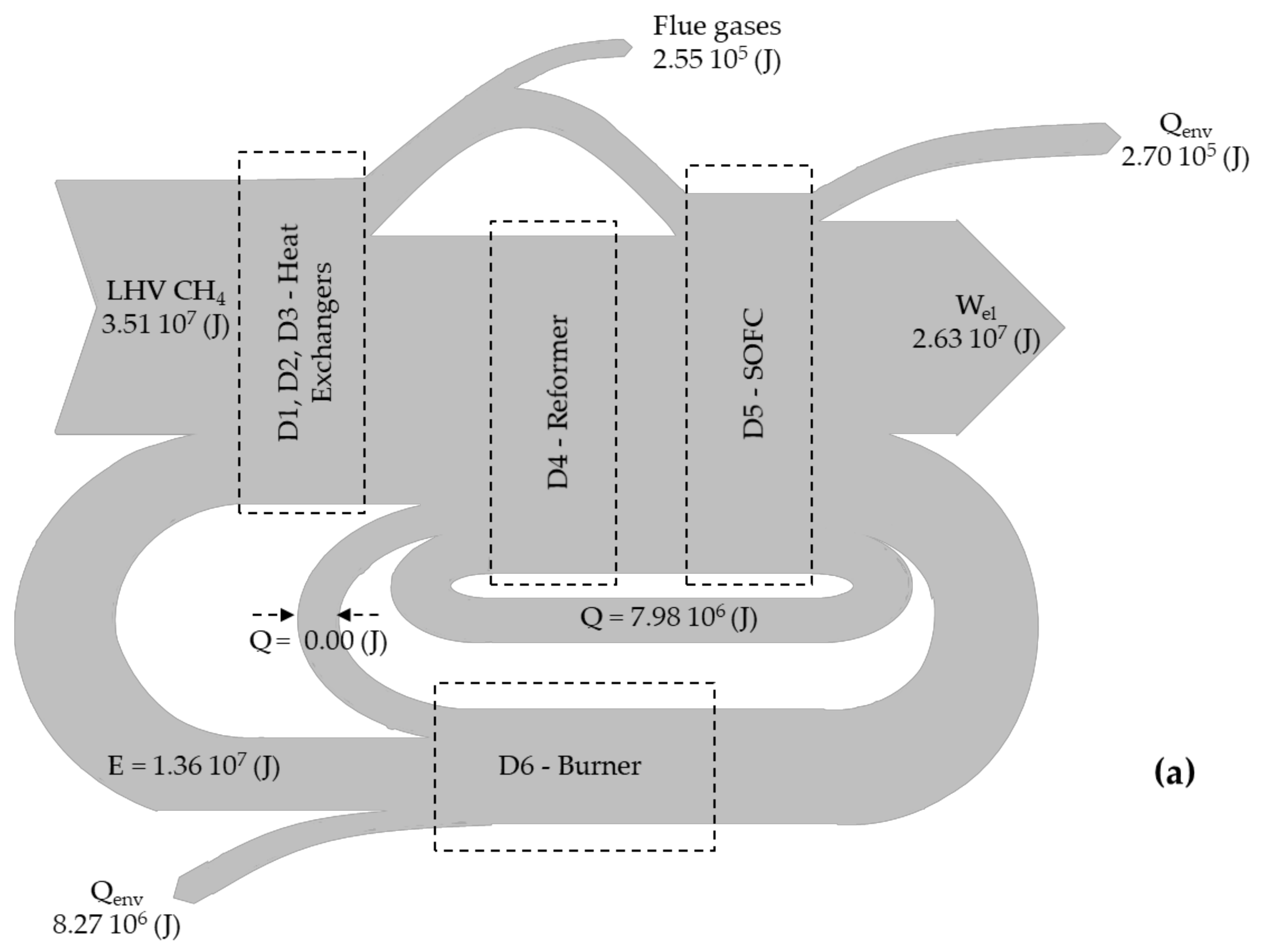
- Energy balance for biogas reformer (see in Figure 1, device D4) [15]:where and are the inlet and outlet temperatures (in K) at each branch of the reformer, is the thermal energy (in J) that is transferred from the burner to the reformer, while and are the enthalpies (in J mol−1) for the water gas shift and the methane reforming reaction, respectively.
