Abstract
L-Arginine (Arg) has been widely used due to its functional properties as a substrate for nitric oxide (NO) generation. However, L-citrulline (CIT), whose main natural source is watermelon, is a non-essential amino acid but which has important health potential. This review provides a comprehensive approach to different studies of the endogenous synthesis of CIT, metabolism, pharmacokinetics, and pharmacodynamics as well as its ergogenic effect in exercise performance. The novel aspect of this paper focuses on the different effects of CIT, citrulline malate and CIT from natural sources such as watermelon on several topics, including cardiovascular diseases, diabetes, erectile dysfunction, cancer, and exercise performance. CIT from watermelon could be a natural food-sourced substitute for pharmacological products and therefore the consumption of this fruit is promoted.
1. Introduction
L-citrulline (CIT) is an organic compound and a non-essential amino acid [], and the body can synthesize it endogenously. CIT is a non-protein amino acid, which is another main metabolic characteristic; it is not one of the 20 primary amino acids encoded by DNA and, therefore, not involved in protein synthesis []. Diet is a poor source of CIT and endogenous synthesis is its main source in the body []. In fact, watermelon is the principal source of that amino acid in the diet; the name CIT comes from Citrullus lanatus (Thunb.), the scientific name for watermelon. CIT concentration in watermelon depends on the type of cultivar and usually ranges between 0.7 and 3.6 g/kg of fresh weight []. CIT is present in both the flesh and in the rind []; during watermelon development, there is a progressive accumulation of CIT in those tissues [], in particular, under stress conditions. In addition, watermelon by-products such as the rind from the fresh-cut watermelon industry can be used for CIT extraction [], improving the sustainability of the fresh-cut industry, and promoting a circular economy model. World watermelon harvest area and production amounted to 3 million hectares and 100 million tons, respectively, in 2019 []. This fruit is mostly appreciated by consumers because of its capability for refreshing them. The United Nations has declared 2021 as the International Year of Fruits and Vegetables. The goal is to raise awareness of the nutritional and health benefits of consuming more fruits and vegetables as part of a diversified, balanced, and healthy diet and lifestyle, as well as to direct policy attention to reducing loss and waste of these highly perishable produce items. It is well known that the colours of fruit and vegetables are often linked to the nutrients and phytochemicals they contain. Therefore, red watermelon can help lower the risk of cancer and improve heart health []; and, this review will cover the benefits of CIT, providing examples when watermelon is consumed and promoting the consumption of this fruit.
There is a growing body of literature that recognizes the importance of CIT in several ways. Many researchers have investigated CIT absorption; CIT’s role as a marker of inflammation on endothelial cells; the therapeutic use of CIT; and the ergogenic effect of CIT in athletes. This review focuses on the metabolism of CIT and its role in health and nutrition in humans, as well as the positive effects in athletes. The main aim of this paper was to review recent research into the CIT synthesis, metabolism and its effects on different diseases and exercise performance, including CIT from natural sources such as watermelon.
2. L-Citrulline Biochemistry
2.1. Endogenous Synthesis of L-Citrulline
De novo formation of CIT occurs in enterocytes [], as described in Figure 1. Hence, CIT concentration is currently considered to be a marker of intestinal function []. The substrates for intestinal CIT synthesis are amino acids derived from the diet, such as glutamine, proline and L-arginine (Arg) []. In enterocytes, part of the dietary and portal blood glutamine is catabolized to CIT by different enzymes (glutaminase, pyrroline-5-carboxylate synthase, ornithine aminotransferase (OAT), ornithine transcarbamylase (OTC) (also called ornithine carbamoyl transferase) and carbamoyl phosphate synthetase-I [,].
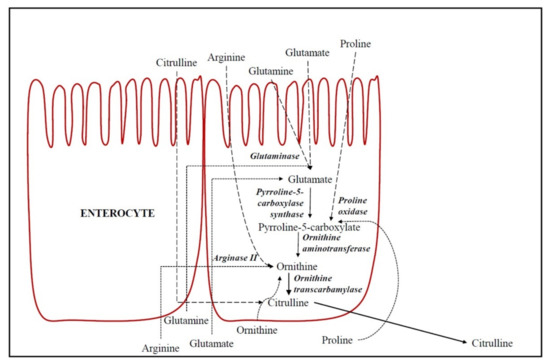
Figure 1.
