Adhesion of Flowable Resin Composites in Simulated Wedge-Shaped Cervical Lesions: An In Vitro Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Resin-Based Restorative Materials and Adhesive Systems
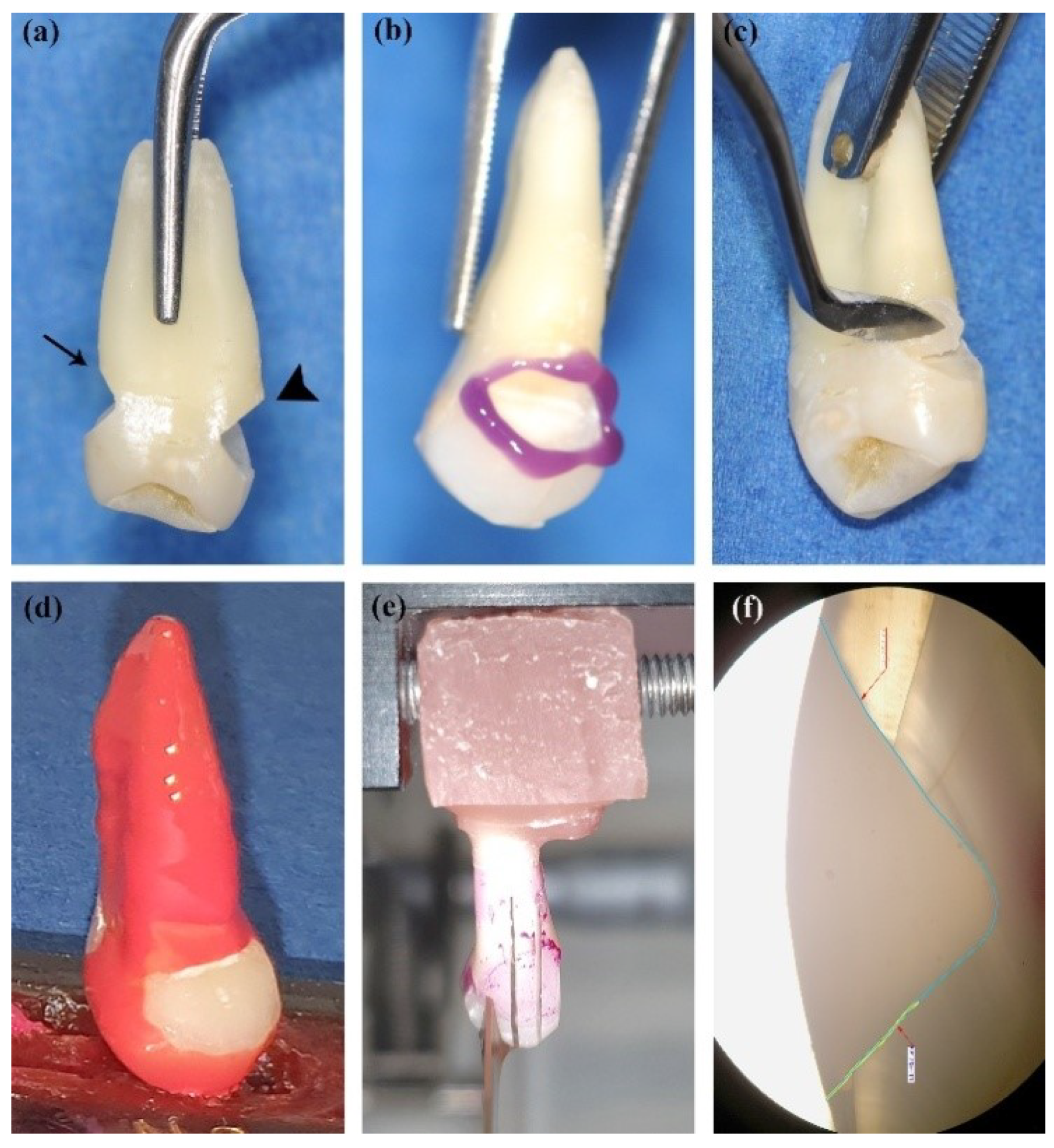
2.3. Cavity Restoration and Sample Preparation
2.4. Microleakage Test
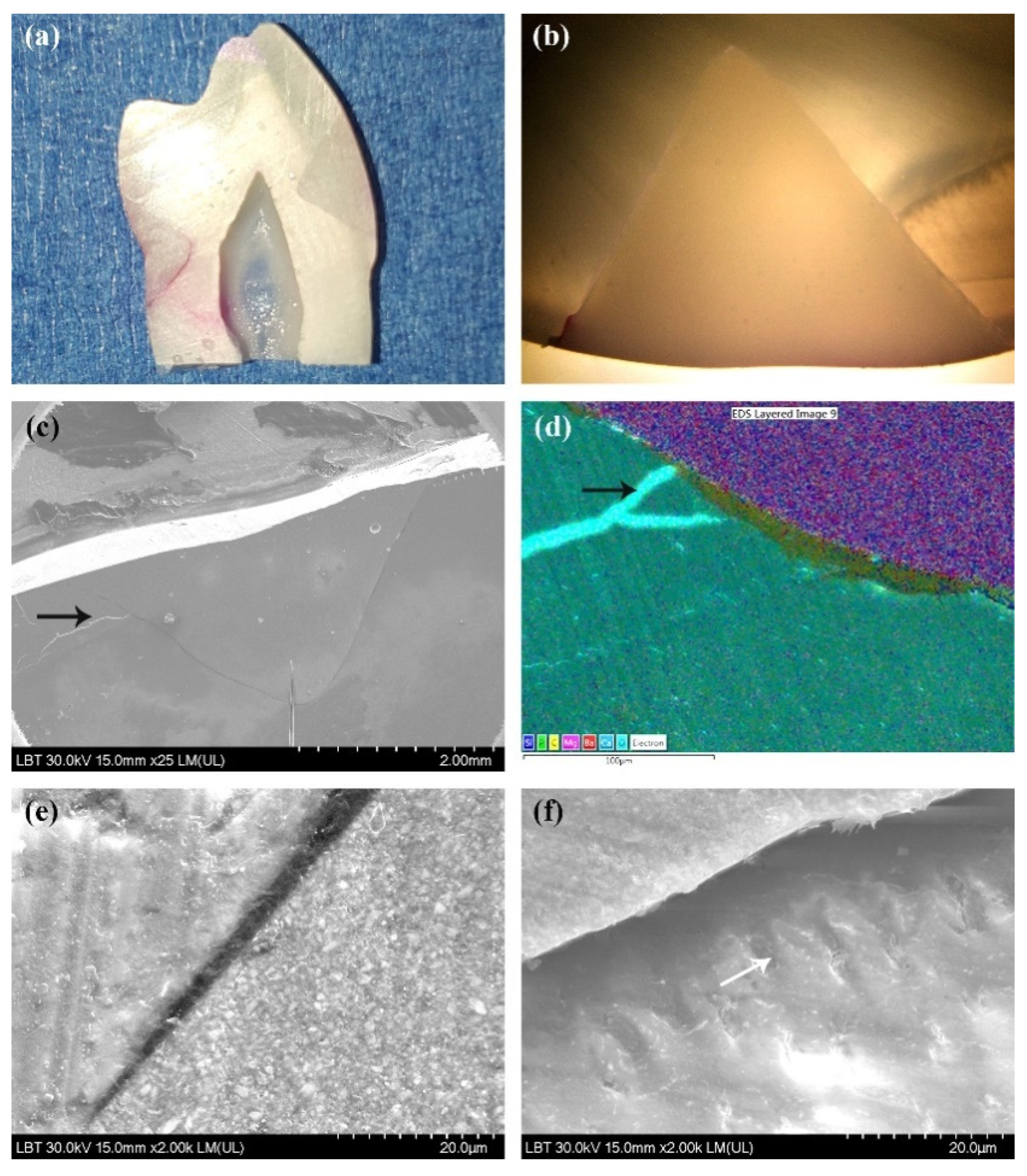
2.5. Scanning Electron Microscope (SEM) and Energy-Dispersive X-ray (EDX) Analyses
2.6. Data Analysis
3. Results
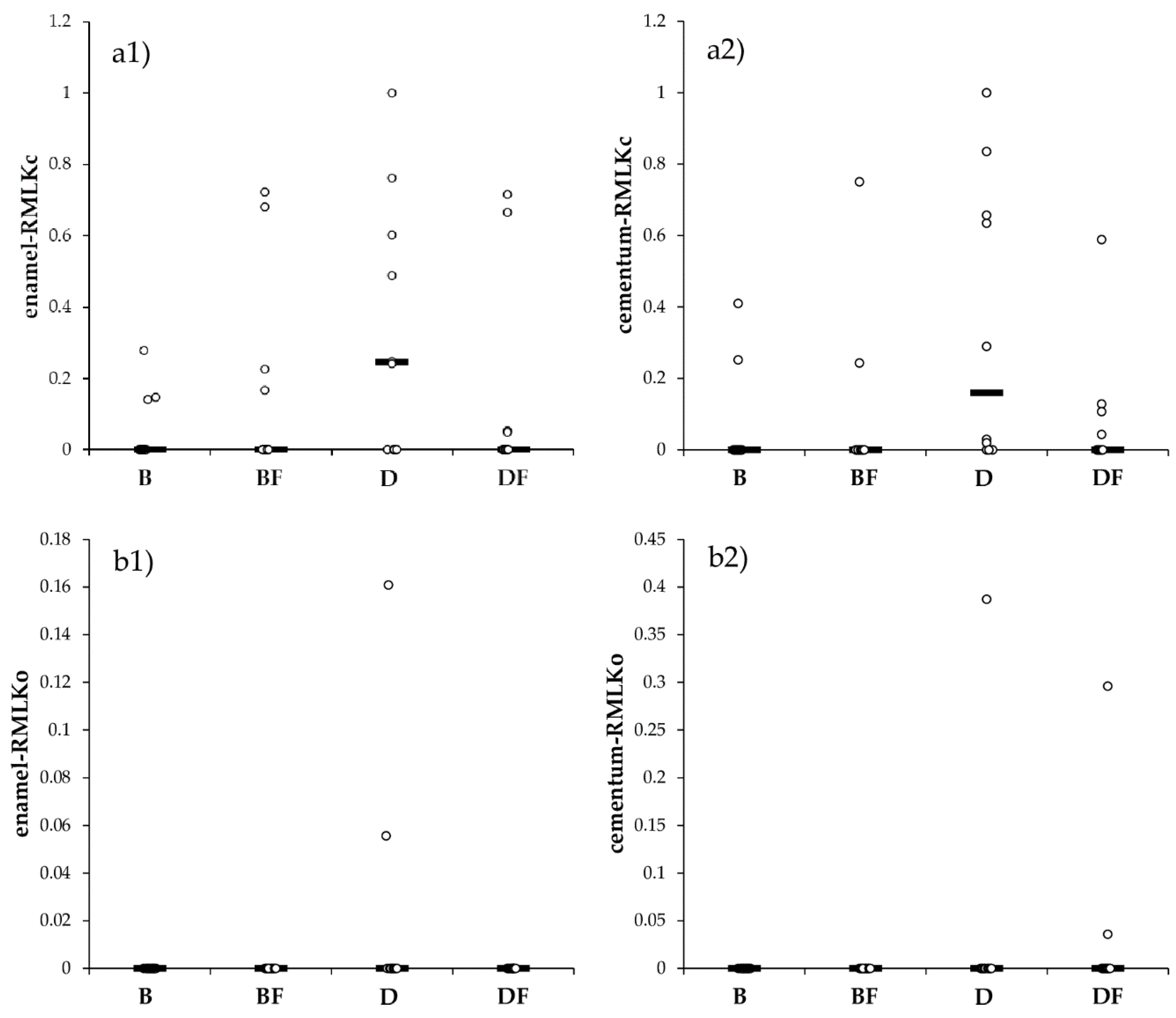
3.1. Microleakage Test
3.2. Scanning Electron Microscopy Results
3.3. Energy-Dispersive X-ray Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peumans, M.; Politano, G. Treatment of Noncarious Cervical Lesions: When, Why, and How. Clin. Res. 2020, 15, 28. [Google Scholar]
- Grippo, J.O. Noncarious Cervical Lesions: The Decision to Ignore or Restore. J. Esthet. Dent. 1992, 4, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.Y.; Zhi, Q.H.; Zhou, Y.; Lin, H.C. Prevalence of Non-Carious Cervical Lesions and Associated Risk Indicators in Middle-Aged and Elderly Populations in Southern China. Chin. J. Dent. Res. Off. J. Sci. Sect. Chin. Stomatol. Assoc. CSA 2015, 18, 41–50. [Google Scholar]
