Orally Administered Activated Charcoal as a Medical Countermeasure for Acute Radiation Syndrome in Rats
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Activated Carbon for Enterosorption
2.1.1. Synthesis of AC
- (1)
- Synthesis of the beads of 2-methyl-5-vinylpiridine copolymer with styrene divinyl benzene (Taiyan Lanlang Technology Industry Corp., Taiyan, China);
- (2)
- Carbonization of these beads at 400 °C in the nitrogen atmosphere in rotary kiln with rotation speed of 0.5 rpm;
- (3)
- “Consolidation” in nitrogen atmosphere at 800 °C;
- (4)
- First activation of the carbonizate by steam at 860 °C in rotary kiln up to bulk density of 0.28 g/cc;
- (5)
- Second activation in fluidized bed in laboratory kiln by steam up to bulk density of 0.12 g/cc;
- (6)
- Sieving.
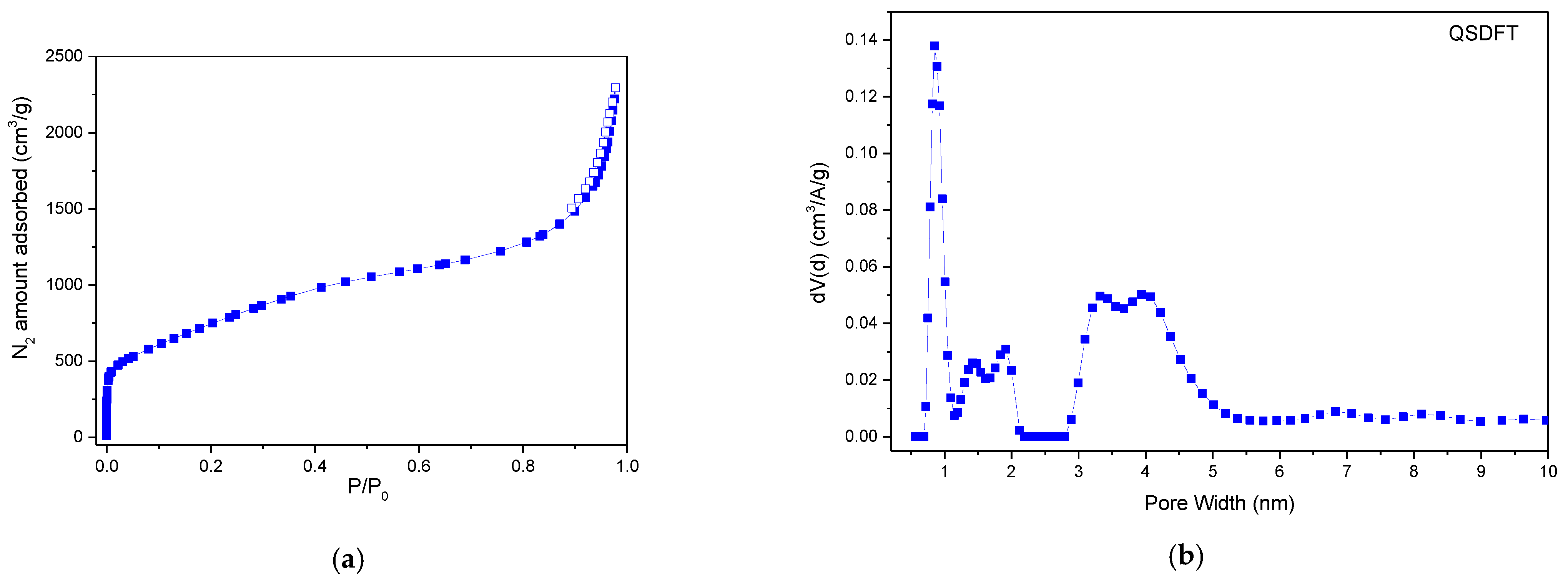
2.1.2. Surface and Porosity Parameters
2.2. Assessment of AC Enterosorbent Efficacy in Animal Model with ARS
2.2.1. Animal Model
2.2.2. Irradiation
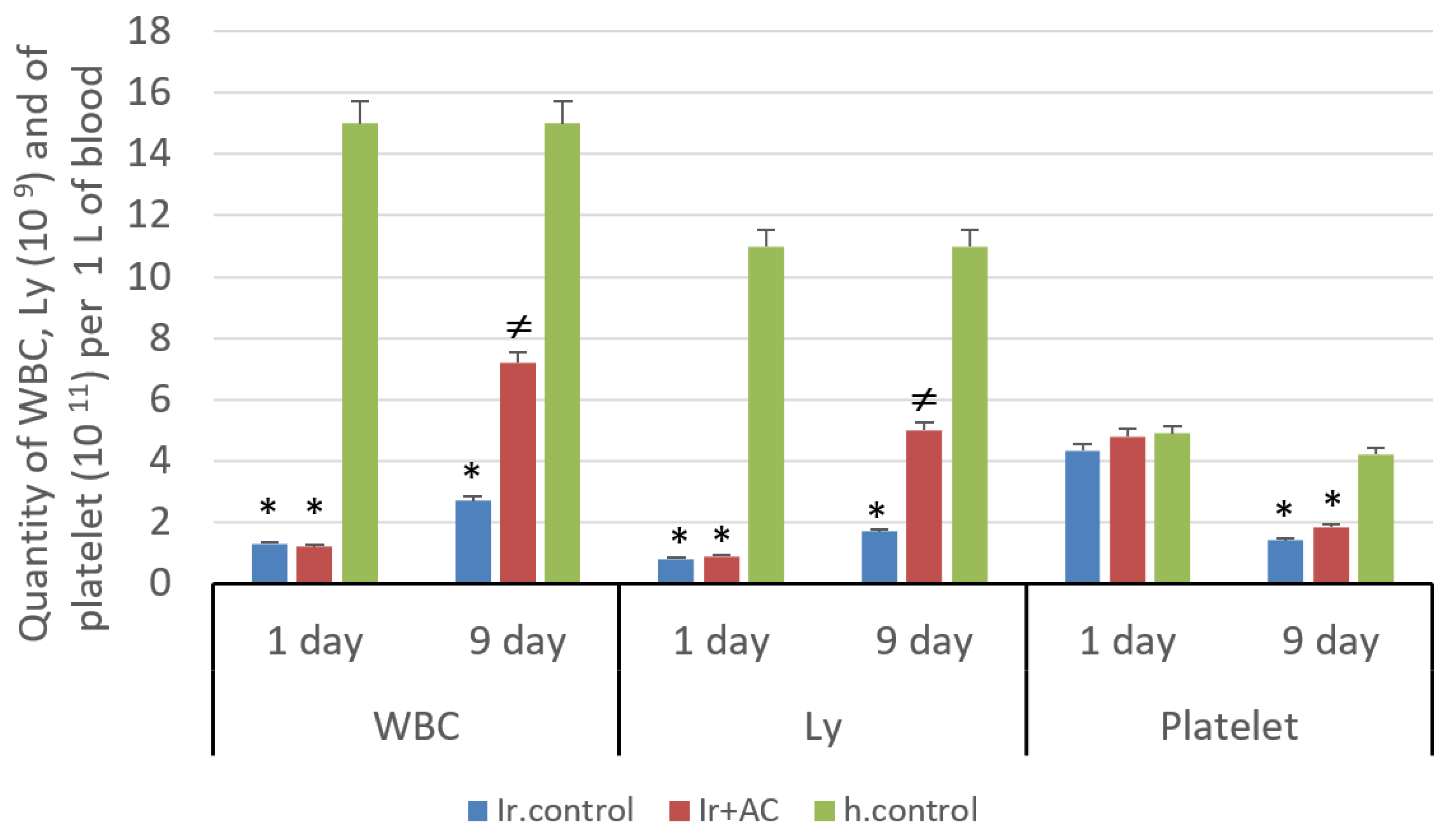
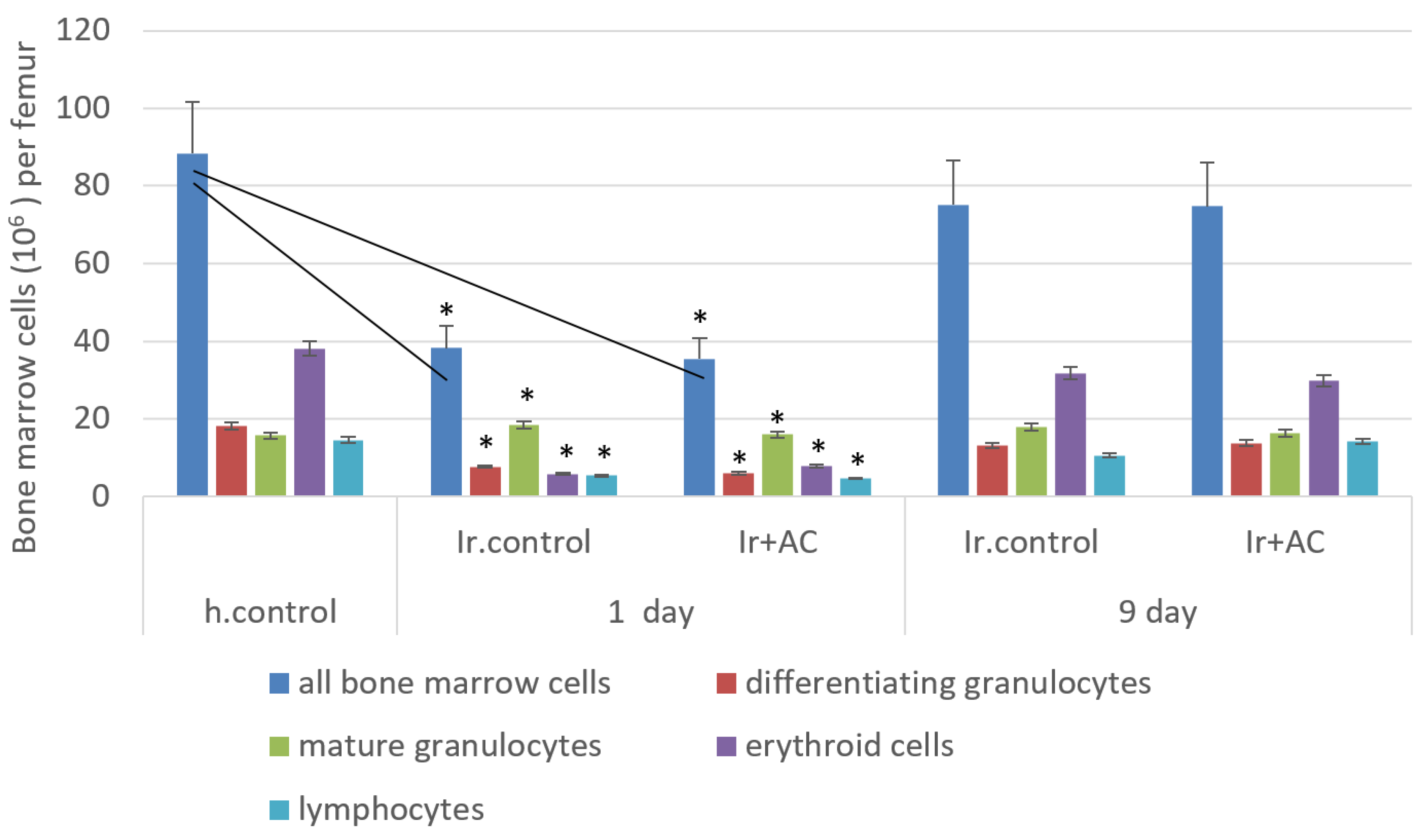
2.2.3. Blood and Bone Marrow Cell Count
2.2.4. Chromosome Aberration Test
2.2.5. Functional Activity of Bone Marrow Cells