- Exergy balance for heat exchangers (see in Figure 1, devices D1, D2 and D3) [16]:where is the total mass (in mol), is the molar fraction of species i, is the temperature difference (in K), is the mean isobaric exergy capacity (in J kmol−1 K−1) of species i, and I is the irreversibility rate (in J).
- Exergy balance for biogas reformer (see in Figure 1, device D4) [16]:where is the exergy (in J) of species i at the state of the environment, is the universal gas constant (= 8.1344 J mol−1 K−1), is the temperature (in K) at the state of the environment, and is the operational temperature (in K) of the SOFC.
- Energy efficiency is calculated through the expression [12]:where is the energetic efficiency, is the electric energy (in J) produced by the SOFC, is the mass of biogas (in mol) entering the system (see Figure 1, path 2), and LHV is the lower heating value of the inlet fuel (= 802.34 kJ mol−1) measured at 298 K.
- Exergy efficiency for each simulated scenario is calculated through the expression:where is the exergetic efficiency, , and are the exergies (in J) of the biogas, the atmospheric air and the water in stream 2, 1 and 3 in Figure 1, respectively.
3. Simulation
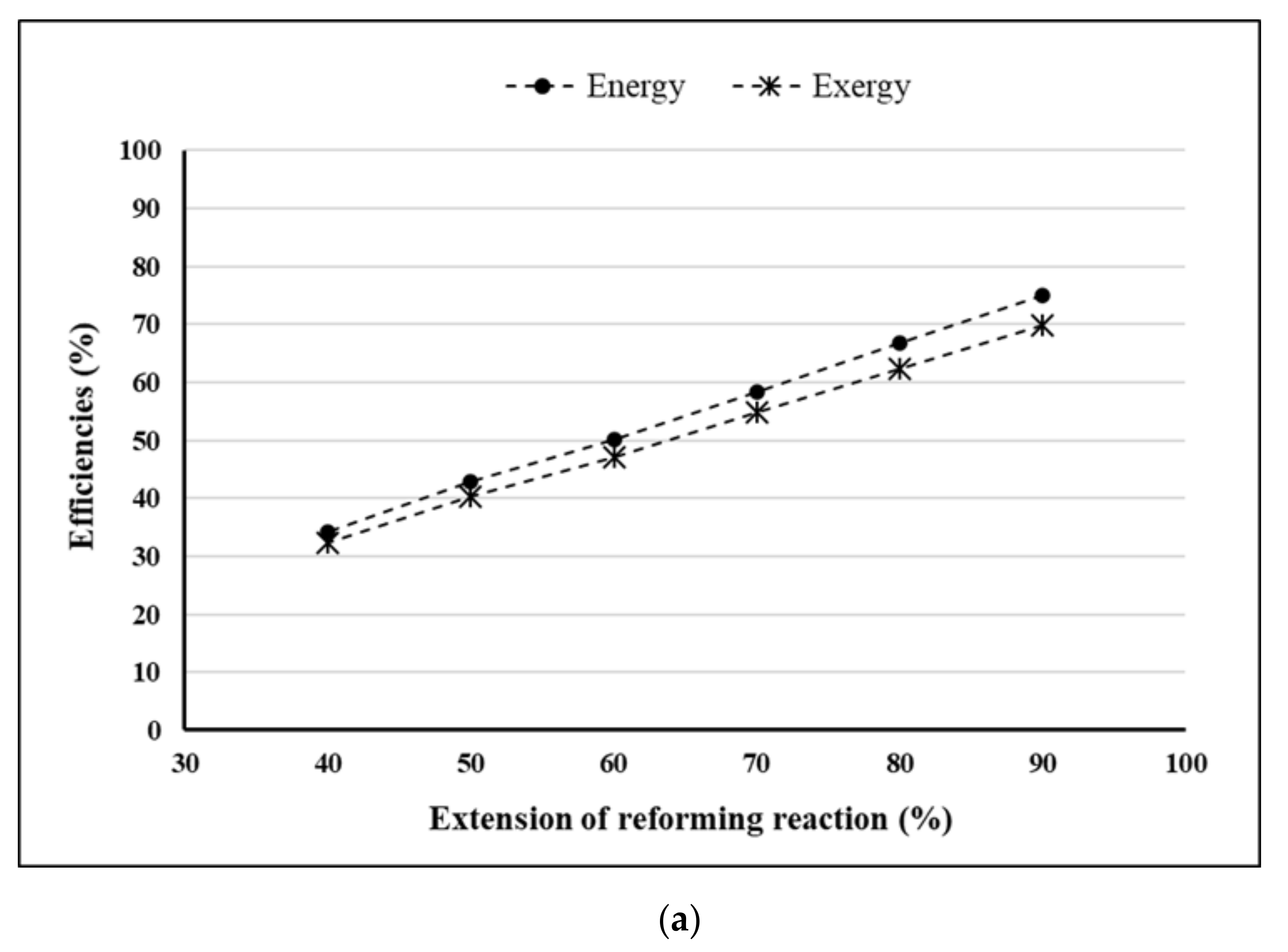
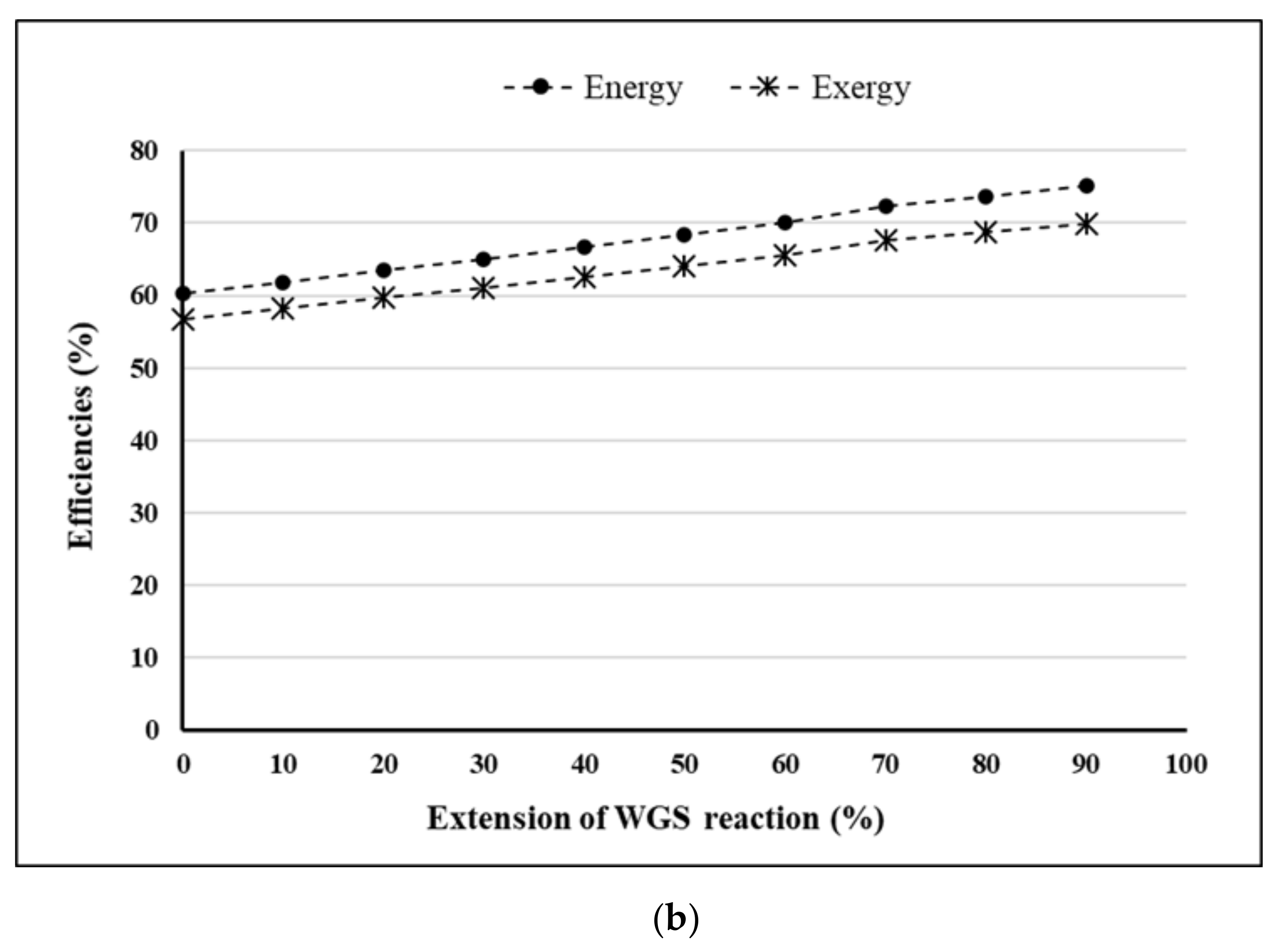
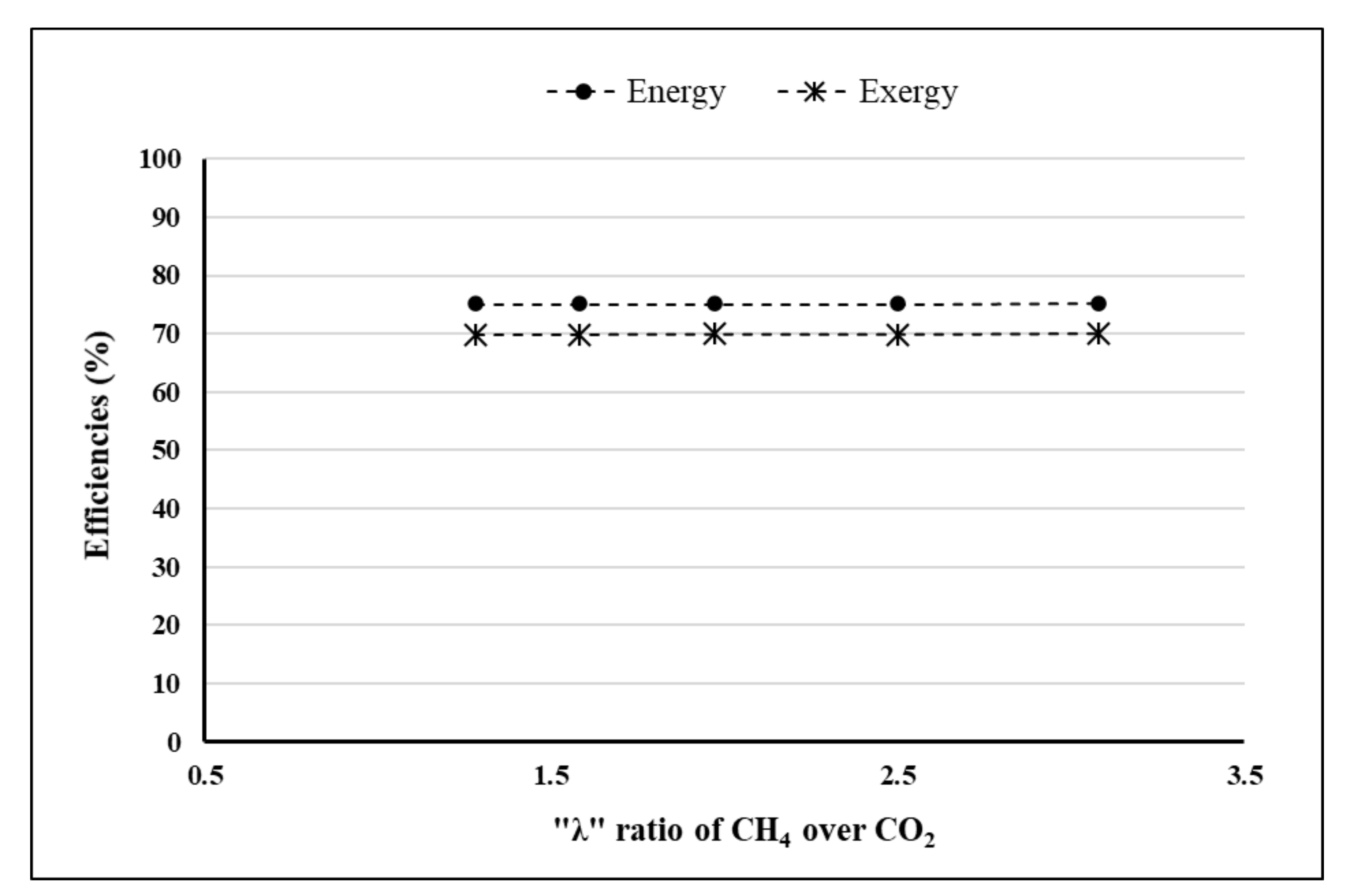
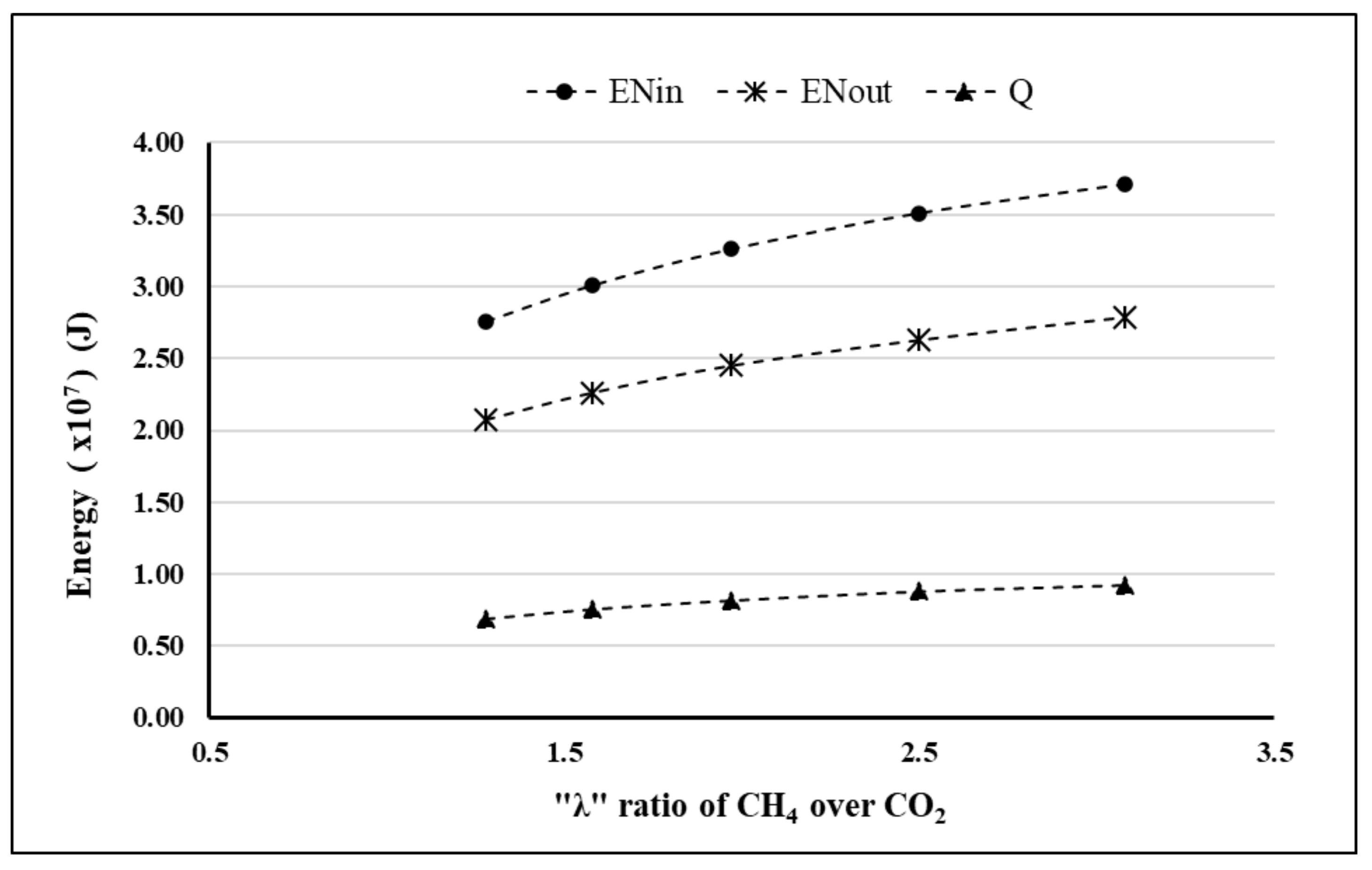
4. Results and Discussion
- λ = 2.5.
- Pressure = 1 Atm (constant).
- Air excess at inlet = 26.80% over the stoichiometric requirement.