- Perez, C.D.R.; Gonzalez, M.R.; Prado, N.A.S.; de Miranda, M.S.F.; Macêdo, M.D.A.; Fernandes, B.M.P. Restoration of Noncarious Cervical Lesions: When, Why, and How. Int. J. Dent. 2012, 2012, 687058. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.P.; Queiroz, L.A.; Mathias, I.F.; Neves, F.L.D.S.; Silveira, C.A.; Bresciani, E.; Jardini, M.A.N.; Sallum, E.A. Resin Composite plus Connective Tissue Graft to Treat Single Maxillary Gingival Recession Associated with Non-Carious Cervical Lesion: Randomized Clinical Trial. J. Clin. Periodontol. 2016, 43, 461–468. [Google Scholar] [CrossRef]
- Peumans, M.; De Munck, J.; Mine, A.; Van Meerbeek, B. Clinical Effectiveness of Contemporary Adhesives for the Restoration of Non-Carious Cervical Lesions. A Systematic Review. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2014, 30, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Pecie, R.; Krejci, I.; García-Godoy, F.; Bortolotto, T. Noncarious Cervical Lesions (NCCL)—A Clinical Concept Based on the Literature Review. Part 2: Restoration. Am. J. Dent. 2011, 24, 183–192. [Google Scholar]
- Correia, A.M.D.O.; Tribst, J.P.M.; Matos, F.D.S.; Platt, J.A.; Caneppele, T.M.F.; Borges, A.L.S. Polymerization Shrinkage Stresses in Different Restorative Techniques for Non-Carious Cervical Lesions. J. Dent. 2018, 76, 68–74. [Google Scholar] [CrossRef]