De novo formation of citrulline in epithelial absorptive cells of the small intestine (enterocytes). Dashed lines indicate the amino acids flow from oral ingestion through enterocytes (apical transport) and dotted lines indicate the amino acids flow from portal blood through enterocytes (basolateral transport).
Glutaminase converts glutamine into glutamate and, therefore, the dietary and portal blood glutamate is catabolized in enterocytes to CIT in a common biosynthetic pathway to glutamine. However, the key regulation enzymes are pyrroline-5-carboxylate synthase and OAT, which are both unique to small intestinal enterocytes []. CIT accounts for 27.6% of metabolized glutamine []. Glutamine is generally considered the main precursor of intestinal CIT []. However, Marini et al. [] demonstrated that dietary Arg was the principal precursor for CIT synthesis and that the proline contribution was low (3.4%), and glutamine was negligible (0.4%). Approximately 40% of dietary Arg is metabolized by arginase II into ornithine in enterocytes. Moreover, proline is also a precursor of CIT in the small intestine in enterocytes of postnatal pigs and healthy adults [,]. Proline is metabolized into P5C (Δ1-l-pyrroline-5-carboxylate) and subsequently into ornithine by proline oxidase and OAT, respectively []. Finally, the ornithine produced in enterocytes from these amino acids is metabolized into CIT by OTC. Ornithine may additionally be a direct CIT precursor from portal blood, above all when the supply of Arg is low []. OTC, in addition to catalyzing the conversion of ornithine into CIT, is a key enzyme in the urea cycle (Figure 1). Therefore, the CIT circulating in the organism is the sum of CIT that we have absorbed from the diet foods plus the CIT production in the de novo synthesis in the intestine, with CIT being a biomarker of the functional small bowel enterocyte mass [].
2.2. L-Citrulline Metabolism
The metabolism of CIT, depending on the tissue distribution of the enzymes involved in it, follows three metabolic pathways (Figure 2): Arg biosynthesis, the Arg-CIT-nitric oxide cycle, and the urea cycle []. There are three key enzymes, two involved in the CIT synthesis, nitric oxide synthase (NOS) and OTC, and one involved in CIT catabolism in mammals, argininosuccinate synthase (ASS) that converts CIT into argininosuccinate, which is used in both metabolic pathways [].
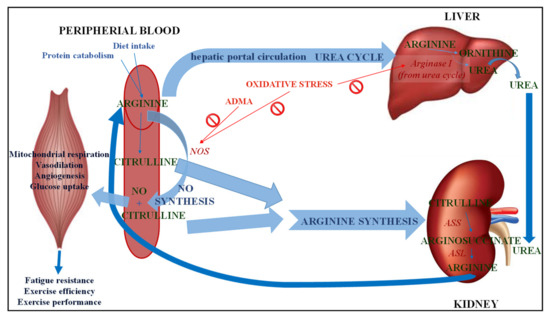
Figure 2.
Metabolism of citrulline. NO: Nitric oxide; NOS: Nitric oxide synthase; ADMA: Asymmetric dimethyl arginine; ASS: Argininosuccinate synthase; ASL: Argininosuccinate lyase.
2.2.1. Arginine
Arginine Biosynthesis
In mammals, Arg is classified as a semi-essential or conditionally essential amino acid, depending on the individual’s health status and stage of development []. Arg is a derivative from endogenous de novo Arg synthesis, body protein breakdown or dietary intake (Figure 2). Arg synthesized from CIT represents 60% of the organism’s de novo Arg synthesis, although only 5–15% is circulating Arg []. Enterocytes release CIT into the portal circulation and transport it to the kidneys, where about 83% CIT catabolizes to Arg in the cells of proximal tubules []. ASS converts CIT into arginosuccinate in the presence of aspartate and ATP and arginosuccinate converts into fumarate and Arg by arginosuccinate lyase (ASL). Arg is synthesized in the intestinal-renal axis, where small intestine epithelial cells participate, along with the kidney proximal tubule cells []. Although other cells types and tissues contain the enzymes ASS and ASL to convert CIT to arginosuccinate and finally to Arg [], the most important synthesis is in the liver and kidney. Moreover, elevated plasma concentrations of CIT can be a marker of kidney dysfunction, since plasma CIT is degraded in the kidney for the renal synthesis of Arg. Arg suffers intense hepatic catabolism, yet CIT is not metabolized by the liver, it seems that giving CIT supplements enables Arg to be administered whilst avoiding its hepatic uptake [].