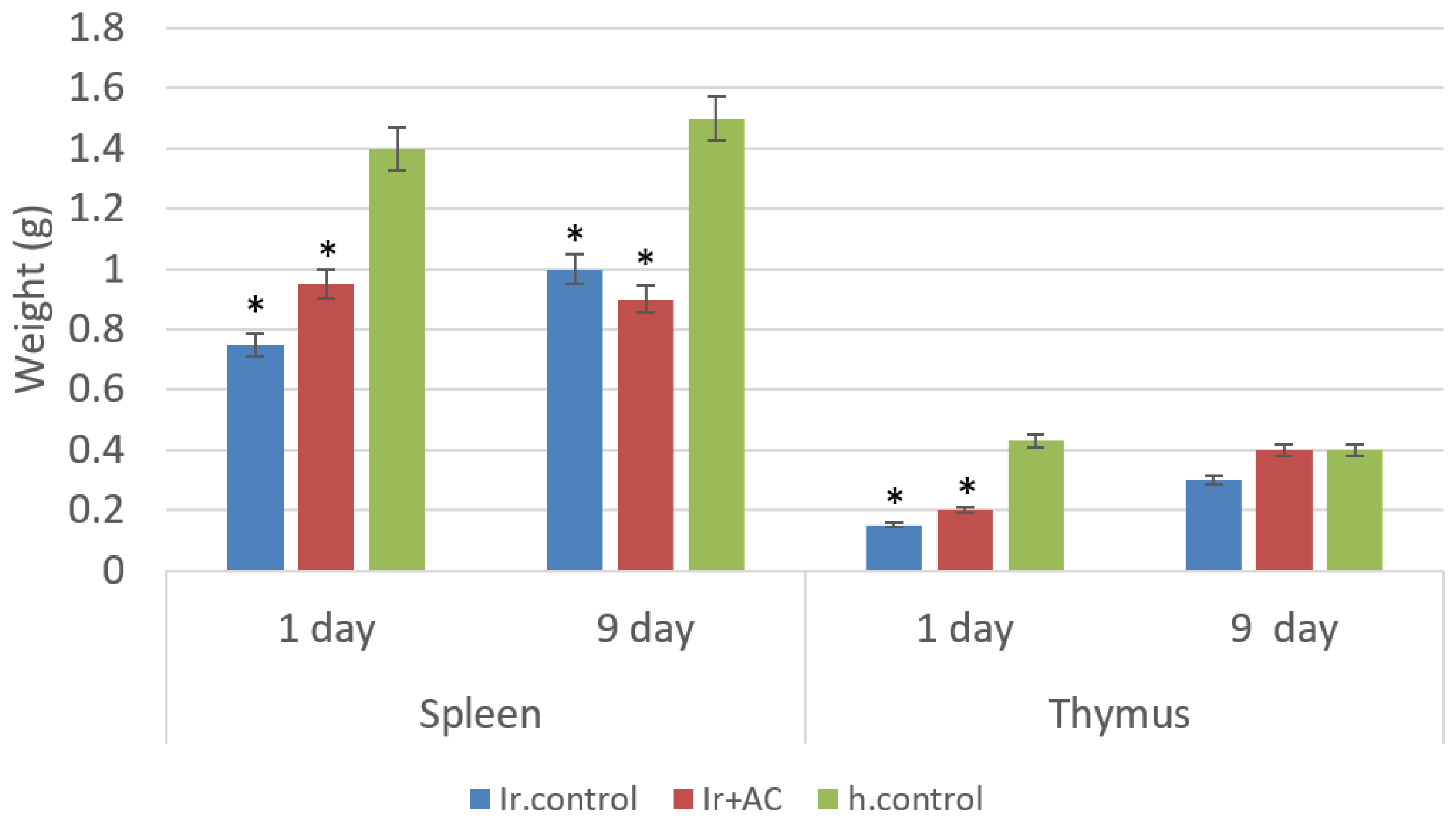
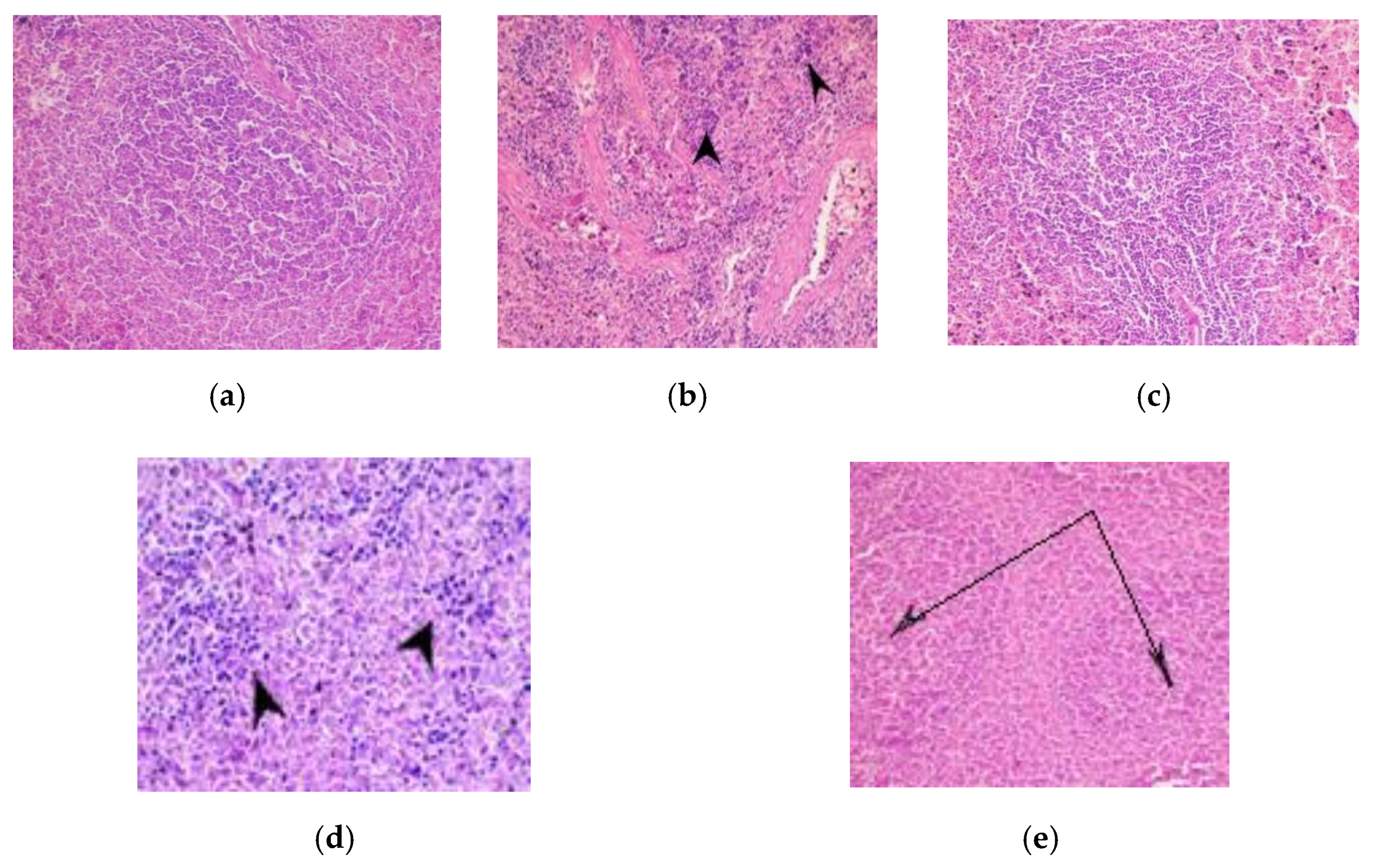
2.2.6. Histology of Organ Tissues
2.2.7. EPR Spin-Trapping Detection of Superoxide Ion—[ O2•−ꜙ]
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bunin, D.I.; Bakke, J.; Green, C.E.; Javitz, H.S.; Fielden, M.; Chang, P.Y. Romiplostim (Nplate®) as an effective radiation countermeasure to improve survival and platelet recovery in mice. Int. J. Radiat. Biol. 2020, 96, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwakura, I. Overview of Radiation-protective Agent Research and Prospects for the Future. Jpn. J. Health Phys. 2017, 52, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Nikolaev, V.; Pinchuk, L.; Umansky, M.; Pinchuk, V.; Burushkina, T.; Petrenko, S.; Rodionova, N.; Snezhkoua, E.; Bichkoua, N. Early Experimental Studies on Hemoperfusion as a Treatment Modality for Acute Radiation Disease. Artif. Organs 1993, 17, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Shevchuk, O.O.; Snezhkova, E.A.; Bardakhivskaya, K.I.; Nikolaev, V.G. Adsorptive treatment of acute radiation sickness: Past achievements and new prospects in Hemoperfusion, Plasmaperfusion