- Water excess at inlet = 6.93% over the stoichiometric requirement.
- Extension of reforming reaction = 90%.
- Extension of WGS reaction = 90%.
- Extension of electrochemical oxidization of H2 = 90%.
- Extension of CO reaction in the SOFC = 15%.
- Temperature of burner = 1043 K.
- Temperature of flue gases = 313.50 K.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| Latin symbols | |
| CP | Molar Isobaric Specific Heat Capacity (J mol−1 K−1) |
| Mean Isobaric Exergy Capacity (J kmol−1 K−1) | |
| Ex | Exergy (J) |
| H | Enthalpy (J) |
| I | Irreversibility (J) |
| IQ | Irreversibility due to heat losses (J) |
| LHV | Lower Heating Value, 802.34 (kJ mol−1) |
| M | Mass (mol) |
| n | Efficiency (%) |
| Q | Thermal Energy (J) |
| R | Gas constant, 8.1344 (J mol−1 K−1) |
| S | Entropy (J) |
| T | Temperature (K) |
| W | Work (J) |
| x | Molar fraction |
| Greek symbols | |
| ΔH | Enthalpy of a reaction (J mol−1) |
| ΔT | Temperature difference (K) |
| ε | Exergy (J) |
| Subscripts | |
| 0 | Property at the state of the environment |
| 3 | Property at path 3 of Figure 1 |
| bio | Biogas |
| burn | Burner |
| burn env | From burner to environment |
| burn ref | From burner to reformer |
| el | Electric |
| en | Energy |
| ex | Exergy |
| i | Index for chemical species |
| in | Input |
| l | Streams throughout a device |
| out | Output |
| prod | Products (chemical elements) |
| r | Chemical reaction |
| react | Reactants (chemical elements) |
| SOFC | Solid Oxide Fuel Cell |
| tot | Total amount of components |
References
- Brouzgou, A.; Demin, A.; Tsiakaras, P. Interconnects for Solid Oxide Fuel Cells. In Advances in Medium and High Temperature Solid Oxide Fuel Cell Technology; Boaro, M., Salvatore, A., Eds.; CISM International Centre for Mechanical Sciences (Courses and Lectures); Springer: Cham, Switzerland, 2017; Volume 574. [Google Scholar] [CrossRef]
- Amiri, A.; Tang, S.; Steinberger-Wilckens, R.; Tadé, M.O. Evaluation of fuel diversity in Solid Oxide Fuel Cell system. Int. J. Hydrog. Energy 2018, 43, 23475–23487. [Google Scholar] [CrossRef] [Green Version]
- Salman, C.A.; Schwede, S.; Thorin, E.; Yan, J. Enhancing biomethane production by integrating pyrolysis and anaerobic digestion processes. Appl. Energy 2017, 204, 1074–1083. [Google Scholar] [CrossRef]
- Hubner, T.; Mumme, J. Integration of pyrolysis and anaerobic digestion—Use of aqueous liquor from digestate pyrolysis for biogas production. Bioresour. Technol. 2015, 183, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, R.; Lombardi, L.; Tribioli, L. Use of biogas from biowaste in a solid oxide fuel cell stack: Application to an off-grid power plant. Renew. Energy 2017, 111, 781–791. [Google Scholar] [CrossRef]
- Radenahmad, N.; Azad, A.T.; Saghir, M.; Taweekun, J.; Abu Bakar, M.S.; Reza, M.R.; Azad, A.K. A review on biomass derived syngas for SOFC based combined heat and power application. Renew. Sustain. Energy Rev. 2020, 119, 109560. [Google Scholar] [CrossRef]
- Van herle, J.; Membrez, Y.; Bucheli, O. Biogas as a fuel source for SOFC co-generators. J. Power Sources 2004, 127, 300–312. [Google Scholar] [CrossRef]
- Akkaya, A.V.; Erdem, H.H.; Sahin, B. An analysis of SOFC/GT CHP system based on exergetic performance criteria. Int. J. Hydrog. Energy 2008, 33, 2566–2577. [Google Scholar] [CrossRef]
- Tang, S.; Amiri, A.; Tadé, M.O. System Level Exergy Assessment of Strategies Deployed for Solid Oxide Fuel Cell Stack Temperature Regulation and Thermal Gradient Reduction. Ind. Eng. Chem. Res. 2019, 58, 2258–2267. [Google Scholar] [CrossRef]