Arginine Catabolism
Arg metabolism is highly compartmentalized. Arginase is a binuclear manganese metalloenzyme which catalyzes Arg hydrolysis to L-ornithine and urea (Figure 2). Two different genetic isoforms of arginase differ in their tissue distribution, subcellular localization, and immunological reactivity. Arginase I is a cytosolic enzyme whose predominant expression lies in the liver, as part of the urea cycle, in which its function is the elimination of nitrogen generated in amino acid metabolism. However, to a much lesser extent, arginase I has been detected in extra-hepatic tissues such as endothelial cells as well as vascular smooth muscle cells []. On the other hand, arginase II is a mitochondrial enzyme that is widely distributed in extrahepatic cells and tissues (such as kidney, small intestine, brain, red blood cells and immune cells) []. Arginase II of the intestinal mucosa has a high activity, in fact the splanchnic area accounts for 40% of the dietary Arg extracted, thus explaining the low plasma concentrations of Arg. Therefore, the constitutive levels of arginase activity in endothelial cells limit the bioavailability of Arg as a substrate for the synthesis of endothelial nitric oxide (NO) by NOS and nitric oxide-dependent vasodilatory function []. Both enzymes, arginase and NOS, compete for the same substrate, and this competition depends on tissue type and the presence of stimuli [].
2.2.2. Nitric Oxide Biosynthesis
Nitric oxide (NO) can be synthesized endogenously in two ways: by a reduction of inorganic nitrate or through NOS enzyme action from Arg, producing CIT as a byproduct (Figure 2) []. For this reaction to take place, oxygen must be present as well as reduced nicotinamide adenine dinucleotide phosphate (NADPH) as cosubstrates and the collaboration of cofactors flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), tetrahydrobiopterin and heme is required. ASS action can recycle the CIT generated as a byproduct to Arg, thereby increasing the Arg available for NO production []. There are three isoforms of NOS, located in different cells of the body: neuronal NOS (NOS1) which is present in neuronal cells, inducible NOS (NOS2) in macrophages and endothelial NOS (NOS3) in endothelial cells []. NOS2 is inducible, Ca2+ independent, and expressed by macrophages and other tissues during inflammation or when stimulated with bacterial endotoxins or inflammatory cytokines. NOS1 and NOS3 are constitutive enzymes regulated by intracellular calcium concentration and by the Ca2+/calmodulin complex. Thus, NOS1 is involved in synaptic plasticity in the central nervous system (CNS), central regulation of blood pressure, smooth muscle relaxation, and vasodilatation via peripheral nitrergic nerves, which are involved in the relaxation of corpus cavernosum and penile erection []. NO generated from NOS1 acts as a neurotransmitter, while NO generated by NOS3 acts as a vasodilator. NOS3 maintains blood vessel dilation, blood pressure control, as well as several other vasoprotective and anti-atherosclerotic effects. Numerous cardiovascular risk factors cause oxidative stress, NOS3 uncoupling, and endothelial dysfunction in the vasculature. NO regulates vascular tone and blood flow by activating soluble guanylate cyclase (sGC) in the vascular smooth muscle. NO relaxes vascular smooth muscle by binding to the heme moiety of cytosolic guanylate cyclase, activating guanylate cyclase and increasing intracellular levels of cyclic guanosine 3′,5′-monophosphate, producing vasodilation [].
It also inhibits platelet aggregation and prevents the adhesion of leukocytes throughout the endothelial cell layer of blood vessels, inhibiting the proliferation of vascular smooth muscle cells by the inhibition of cytochrome oxidase, regulating the consumption of mitochondrial oxygen [,]. Abnormal vascular NO production and transport cause endothelial dysfunction with several cardiovascular pathologies such as hypertension, atherosclerosis and angiogenesis-associated disorders []. NO synthesis depends on Arg availability, which can be affected by: intracellular synthesis of Arg from CIT (which in turn depends on CIT being available), transport of extracellular Arg (cationic amino acid) and arginase activity (which competes with NOS for Arg). Additionally, asymmetric dimethylarginine (ADMA) is an endogenous inhibitor for all three NOS isoforms and has been reported as a risk factor for all causes of cardiovascular mortality [].