and other Clinical Uses of General, Biospecific, Immuno and Leucocyte Adsorbents. In Regenerative Medicine, Artificial Cells and Nanomedicine; Chang, T.M.S., Endo, Y., Nikolaev, V.G., Tani, T., Yu, Y., Zheng, W.-H., Eds.; World Scientific Publishing: Hackensack, NJ, USA, 2017; Volume 4, pp. 245–256. [Google Scholar] [CrossRef]
- Zakharash, M.P.; Ivanova, N.V.; Maslenny, V.N.; Sakhno, L.A.; Sklyarenko, V.G.; Shmatko, I.I.; Snezhkova, E.A.; Bardakhivskaya, K.I.; Sarnatskaya, V.V.; Nikolaev, V.G. Progress and prospects of adsorption therapy in the treatment of postradiation neurosomatic polypathia. Int. J. Radiat. Med. 2003, 5, 256–262. [Google Scholar]
- Pavlenko, D.; Giasafaki, D.; Charalambopoulou, G.; Van Geffen, E.; Gerritsen, K.G.F.; Steriotis, T.; Stamatialis, D. Carbon Adsorbents With Dual Porosity for Efficient Removal of Uremic Toxins and Cytokines from Human Plasma. Sci. Rep. 2017, 7, 14914. [Google Scholar] [CrossRef] [PubMed]
- Sarnatskaya, V.; Mikhailenko, V.; Prokopenko, I.; Gerashchenko, B.I.; Shevchuk, O.; Yushko, L.; Glavin, A.; Makovetska, L.; Sakhno, L.; Sydorenko, O.; et al. The effect of two formulations of carbon enterosorbents