- Peumans, M.; De Munck, J.; Van Landuyt, K.L.; Poitevin, A.; Lambrechts, P.; Van Meerbeek, B. A 13-Year Clinical Evaluation of Two Three-Step Etch-and-Rinse Adhesives in Non-Carious Class-V Lesions. Clin. Oral Investig. 2012, 16, 129–137. [Google Scholar] [CrossRef]
- Luque-Martinez, I.V.; Mena-Serrano, A.; Muñoz, M.A.; Hass, V.; Reis, A.; Loguercio, A.D. Effect of Bur Roughness on Bond to Sclerotic Dentin with Self-Etch Adhesive Systems. Oper. Dent. 2013, 38, 39–47. [Google Scholar] [CrossRef]
- Borges, A.; Borges, A.; Xavier, T.; Bottino, M.; Platt, J. Impact of Quantity of Resin, C-Factor, and Geometry on Resin Composite Polymerization Shrinkage Stress in Class V Restorations. Oper. Dent. 2014, 39, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Eliguzeloglu, E.; Eraslan, O.; Omurlu, H.; Eskitascioglu, G.; Belli, S. The Effect of Cavity Shape and Hybrid Layer on the Stress Distribution of Cervical Composite Restorations. Eur. J. Dent. 2011, 5, 180–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Correia, A.; Bresciani, E.; Borges, A.; Pereira, D.; Maia, L.; Caneppele, T. Do Tooth- and Cavity-Related Aspects of Noncarious Cervical Lesions Affect the Retention of Resin Composite Restorations in Adults? A Systematic Review and Meta-Analysis. Oper. Dent. 2020, 45, E124–E140. [Google Scholar] [CrossRef]