2.2.3. Urea Cycle
The urea cycle (Figure 2), taking place in the liver, detoxifies ammonia from nitrogen from enteral sources (dietary protein) and muscle, thus metabolizing it into urea []. CIT metabolism in the liver consists of a highly compartmentalized metabolism, which is not connected with the other metabolic pathways that involve CIT []. Moreover, hepatocytes implicated in the urea cycle cannot take up CIT from the portal circulation (1). The two enzymes that control the first reactions of the urea cycle, carbamoyl phosphate synthetase (CPS) and OTC, are not exclusive to the liver but are also present in the small intestine. OTC joins carbamoyl phosphate and L-ornithine to produce CIT []. OTC deficiency could cause hypocitrullinemia, hyperammonemia and higher glutamine, alanine and lysine plasma concentrations, and even lead to coma or death []. However, this CIT pool is particularly labile: cytoplasmic ASS converts all synthesized CIT into argininosuccinate, and CIT is not released into general circulation [,]. Argininosuccinate is then transformed into Arg and fumarate by ASL. The cycle reaches its end when enzyme arginase metabolizes Arg into L-ornithine, thus liberating urea. Ornithine is mostly recycled while urea is synthesized, ultimately coming from de novo synthesis in the small intestine, although food sources may provide minor quantities. Finally, urea enters the kidneys to be excreted, thus completing the urea cycle, as a consequence of liver and kidney collaboration.
3. L-Citrulline in Muscles
CIT supplementation in elderly malnourished rats increases muscle protein content by the stimulation of protein synthesis, but not of liver protein synthesis. This explains why whole-body protein synthesis does not change. On the other hand, neither protein mass nor protein synthesis are affected by CIT supplementation in the splanchnic area, thus confirming that that area is bypassed []. Additionally, branched-chain amino acids (BCAAs; leucine (LEU), valine, isoleucine), specifically LEU, are able to stimulate protein synthesis and decrease protein catabolism []. Le Plénier et al. [], showed that in adult fasted rats, both LEU and CIT (despite having differing chemical structures and metabolisms) can stimulate muscle protein synthesis (MPS), but that LEU stimulates MPS in the post-prandial state and CIT in the post-absorptive state. Additionally, Faure et al. [] demonstrated, through modeling protein-energy malnutrition in aged rats in the post-absorptive state that a CIT-enriched diet, increased muscle mass and muscle function was associated with improvement in both motor activity and maximal tetanic isometric force.
In humans, Jourdan et al. [] were the first to demonstrate the muscle protein synthesis stimulated by CIT. In that study, increased muscle protein synthesis 25% was observed in healthy volunteers after three days on a relatively low-protein diet with CIT compared to a non-essential amino acid iso-nitrogenous mixture. This increased muscle protein synthesis appears to specifically affect CIT on muscle and is not an effect of increased nitrogen intake, as CIT did not affect whole-body protein synthesis []. This may be due to the specific effect of CIT on myofibrillar protein synthesis and expression in the muscle, which is the protein fraction involved in muscle contractility [].
4. Pharmacokinetics and Pharmacodynamics of L-Citrulline
The characteristics of the pharmacokinetic parameters of CIT resemble those of related amino acids, Arg and ornithine, but not Cmax (peak concentration) which is several times greater in CIT than in Arg or ornithine []. Therefore, unlike Arg, the intestine or liver do not metabolize CIT; it is not a substrate for the arginase enzymes because CIT bypasses splanchnic extraction [] and also inhibits its activity []. This could explain why plasma Arg levels rise rapidly and significantly when administered together, giving rise to the suggestion that Arg could pass through the gastrointestinal tract and liver without suffering the influence of intestinal and hepatic-first pass effects, which is likely to be due to CIT inhibiting arginase activity []. Oral CIT administration can be used to enhance systemic CIT and Arg availability; it is bioavailable and losses in urine are very low []. In a combined sample of middle-aged women and men, Schwedhelm et al. [] found that oral CIT supplementation, at a dose of 1.5 g twice daily for one week, increased the plasma concentrations of CIT and Arg in a dose-dependent manner and augmented plasma Arg levels with greater effectiveness than by supplementing Arg in healthy subjects. Moinard et al. [] found a similar behavior in healthy elderly subjects when a single dosing caused a marked increase in Arg availability when CIT (10 g) was administered, compared with Arg (9.94 g) administration itself. Previously, in a study performed in healthy men, using oral loads (2, 5, 10, or 15 g CIT) administered at random, Moinard et al. [], had found that the plasma CIT concentration increased rapidly and massively (10-fold at the 2 g load to 100-fold at the 15 g load) and returned to baseline values within 5–8 h post-loading. These authors demonstrated that CIT was well tolerated (no side effects) and did not induce gastrointestinal disorders at a high dose (i.e., 15 g), thus suggesting that intestinal absorption of CIT is not a limiting step. However, Arg production was lower than expected when 15 g of CIT was administered. This could be explained by the decreased renal conversion of CIT to Arg and/or, a possible saturation of its transporters a certain dose of CIT. Several authors have proposed that CIT uptake uses a different transport system to Arg which is mainly transported through Na+-independent cationic amino acid transporters (CAT-1, 2 and 3) []. CIT is transported across the enterocytes to the portal circulation, likely using the Na+-dependent, neutral amino acid, including the ASC or B0,+-amino acid transporters located in those cells [,,,].