on oxidative stress indexes and molecular conformation of serum albumin in experimental animals exposed to CCl4. Heliyon 2020, 6, e03126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, S.; Rudrawar, S.; Zunk, M.; Bernaitis, N.; Arora, D.; McDermott, C.M.; Anoopkumar-Dukie, S. Protection against Radiotherapy-Induced Toxicity. Antioxidants 2016, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Kiang, J.G.; Olabisi, A.O. Radiation: A poly-traumatic hit leading to multi-organ injury. Cell Biosci. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacNaughton, W.K. Review article: New insights into the pathologenesis of radiation-induced intestinal dysfunction. Aliment. Pharmacol. Ther. 2000, 14, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G. Adult Mesenchymal Stem Cells and Radiation Injury. Health Phys. 2016, 111, 198–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dainiak, N. Hematologic consequences of exposure to ionizing radiation. Exp. Hematol. 2002, 30, 513–528. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- OECD. Test No. 475: Mammalian Bone Marrow Chromosomal Aberration Test; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- Babić, N.; Peyrot, F. Molecular Probes for Evaluation of Oxidative Stress by In Vivo EPR Spectroscopy and Imaging: State-of-the-Art and Limitations. Magnetochemistry 2019, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Acharya, S.S.; Fendler, W.; Watson, J.; Hamilton, A.; Dipanjan, C.; Gaudiano, E.; Moskwa, P.; Bhanja, P.; Saha, S.; Guha, C.; et al. Serum microRNAs are early indicators of survival after radiation-induced hematopoietic injury. Sci. Transl. Med. 2015, 7, 287ra69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Diz, D.I.; Robbins, M.E. Oxidative damage pathways in relation to normal tissue injury. Br. J. Radiol. 2007, 80, S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, B.; Wang, H.; Meng, L.; Zhao, Q.; Li, X.; Xin, Y.; Jiang, X. Radiation-Induced Normal Tissue Damage: Oxidative Stress and Epigenetic Mechanisms. Oxid. Med. Cell. Longev. 2019, 3010342. [Google Scholar] [CrossRef] [PubMed]



| Groups of Rats | Mitotic Index of Bone Marrow Cells | ||
|---|---|---|---|
| All Cells | Erythroid Cells | White Cells | |
| Ir control, (n = 5) | 8.8 ± 1.0 | 4.2 ± 1.2 * | 4.6 ± 1.6 |
| Ir+AC, (n = 5) | 13.8 ± 1.5 ** | 7.0 ± 1.4 | 6.8 ± 0.7 |