- Athanasiou, C.; Vakouftsi, E.; Coutelieris, F.A.; Marnellos, G.; Zabaniotou, A. Efficiencies of olive kernel gasification combined cycle with solid oxide fuel cells (SOFCs). Chem. Eng. J. 2009, 149, 183–190. [Google Scholar] [CrossRef]
- Prodromidis, G.N.; Coutelieris, F.A. Thermodynamic analysis of biogas fed solid oxide fuel cell power plants. Renew. Energy 2017, 108, 1–10. [Google Scholar] [CrossRef]
- Prodromidis, G.N.; Coutelieris, F.A. Solid Oxide Fuel Cell systems for electricity generation: An optimization prospect. Renew. Energy 2020, 146, 38–43. [Google Scholar] [CrossRef]
- Szargut, J. Chemical Exergies of the Elements. Appl. Energy 1989, 32, 269–285. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Bejan, A.; Tsatsaronis, G.; Moran, M. Thermal Design and Optimization, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Kotas, T.J. The Exergy Method of Thermal Plant Analysis; Krieger: Malabar, FL, USA, 1995. [Google Scholar]
- Vita, A.; Italiano, C.; Fabiano, C.; Lagana, M.; Pino, L. Biogas as renewable raw material for syngas production by tri-reforming process over NiCeO2 catalysts: Optimal operative condition and effect of nickel content. Fuel Process Technol. 2014, 127, 47–58. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Katsoni, A.; Diamantopoulos, E.; Mantzavinos, D.; Delimitris, A. Dry reforming of methane: Catalytic performance and stability of Ir catalysts supported on γ-Al2O3, Zr0.92Y0.08O2–δ (YSZ) or Ce0.9Gd0.1O2–d (GDC) supports. Top. Catal. 2015, 58, 1228–1241. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Zhang, Y.; Xu, Y.; Shang, S.; Yin, Y. Ni-Co catalyst derived from layered double hydroxides for dry reforming of methane. Int. J. Hydrog. Energy 2015, 40, 16115–16126. [Google Scholar] [CrossRef]
- Broun, B.; Sattler, M. A comparison of greenhouse gas emissions and potential electricity recovery from conventional and bioreactor landfills. J. Clean. Prod. 2016, 112, 2664–2673. [Google Scholar] [CrossRef]
- Del Valle-Zermeno, R.; Romero-Guiza, M.S.; Chimenos, J.M.; Formosa, J.; Mata-Alvarez, J.; Astals, S. Biogas upgrading using MSWI bottom ash: An integrated municipal solid waste management. Renew. Energy 2015, 80, 184–189. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Fabiano, C.; Lagana, M.; Pino, L. Influence of Ce-precursor and fuel on structure and catalytic activity of combustion synthesized Ni/CeO2 catalysts for biogas oxidative steam reforming. Mater. Chem. Phys. 2015, 163, 337–347. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Product. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. iScience 2020, 23, 101082. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Tzounis, L.; Sebastian, V.; Stolojan, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. The relationship between reaction temperature and carbon deposition on nickel catalysts based on Al2O3, ZrO2 or SiO2 supports during the biogas dry reforming reaction. Catalysts 2019, 9, 676. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.S.; Song, J.; Kim, K.; Park, C.S.; Raju, A.S.K. CO2 conversion to syngas through the steam-biogas reforming process. J. CO2 Util. 2018, 25, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Walker, D.M.; Pettit, S.L.; Wolan, J.T.; Kuhn, J.N. Synthesis gas production to desired hydrogen to carbon monoxide ratios by tri-reforming of methane using Ni-MgO-(Ce, Zr) O2 catalysts. Appl. Catal. A Gen. 2012, 445–446, 61–68. [Google Scholar] [CrossRef]