According to the pharmacodynamic CIT parameters, this amino acid is well tolerated and under short-term application, no side effects such as gastrointestinal disorders were observed, even when high doses (i.e., 15 g) were applied. This is a positive feature of CIT, since in previous studies the administration of related amino acids (>9 g/day of Arg for 1 week or >10 g/day of ornithine in a single dose) caused nausea, vomiting or diarrhea []. The explanation for this may lie in the rapid saturation of intestinal absorption of ornithine and Arg, with high loads inducing osmotic diarrhea []. In contrast to Arg and ornithine, intestinal CIT absorption is not a limiting factor in CIT bioavailability, since CIT is well tolerated and recognized as safe for oral consumption [,]. The highest doses of CIT reported are 0.18 g/kg/day (approximately 12.6 g/day in a person of 70 kg) for 7 days [] and a single dose of 15 g [].
5. Ergogenic Effect of L-Citrulline Supplements
Ergogenic supplements are aids that can act in the production of metabolic energy, controlling its use and making it more efficient, to increase an athlete’s performance []. The ergogenic effects of CIT could be due to several mechanisms (Figure 2): (1) CIT is the forerunner of Arg renal synthesis. Therefore, oral CIT supplementation increases plasma concentrations of Arg, increasing its bioavailability as a substrate for NO synthesis [] and, likewise, CIT is an effective substitute to restore NO production when there is limited ARG availability []; (2) in the urea cycle, arginase catabolizes the Arg produced from CIT into ornithine and urea. Therefore, CIT supplementation improves the ammonia homeostasis to remove ammonia []. Ammonia is associated with muscle fatigue due to its ability to increase anaerobic glycolysis and produce lactic acid during high intensity exercise []. Based on these mechanisms, several authors have postulated that CIT can augment exercise performance by either improving skeletal muscle oxygen consumption or by lowering lactic acid production [,,]. CIT supplementation (10 g/day) combined with 12-week high-intensity interval training induced greater improvements in upper limbs muscle strength and walking speed in a specific population such as dynapenic-obese elderly []. One week CIT supplementation (6 g/day) provided a modest improvement in the endurance cycling performance in male trained cyclists, increasing the average heart rate, rating of perceived exertion, and power throughout the time trial []. A second study (similar doses) showed improved oxygen uptake kinetics during walking in men, although not in women [].
5.1. Ergogenic Effect of NO from L-Citrulline Supplements
Numerous bodily functions such as regulating vasodilatation, blood flow, mitochondrial respiration and platelet function are significantly influenced by NO. In mitochondrial respiration, NO inhibits cytochrome C oxidase, which decreases oxygen consumption and helps in the distribution of oxygen in the skeletal muscles. Hence, increasing the NO available could enhance oxygen and nutrient delivery to active muscles and thus reduce the ATP cost of muscle force production, leading to improved physiological responses relating to endurance performance and recovery []. The synthesis of NO by NOS enzyme action can be limited by the availability of substrate Arg and the activity of arginase []. NO can be increased through administering oral Arg supplements, albeit with the drawback of intense catabolism of Arg both in the gastrointestinal tract as well as in the liver where arginase catabolizes it and transforms it into ornithine and urea. However, an alternative to that is oral supplementation of CIT, with the advantage that arginase enzymes do not catabolize CIT and, moreover, arginase activity is inhibited []. Previous in vitro and in vivo studies have shown CIT to suppress arginase activity, acting as a strong allosteric inhibitor []. It has been shown that 2 g/day [] or 3 g/day [] of oral CIT supplementation for 7 days increased the plasma Arg concentration and elevated NO-dependent signaling. Studies in prolonged exercise have shown that CIT supplementation may increase plasma urea concentration and NO production [], while suppressing exercise-induced hyperammonemia []. As a consequence, oxygen and nutrients are better delivered to active muscles by CIT, so physical exercise is better tolerated by increasing NO [], albeit with a modest effect in submaximal exercises in older men [].