| healthy control, (n = 5) | 11.6 ± 2.0 | 6.8 ± 1.6 | 4.8 ± 1.6 |
| Groups of Rats | Number of Analyzed Metaphases | Aberrations per 100 Metaphases | Total Aberrations | |||
|---|---|---|---|---|---|---|
| Chromatid Type (M ± SE) | Chromosome Type, (M ± SE) | |||||
| Paired Fragments | Dicentrics | Rings | ||||
| Ir control, (n = 5) | 755 | 1.9 ± 0.2 | 2.3 ± 0.2 | 0.1 ± 0.1 | 0 | 4.28 ± 0.3 * |
| Ir+AC, (n = 5) | 705 | 1.5 ± 0.3 | 0.9 ± 0.3 | 0 | 0 | 2.2 ± 0.2 ** |
| healthy control, (n = 5) | 740 | 1.2 ± 0.3 | 0.3 ± 0.2 | 0 | 0 | 1.6 ± 0.3 |
| Groups of Rats | Liver | Brain | ||
|---|---|---|---|---|
| 1st Day | 9th Day | 1st Day | 9th Day | |
| Ir control, (n = 5) | 1.5 ± 0.2 * | 1.7 ± 0.2 * | 5.0 ± 0.4 * | 5.1 ± 0.7 * |
| Ir+AC, (n = 5) | 0.9 ± 0.2 ** | 1.0 ± 0.2 ** | 4.6 ± 0.2 * | 5.2 ± 0.5 * |
| healthy control, (n = 5) | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snezhkova, E.; Rodionova, N.; Bilko, D.; Silvestre-Albero, J.; Sydorenko, A.; Yurchenko, O.; Pakharenko, M.; Alavijeh, M.; Bardakhivska, K.; Riabchenko, N.; et al. Orally Administered Activated Charcoal as a Medical Countermeasure for Acute Radiation Syndrome in Rats. Appl. Sci. 2021, 11, 3174. https://doi.org/10.3390/app11073174