- Heintze, S.D.; Blunck, U.; Göhring, T.N.; Rousson, V. Marginal Adaptation in Vitro and Clinical Outcome of Class V Restorations. Dent. Mater. 2009, 25, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Leknes, K.N. The Influence of Anatomic and Iatrogenic Root Surface Characteristics on Bacterial Colonization and Periodontal Destruction: A Review. J. Periodontol. 1997, 68, 507–516. [Google Scholar] [CrossRef]
- Szesz, A.; Parreiras, S.; Martini, E.; Reis, A.; Loguercio, A. Effect of Flowable Composites on the Clinical Performance of Non-Carious Cervical Lesions: A Systematic Review and Meta-Analysis. J. Dent. 2017, 65, 11–21. [Google Scholar] [CrossRef]
- Baroudi, K.; Rodrigues, J.C. Flowable Resin Composites: A Systematic Review and Clinical Considerations. J. Clin. Diagn. Res. JCDR 2015, 9, ZE18–ZE24. [Google Scholar] [CrossRef]
- Labella, R.; Lambrechts, P.; Van Meerbeek, B.; Vanherle, G. Polymerization Shrinkage and Elasticity of Flowable Composites and Filled Adhesives. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1999, 15, 128–137. [Google Scholar] [CrossRef]
- Brochures—Shofu. Available online: https://www.shofu.com/wp-content/uploads/Giomer-BRO-US.pdf (accessed on 17 December 2020).