5.2. Ergogenic Effect of Urea Cycle from L-Citrulline Supplements
The urea cycle detoxifies the ammonia accumulated in skeletal muscles from high intensity exercise [,]. Experimental studies in rats have proven that supplementation using a CIT/Arg/ornithine mix suppressed blood ammonia from accumulating following exercise and in a swimming activity it lengthened the time before becoming exhausted, by about 60% []. CIT supplementation, by buffering ammonia through the urea cycle, enhances the aerobic utilization of pyruvate and, therefore, decreases lactate production via the anaerobic pathway. Conversely, the central nervous system is instrumental in the development of exercise-induced fatigue []. The cerebral concentration of serotonin is increased during physical exercise, which can contribute to fatigue developing in the central nervous system []. The cerebral synthesis of serotonin requires tryptophan as a precursor, and it seems that tryptophan transportation through the blood–brain barrier is the factor limiting the speed of synthesis of cerebral serotonin []. BCAAs have been proposed as a means of alleviating central fatigue because of their capacity to compete with tryptophan, in traversing the blood-brain barrier using the same L-system transporter. However, studies in humans have not demonstrated the ergogenic effect of BCAA supplementation during physical exercise, due to excess hyperammonemia to the increased BCAA metabolism when exercising []. In humans, there is an increased brain absorption and accumulation of ammonia during prolonged exercise []; this seems to be able to induce central fatigue through alterations in cerebral energy metabolism and neurotransmission and signaling pathways within the neuron []. Thus, it appears that the lower plasma tryptophan/BCAA ratio following BCAA supplements could decrease the cerebral uptake of tryptophan and, consequently, serotonin synthesis, thereby preventing central fatigue during prolonged exercise [].
Combining BCAA and Arg supplements on the performance of intermittent sprints in simulated handball matches on two consecutive days in well-trained athletes showed a significantly lower plasma tryptophan/BCAA ratio following the activity on both days in the group that took the combined supplementation of BCAA + Arg, describing lower rates of perceived exertion (RPE), using Borg’s 20-point scale, possibly by alleviating central fatigue []. Therefore, both Arg and CIT may decrease exercise-related build-ups of ammonia by increasing the urea cycle and NO biosynthesis []. Chen et al. [] studied how combining BCAA, Arg and CIT supplements affected central fatigue by monitoring three simulated matches in well-trained taekwondo athletes. Their results showed that supplementing BCAA and Arg and CIT achieved a significantly lower plasma free tryptophan/BCAA ratio and raised NO concentrations. Moreover, those supplements led to no additional ammonia accumulation, potentially with mediation of a raised NO production from Arg and CIT. The ergogenic effect may be potentially caused by mechanisms such as central fatigue alleviation (by means of inhibiting cerebral serotonin synthesis by BCAA) and preventing excess hyperammonemia, through increased urea genesis by Arg and CIT.