Snezhkova E, Rodionova N, Bilko D, Silvestre-Albero J, Sydorenko A, Yurchenko O, Pakharenko M, Alavijeh M, Bardakhivska K, Riabchenko N, et al. Orally Administered Activated Charcoal as a Medical Countermeasure for Acute Radiation Syndrome in Rats. Applied Sciences. 2021; 11(7):3174. https://doi.org/10.3390/app11073174
Chicago/Turabian StyleSnezhkova, Elisaveta, Natalia Rodionova, Dennis Bilko, Joaquin Silvestre-Albero, Alexey Sydorenko, Olga Yurchenko, Marharyta Pakharenko, Mo Alavijeh, Kvitoslava Bardakhivska, Natalia Riabchenko, and et al. 2021. "Orally Administered Activated Charcoal as a Medical Countermeasure for Acute Radiation Syndrome in Rats" Applied Sciences 11, no. 7: 3174. https://doi.org/10.3390/app11073174
APA StyleSnezhkova, E., Rodionova, N., Bilko, D., Silvestre-Albero, J., Sydorenko, A., Yurchenko, O., Pakharenko, M., Alavijeh, M., Bardakhivska, K., Riabchenko, N., & Nikolaev, V. (2021). Orally Administered Activated Charcoal as a Medical Countermeasure for Acute Radiation Syndrome in Rats. Applied Sciences, 11(7), 3174. https://doi.org/10.3390/app11073174