- Elsayed, N.H.; Maiti, D.; Joseph, B.; Kuhn, J.N. Precious metal doped Ni-Mg/ceria-zirconia catalysts for methane conversion to syngas by low temperature bi-reforming. Catal. Lett. 2018, 148, 1003–1013. [Google Scholar] [CrossRef]
- Rahmat, N.; Yaakob, Z.; Rahman, N.A.; Jahaya, S.S. Renewable hydrogen-rich syngas from CO2 reforming of CH4 with steam over Ni/MgAl2O4 and its process optimization. Int. J. Environ. Sci. Technol. 2019, 17, 843–856. [Google Scholar] [CrossRef]
- Vakouftsi, E.; Marnellos, G.; Athanassiou, C.; Coutelieris, F.A. CFD modeling of a biogas fuelled SOFC. Solid State Ion. 2011, 192, 458–463. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Szargut, J.; Kolenda, Z.; Taramin, K. Co-ordination of material and energy balances as a basis of combustion process optimization. Int. J. Energ. Res. 1981, 5, 253–261. [Google Scholar] [CrossRef]



| Sources of Biogas | CH4 Content (%) | CO2 Content (%) | H2O, N2, O2, H2, H2S (%) |
|---|---|---|---|
| Organic raw materials | 45–75 | 25–55 | Mainly H2O: 2–7%, while the other impurities appear at less than 2%, analogous to primary source. |
| Anaerobic digestion of waste materials | 45–70 | 30–55 | |
| Urban organic waste | 40–70 | 30–60 | |
| Solid waste in landfill | 40–60 | 40–60 | |
| Organic fraction of municipal waste | 55–70 | 30–45 | |
| Lignocellulosic biomasses and sewage sludge | 55–70 | 30–45 |
| Branch | Elements | Temperature (K) | Mass Flux (Kg/s) | Enthalpy (×106 J) |
|---|---|---|---|---|
| 1 | Atmospheric Air (O2 and N2) | 298.00 | 11.55 | +0.0044 |
| 2 | Biogas (CH4, CO2 and H2O) | 298.00 | 1.00 | −6.0400 |
| 3 | Water (H2O) | 298.00 | 1.44 | −21.0000 |
| 4 | Biogas (CH4, CO2 and H2O) | 1100.00 | 1.00 | −3.7600 |
| 5 | CH4, CO2, H2O, H2, CO | 1120.00 | 2.44 | −12.7000 |
| 6 | Steam (H2O) | 1100.00 | 1.44 | −16.9000 |
| 7 | CH4, CO2, H2O, H2, CO, O2 and N2 | 1025.00 | 13.94 | −38.6000 |
| 8 | CH4, CO2, H2O, H2, CO, O2 and N2 | 1043.50 | 13.94 | −46.9000 |
| 9 | CH4, CO2, H2O, H2, CO, O2 and N2 | 921.50 | 13.94 | −49.2750 |
| 10 | CH4, CO2, H2O, H2, CO, O2 and N2 | 803.00 | 13.94 | −51.5490 |
| 11 | CH4, CO2, H2O, H2, CO, O2 and N2 | 313.50 | 13.94 | −60.2390 |
| 12 | Atmospheric Air (O2 and N2) | 1000.00 | 13.94 | +8.6900 |
| Energy efficiency (%) | 74.98 | |||
| Exergy efficiency (%) | 69.90 | |||
| Total Thermal Losses to the environment (×106 J) | 8.80 | |||
| Thermal Losses to the environment through devices (% of energy entering the system) | 24.33 | |||
| Thermal Losses to the environment through flue gases, see Branch 11, Figure 1 (% of energy entering the system) | 0.73 | |||
| Energy efficiency (%) | 76.20 |
| Exergy efficiency (%) | 71.16 |
| Thermal Losses to the environment (% of energy entering the system) | 20.77 |
| Energy of flue gases, at T11, emitted to the environment (% of energy entering the system) | 3.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prodromidis, G.N.; Coutelieris, F.A. The Effect of Biogas Origin on the Electricity Production by Solid Oxide Fuel Cells. Appl. Sci. 2021, 11, 3112. https://doi.org/10.3390/app11073112
Prodromidis GN, Coutelieris FA. The Effect of Biogas Origin on the Electricity Production by Solid Oxide Fuel Cells. Applied Sciences. 2021; 11(7):3112. https://doi.org/10.3390/app11073112
Chicago/Turabian StyleProdromidis, George N., and Frank A. Coutelieris. 2021. "The Effect of Biogas Origin on the Electricity Production by Solid Oxide Fuel Cells" Applied Sciences 11, no. 7: 3112. https://doi.org/10.3390/app11073112