6. Studies with Citrulline Malate (CM)
CM is a pharmaceutical compound (Stimol® Biocodex, Gentilly, France), authorized for treating asthenia, with the recommended dose being 1 g thrice daily []. Higher rates of ATP production during CM supplementation have been demonstrated in active human skeletal muscle using magnetic resonance spectroscopy [], this result is thought to be driven by malate’s role in mitochondrial oxidative metabolism []. In accordance with this, several authors have reported the positive effects of CM in sports [,,,]. Bendahan et al. [] concluded that supplementation using CM (6 g/day for 15 days) significantly reduced fatigue sensation, due to an increased (34%) oxidative ATP production rate during exercise and subsequent increase (20%) in the post-exercise phosphocreatine recovery rate. As a result, aerobic energy production in muscles and exercise tolerance are improved, significantly boosting aerobic performance and prolonging muscular fatigue onset. Moreover, CM decreased the blood lactate concentration and perception of fatigue following muscular effort in high-performance athletes, enabling more rapid recovery, and adapting training with no deleterious effects []. Accordingly, oral administration of CM in healthy rats, 1 g/kg thrice daily for 48 h, led to an improved muscular efficiency, enhancing specific muscle force production by 23%, associated with a significant fall in phosphocreatine (PCr) (28%) as well as in oxidative (32%) costs of contraction []. Additionally, Perez-Guisado and Jakeman [] showed a fall of 40% in muscle soreness 24 and 48 h after a pectoral training session and a higher percentage response, over 90%, was reached using just one 8 g dose of supplementary CM. Moreover, other authors described less fatigue in working muscles, enabling them to carry out a larger number of repetitions on exercise performance during lower-body dynamic resistance exercise under CM supplementation (8 g) []. In addition, CM supplementation in cyclists with dose acute CM (6 g) 2 h prior to exercise raised plasma Arg concentration and increased Arg-derived metabolites such as nitrite, ornithine and urea []. By contrast, Cunniffe et al. [], showed that acute supplementation of 12 g CM failed to attenuate fatigue induced by repeated high-intensity cycling or prolonging the time to exhaustion in well-trained males. Later, da Silva et al. [], showed CM supplementation failed to improve the muscle recovery process after a high-intensity RE session in untrained young adult men. Recently, Casonatto et al. [], studied if CM supplementation (6 g) had an impact in the acute post-exercise blood pressure response in hypertensive adults, obtaining a potential positive effect.
7. Other Positive Effects of L-Citrulline
7.1. L-Citrulline and Cardiovascular Diseases
Endothelial cell dysfunction, defined as impaired endothelium-dependent vasodilatation caused by decreased NO bioavailability, is a primary cause of disability and death associated with cardiovascular disease [,,]. In the vascular endothelial cells, synthesis of NO by NOS3 is essential in maintaining the dilation of blood vessels, controlling blood pressure, and also presents other vasoprotective and anti-atherosclerotic effects []. A strong association has been found between NO deficiency in the vascular endothelium and cardiovascular diseases, including hypertension, atherosclerosis, and diabetic vascular disease. This suggests that increased NO production could have a key role in slowing the progression of these diseases or their component processes [,,]. Arg can be administered as a substrate in order to reach the NO levels to achieve good cardiovascular function. However, some studies have shown the drawbacks of oral supplementation with Arg: (1) Arg undergoes intense catabolism by the arginase enzymes of the gastrointestinal and hepatic systems [,]. (2) High levels of Arg have been shown to induce arginase enzymes, thus further increasing Arg catabolism. Moreover, disease conditions including diabetes, trauma, pulmonary hypertension, sickle cell, heart failure, and even aging enhance arginase expression and activity, thus negatively affecting Arg therapy efficacy []. Conversely, CIT does not present such drawbacks, so it would be an adequate supplement in the diseases associated with Arg deficiencies. Consequently, CIT supplementation achieved better NO levels than supplementing Arg through the inhibition of reactive oxygen species (ROS) production and arginase II protein expression [].
Furthermore, chronic administration of a combination of CIT and Arg can revoke the effect on high cholesterol, and undo atherosclerosis progression in rabbits []. CIT supplementation (800 mg/day for 8 weeks) improved endothelial dysfunction in patients diagnosed with vasospastic angina, probably due to potentiating NO-dependent reactions and decreasing the state of lipoprotein oxidation in humans []. However, in another study, Kim et al. [] reported that acute CIT supplementation (10 g) was ineffective at improving vascular endothelial dysfunction in the older heart failure adults. These results could imply that factors other than NO synthesis per se may play a role in vascular dysfunction and/or that longer-term supplementation of either Arg or CIT is required in order to reverse the impairment in peripheral vascular function in the older heart failure adults. Recently, Le Roux-Mallouf et al. [] demonstrated that chronic NO precursor supplementation (nitrate-rich salad and citrulline, 520 mg nitrate and 6 g citrulline) in healthy older individuals can reduce resting blood pressure and increase cycling performance by improving cardiorespiratory responses. Wong et al. [] studied the effect of CIT supplementation on resting heart rate variability (HRV) and blood pressure in obese postmenopausal women, as HRV has been proven to be negatively influenced by menopause and obesity in women, which has been associated with adverse cardiac events and mortality. Those authors demonstrated that 8 weeks of CIT supplementation (6 g/day in two doses) improved cardiac autonomic function in sedentary obese postmenopausal women. Therefore, although these results could be clinically useful, there are many factors that greatly influence the positive CIT results, improving cardiovascular diseases in humans.