- Abiodun-Solanke, I.; Ajayi, D.; Arigbede, A. Nanotechnology and Its Application in Dentistry. Ann. Med. Health Sci. Res. 2014, 4 (Suppl. 3), S171–S177. [Google Scholar] [CrossRef]
- Peumans, M.; De Munck, J.; Van Landuyt, K.L.; Kanumilli, P.; Yoshida, Y.; Inoue, S.; Lambrechts, P.; Van Meerbeek, B. Restoring Cervical Lesions with Flexible Composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2007, 23, 749–754. [Google Scholar] [CrossRef]
- Shaalan, O.O.; Abou-Auf, E.; El Zoghby, A.F. Clinical Evaluation of Flowable Resin Composite versus Conventional Resin Composite in Carious and Noncarious Lesions: Systematic Review and Meta-Analysis. J. Conserv. Dent. JCD 2017, 20, 380–385. [Google Scholar] [CrossRef]
- Heintze, S.D. Clinical Relevance of Tests on Bond Strength, Microleakage and Marginal Adaptation. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, 59–84. [Google Scholar] [CrossRef]
- Gayatri, C.; Rambabu, T.; Sajjan, G.; Battina, P.; Priyadarshini, M.S.; Sowjanya, B.L. Evaluation of Marginal Adaptation of a Self-Adhering Flowable Composite Resin Liner: A Scanning Electron Microscopic Study. Contemp. Clin. Dent. 2018, 9 (Suppl. 2), S240–S245. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Mazzoleni, S.; Fantin, F.; Favero, L.; De Francesco, M.; Stellini, E. Evaluation of three different manual techniques of sharpening curettes through a scanning electron microscope: A randomized controlled experimental study. Int. J. Dent. Hyg. 2015, 13, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.; Stratul, S.I.; Rusu, D.; Boariu, M.; Soanca, A.; Balazsi, R.; Suciu, M.; Moldovan, M.; Bulboacă, A.E. Investigations on the Adhesion of New Composites for Restoring Cervical Lesions Using Energy Dispersive X-ray Analysis and Scanning Electron Microscopy. Sci. Rep. 2019, 9, 9853. [Google Scholar] [CrossRef] [PubMed]
- Paradella, T.C.; Koga-Ito, C.Y.; Jorge, A.O.C. Ability of Different Restorative Materials to Prevent in Situ Secondary Caries: Analysis by Polarized Light-Microscopy and Energy-Dispersive X-ray. Eur. J. Oral Sci. 2008, 116, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.P.; Pai, V.S.; Usha, G.; Nadig, R.R. Comparative Evaluation of Smear Layer Removal by Chitosan and Ethylenediaminetetraacetic Acid When Used as Irrigant and Its Effect on Root Dentine: An in Vitro Atomic Force Microscopic and Energy-Dispersive X-Ray Analysis. J. Conserv. Dent. JCD 2017, 20, 245–250. [Google Scholar] [CrossRef]
- Abdolahpur Monikh, F.; Chupani, L.; Vijver, M.G.; Vancová, M.; Peijnenburg, W.J.G.M. Analytical approaches for characterizing and quantifying engineered nanoparticles in biological matrices from an (eco)toxicological perspective: Old challenges, new methods and techniques. Sci Total Environ. 2019, 660, 1283–1293. [Google Scholar] [CrossRef]
- Son, D.; Cho, S.; Nam, J.; Lee, H.; Kim, M. X-ray-Based Spectroscopic Techniques for characterization of Polymer Nanocomposite Materials at a Molecular Level. Polymers 2020, 12, 1053. [Google Scholar] [CrossRef]
- Soanca, A.; Rominu, M.; Moldovan, M.; Bondor, C.; Nicola, C.; Roman, A. Microscopic Evaluation of the Interface between Composite Biomaterials and Dentin Biostructure. Dig. J. Nanomater. Biostructures 2011, 6, 383–392. [Google Scholar]
- Mixson, J.M.; Richards, N.D.; Mitchell, R.J. Effects of Dentin Age and Bonding on Microgap Formation. Am. J. Dent. 1993, 6, 72–76. [Google Scholar]
- Popovici, A.; Nicola, C.; Moldovan, M.; Bondor, C.; Badet, C.; Roman, A.; Baciut, G.; Bran, S. A Microleakage Comparative Study for Indirect Composite Restorations on Posterior Teeth. J. Optoelectron. Adv. Mater. 2009, 11, 490–493. [Google Scholar]
- Zhou, L.; Tan, J.; Hu, B.; Feng, H. Ultrastructural study of sclerotic dentin in non-carious cervical lesions disposed by a total-etching dentin adhesive. Beijing Da Xue Xue Bao 2004, 36, 319–321. [Google Scholar]
- Spencer, P.; Ye, Q.; Song, L.; Parthasarathy, R.; Boone, K.; Misra, A.; Tamerler, C. Threats to Adhesive/Dentin Interfacial Integrity and Next Generation Bio-Enabled Multifunctional Adhesives. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.; Kawano, Y.; Braga, R.R. Contraction Stress Related to Composite Inorganic Content. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010, 26, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Yadav, M. Marginal microleakage properties of active bioactive restorative and nanohybrid composite resin using two different adhesives in non carious cervical lesions—An in vitro study. J. West. Afr. Coll. Surg. 2017, 7, 1–14. [Google Scholar] [PubMed]
- Gladys, S.; Van Meerbeek, B.; Lambrechts, P.; Vanherle, G. Microleakage of Adhesive Restorative Materials. Am. J. Dent. 2001, 14, 170–176. [Google Scholar]
- Jang, K.T.; Chung, D.H.; Shin, D.; García-Godoy, F. Effect of Eccentric Load Cycling on Microleakage of Class V Flowable and Packable Composite Resin Restorations. Oper. Dent. 2001, 26, 603–608. [Google Scholar]
- Fruits, T.J.; VanBrunt, C.L.; Khajotia, S.S.; Duncanson, M.G. Effect of Cyclical Lateral Forces on Microleakage in Cervical Resin Composite Restorations. Quintessence Int. Berl. Ger. 1985 2002, 33, 205–212. [Google Scholar]
- Hussainy, S.N.; Nasim, I.; Thomas, T.; Ranjan, M. Clinical Performance of Resin-Modified Glass Ionomer Cement, Flowable Composite, and Polyacid-Modified Resin Composite in Noncarious Cervical Lesions: One-Year Follow-Up. J. Conserv. Dent. JCD 2018, 21, 510–515. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Mitchem, J.C. Relationship between Composite Contraction Stress and Leakage in Class V Cavities. Am. J. Dent. 2003, 16, 239–243. [Google Scholar]
- Braga, R.R.; Hilton, T.J.; Ferracane, J.L. Contraction Stress of Flowable Composite Materials and Their Efficacy as Stress-Relieving Layers. J. Am. Dent. Assoc. 2003, 134, 721–728. [Google Scholar] [CrossRef]
- Schwendicke, F.; Göstemeyer, G.; Blunck, U.; Paris, S.; Hsu, L.-Y.; Tu, Y.-K. Directly Placed Restorative Materials: Review and Network Meta-Analysis. J. Dent. Res. 2016, 95, 613–622. [Google Scholar] [CrossRef]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A Critical Review of the Durability of Adhesion to Tooth Tissue: Methods and Results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef]
- Van Landuyt, K.; De Munck, J.; Coutinho, E.; Peumans, M.; Lambrechts, P.; Van Meerbeek, B. Bonding to Dentin: Smear Layer and the Process of Hybridization. In Dental Hard Tissues and Bonding; Eliades, G., Watts, D., Eliades, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 89–122. [Google Scholar] [CrossRef]
- Oliveira, S.S.A.; Marshall, S.J.; Hilton, J.F.; Marshall, G.W. Etching Kinetics of a Self-Etching Primer. Biomaterials 2002, 23, 4105–4112. [Google Scholar] [CrossRef]
- Inoue, S.; Vargas, M.A.; Abe, Y.; Yoshida, Y.; Lambrechts, P.; Vanherle, G.; Sano, H.; Van Meerbeek, B. Microtensile Bond Strength of Eleven Contemporary Adhesives to Dentin. J. Adhes. Dent. 2001, 3, 237–245. [Google Scholar]
- Amaireh, A.I.; Al-Jundi, S.H.; Alshraideh, H.A. In Vitro Evaluation of Microleakage in Primary Teeth Restored with Three Adhesive Materials: ACTIVATM, Composite Resin, and Resin-Modified Glass Ionomer. Eur. Arch. Paediatr. Dent. Off. J. Eur. Acad. Paediatr. Dent. 2019, 20, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Crim, G.A.; Mattingly, S.L. Evaluation of Two Methods for Assessing Marginal Leakage. J. Prosthet. Dent. 1981, 45, 160–163. [Google Scholar] [CrossRef]
- Ferrari, M.; Cagidiaco, M.C.; Davidson, C.L. Resistance of Cementum in Class II and V Cavities to Penetration by an Adhesive System. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1997, 13, 157–162. [Google Scholar] [CrossRef]
- Walter, C.; Kress, E.; Götz, H.; Taylor, K.; Willershausen, I.; Zampelis, A. The anatomy of non-carious cervical lesions. The anatomy of non-carious cervical lesions. Clin. Oral Investig. 2014, 18, 139–146. [Google Scholar] [CrossRef]
- Brännström, M.; Nordenvall, K.J.; Malmgren, O. The Effect of Various Pretreatment Methods of the Enamel in Bonding Procedures. Am. J. Orthod. 1978, 74, 522–530. [Google Scholar] [CrossRef]
- Kubo, S.; Yokota, H.; Yokota, H.; Hayashi, Y. Challenges to the Clinical Placement and Evaluation of Adhesively-Bonded, Cervical Composite Restorations. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Palamara, J.E.A.; Palamara, D.; Messer, H.H.; Tyas, M.J. Tooth Morphology and Characteristics of Non-Carious Cervical Lesions. J. Dent. 2006, 34, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Karan, K.; Yao, X.; Xu, C.; Wang, Y. Chemical Profile of the Dentin Substrate in Non-Carious Cervical Lesions. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2009, 25, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Raskin, A.; D’Hoore, W.; Gonthier, S.; Degrange, M.; Déjou, J. Reliability of in Vitro Microleakage Tests: A Literature Review. J. Adhes. Dent. 2001, 3, 295–308. [Google Scholar]
- Watts, D.C. Radiopacity vs. Composition of Some Barium and Strontium Glass Composites. J. Dent. 1987, 15, 38–43. [Google Scholar] [CrossRef]
- Habib, E.; Wang, Y.; Zhu, M.; Zhu, X. Inorganic Fillers for Dental Resin Composites—Present and Future. ACS Biomater. Sci. Eng. 2015, 2. [Google Scholar] [CrossRef]
- Che, B.; Rickman, L.J.; Satterthwaite, J.D. Adhesives for the restoration of non-carious cervical lesions: A systematic review. J. Dent. 2012, 40, 443–452. [Google Scholar] [CrossRef]



| Type of Material | Restorative Material/Abbreviation | Manufacturer | Matrix Monomers | Filler Content | Adhesive System |
|---|---|---|---|---|---|
| Low shrinkage nano-hybrid composite resin | Beautifil II® LS (B) | Shofu Dental Corporation, JAPAN | Bis-GMA TEGDMA UDMA Bis-MPEPP | 83% weight S-PRG filler based on Fluoroboroalumino–silicate glass mean particle size 0.4 μm Polymerization initiator, Pigments and others | BeautiBond (Shofu, Japan) “All-in-One” 7th Generation -pH 2.4 -HEMA-free -Bis-GMA -TEGDMA -Phosphonic and carboxylic acid monomers -Acetone -Distilled water |
| Low flow nano-hybrid composite resin | Beautifil Flow Plus® F03 (BF) | Shofu Dental Corporation, JAPAN | Bis-GMA TEGDMA | 67% weight S-PRG filler based on Fluoroboroalumino–silicate glass Polymerization initiator, pigments and others | |
| Microhybrid composite resin | Dynamic Plus® (D) | President Dental, GERMANY | Bis-GMA TEGDMA | 80% weight Barium aluminosilicate—mean particle size ≤ 1 µm Fumed silica—mean particle size ≤ 0.04 µm | Prebond SE (President Dental, Germany) “All-in-One” 7th Generation -pH 3 -functional MDP monomers -4-META -HEMA -Ethanol -Isomer\Bis-GMA -TEGDMA -Aliphatic UDMA -Photoinitiators, water |
| Microhybrid composite | Dynamic Flow (DF) | President Dental, GERMANY | BIS-GMA UDMA. Bis-EMA TMPTMA | Barium aluminum borosilicate <60% |
| Material | MLKc | MLKo | LAI | RMLKc | RMLKo |
|---|---|---|---|---|---|
| B (n = 10) | |||||
| {Min to Max} | {0 to 431) | {0 to 0) | {1546 to 2138) | {0 to 0.28) | {0 to 0) |
| Median (Q1 to Q3) | 0 (0 to 187.5) | 0 (0 to 0) | 1948 (1751.3 to 2028.5) | 0 (0 to 0.11) | 0 (0 to 0) |
| Mean (SD) | 96.4 (161.7) | 0 (0) | 1894.2 (190.8) | 0.06 (0.1) | 0 (0) |
| BF (n = 10) | |||||
| {Min to Max} | {0 to 1347} | {0 to 0} | {1252 to 2010} | {0 to 0.72} | {0 to 0} |
| Median (Q1 to Q3) | 0 (0 to 417.3) | 0 (0 to 0) | 1818 (1630 to 1927.5) | 0 (0 to 0.21) | 0 (0 to 0) |
| Mean (SD) | 301.5 (474.4) | 0 (0) | 1752.3 (235.1) | 0.18 (0.29) | 0 (0) |
| D (n = 10) | |||||
| {Min to Max} | {0 to 1856} | {0 to 300} | {1788 to 2007} | {0 to 1} | {0 to 0.16} |
| Median (Q1 to Q3) | 470 (0 to 1083.3) | 0 (0 to 0) | 1862 (1852.3 to 1887.3) | 0.24 (0 to 0.57) | 0 (0 to 0) |
| Mean (SD) | 626.9 (675.7) | 40.2 (96.7) | 1875.8 (60) | 0.33 (0.36) | 0.02 (0.05) |
| DF (n = 10) | |||||
| {Min to Max} | {0 to 1364} | {0 to 0} | {1576 to 2040} | {0 to 0.72} | {0 to 0} |
| Median (Q1 to Q3) | 0 (0 to 102.3) | 0 (0 to 0) | 1884.5 (1820.5 to 1945.8) | 0 (0 to 0.05) | 0 (0 to 0) |
| Mean (SD) | 279.2 (538.6) | 0 (0) | 1862.6 (134.2) | 0.15 (0.29) | 0 (0) |
| p-value * | 0.2488 | 0.1044 | 0.4997 | 0.3128 | 0.1044 |
| Material | MLKc | MLKo | LAI | RMLKc | RMLKo |
|---|---|---|---|---|---|
| B (n = 10) | |||||
| {Min to Max} | {0 to 784) | {0 to 0) | {1527 to 2017) | {0 to 0.41) | {0 to 0) |
| Median (Q1 to Q3) | 0 (0 to 0) | 0 (0 to 0) | 1875 (1755.25 to 1965.25) | 0 (0 to 0) | 0 (0 to 0) |
| Mean (SD) | 129 (279.7) | 0 (0) | 1845.4 (152.9) | 0.07 (0.14) | 0 (0) |
| BF (n = 10) | |||||
| {Min to Max} | {0 to 1440} | {0 to 0} | {1529 to 2081} | {0 to 0.75} | {0 to 0} |
| Median (Q1 to Q3) | 0 (0 to 0) | 0 (0 to 0) | 1881.5 (1743.5 to 2026.8) | 0 (0 to 0) | 0 (0 to 0) |
| Mean (SD) | 188 (461.1) | 0 (0) | 1857.1 (199.3) | 0.1 (0.24) | 0 (0) |
| D (n = 10) | |||||
| {Min to Max} | {0 to 2291} | {0 to 898} | {1504 to 2319} | {0 to 1} | {0 to 0.39} |
| Median (Q1 to Q3) | 366.5 (10.5 to 1193.8) | 0 (0 to 0) | 1912.5 (1822 to 2128.3) | 0.16 (0 to 0.65) | 0 (0 to 0) |
| Mean (SD) | 666.5 (788) | 89.8 (284) | 1957.9 (257.9) | 0.35 (0.4) | 0.04 (0.12) |
| DF (n = 10) | |||||
| {Min to Max} | {0 to 998} | {0 to 645} | {1231 to 2278} | {0 to 0.59} | {0 to 0.3} |
| Median (Q1 to Q3) | 0 (0 to 197) | 0 (0 to 0) | 1942.5 (1701.5 to 2149.8) | 0 (0 to 0.09) | 0 (0 to 0) |
| Mean (SD) | 157 (311.7) | 71.5 (202.7) | 1876.9 (330.5) | 0.09 (0.18) | 0.03 (0.09) |
| p-value * | 0.0713 | 0.2928 | 0.7689 | 0.0653 | 0.2928 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bănuț Oneț, D.; Barbu Tudoran, L.; Delean, A.G.; Șurlin, P.; Ciurea, A.; Roman, A.; Bolboacă, S.D.; Gasparik, C.; Muntean, A.; Soancă, A. Adhesion of Flowable Resin Composites in Simulated Wedge-Shaped Cervical Lesions: An In Vitro Pilot Study. Appl. Sci. 2021, 11, 3173. https://doi.org/10.3390/app11073173
Bănuț Oneț D, Barbu Tudoran L, Delean AG, Șurlin P, Ciurea A, Roman A, Bolboacă SD, Gasparik C, Muntean A, Soancă A. Adhesion of Flowable Resin Composites in Simulated Wedge-Shaped Cervical Lesions: An In Vitro Pilot Study. Applied Sciences. 2021; 11(7):3173. https://doi.org/10.3390/app11073173
Chicago/Turabian StyleBănuț Oneț, Diana, Lucian Barbu Tudoran, Ada Gabriela Delean, Petra Șurlin, Andreea Ciurea, Alexandra Roman, Sorana D. Bolboacă, Cristina Gasparik, Alexandrina Muntean, and Andrada Soancă. 2021. "Adhesion of Flowable Resin Composites in Simulated Wedge-Shaped Cervical Lesions: An In Vitro Pilot Study" Applied Sciences 11, no. 7: 3173. https://doi.org/10.3390/app11073173
APA StyleBănuț Oneț, D., Barbu Tudoran, L., Delean, A. G., Șurlin, P., Ciurea, A., Roman, A., Bolboacă, S. D., Gasparik, C., Muntean, A., & Soancă, A. (2021). Adhesion of Flowable Resin Composites in Simulated Wedge-Shaped Cervical Lesions: An In Vitro Pilot Study. Applied Sciences, 11(7), 3173. https://doi.org/10.3390/app11073173