7.2. L-Citrulline and Diabetes
Endothelial cell dysfunction is the initial step in cardiovascular diseases such as hypertension and diabetes mellitus. The main causes of diabetic complications are alterations in vascular structures and functions, which is why preventing these complications is so important. Reduced availability of Arg to the endothelial nitric oxide synthase (eNOS o NOS3) was linked to vascular dysfunction in diabetes and several other diseases []. High plasma glucose level can lead to increased ROS and decreased NO bioavailability and also cause the development of diabetic atherosclerosis. NOS substrates can slow down endothelial senescence under high glucose through NO, possibly due to interference with the redox balance of endothelial cells []. Arg is the common substrate for both enzymes NOS3 and arginase, which therefore compete. Consequently, the activation of arginase enzyme decreases Arg availability for NOS3 and NO production in tissues as well as in cells []. Previous authors reported that the administration of CIT, norvaline and ornithine in diabetic animals alleviated the hypertension associated with diabetes mellitus []. According to this, CIT and Arg plus CIT supplementation retarded high glucose-induced endothelial senescence []. Additionally, after 4 weeks of dietary supplementation in drinking water with Arg (0.2%) or watermelon rich in CIT (63%) enhanced endothelium-dependent relaxation in Zucker diabetic fatty rats []. Moreover, in a study involving patients with type 2 diabetes mellitus and microvascular complications, inhibiting the arginase enzyme improved resistance and conduit artery endothelial function. The increased arginase activity decreases the bioavailability of Arg as a substrate of NOS, therefore reducing the production of NO, and this has also been shown to be linked to increased reactive oxygen species production []. This may be due to the fact that NOS produces superoxide when there is a deficit of substrate Arg, this phenomenon is known as “eNOS uncoupling”, and further reduces the bioavailability of NO []. In addition, the inhibition of arginase can be carried out via direct or indirect mechanisms. The direct protective mechanism involves maintaining endothelial-dependent relaxation and generating NO, and the indirect mechanism is by inhibiting insulin resistance and hypertriglyceridemia []. On the other hand, in vivo experimental tests on mice demonstrated that Arg and CIT supplementation both enhanced the microcirculation in organs, through increased Arg availability and NO production; this was studied in conditions of acute Arg deficit, induced by the injection of the enzyme arginase []. However, You et al. [] reported that chronic Arg (1.5%) or CIT (1.66%) supplementation (in drinking water for 9 weeks) did not prevent or reduce renal injury in a model of type I diabetes in diabetic mice, despite greatly increasing kidney and plasma Arg levels. Finally, a recent systematic review concluded that CIT and watermelon extract may beneficially affect glycemic status and inflammation in diabetes mellitus, although studies on their antioxidant and hypolipidemic effects in diabetic patients are scarce [].
7.3. L-Citrulline and Erectile Dysfunction
Erectile dysfunction refers to the inability to achieve and maintain an erection sufficient to enable satisfactory sexual intercourse. NO is a physiological signal essential to penile erection [], since it acts as a neurotransmitter in the penile non-adrenergic non-cholinergic nerve fibers, as well as a vasodilator of smooth muscle cells of the penile arteries, sinusoids, and trabeculae []. Sexual stimulation releases NO to the smooth muscle of the penis, activating soluble guanylate cyclase to convert guanosine triphosphate into cyclic guanosine monophosphate (cGMP). This subsequently activates a specific protein kinase that produces phosphorylation of proteins and ion channels; cytosolic calcium is reduced because of the whole process, and penile smooth muscle is relaxed. Phosphodiesterase type 5 enzymes (PDE-5), hydrolyze cGMP to inactive GMP and, therefore, the erection of the penis ceases []. PDE-5 inhibitors enhance NO activity and are currently the most effective oral drugs to treat male erectile dysfunction. Interestingly, Lacchini and Tanus-Santos [] found that patients with vasculogenic erectile dysfunction presented increased plasma levels of arginase type II. Therefore, it would seem that these patients could benefit from Arg supplements. Previously, Shiota et al. [], studied a rat model with acute arteriogenic erectile dysfunction, demonstrating that oral CIT supplementation improved erectile function by increasing NO plasma concentrations. Shirai et al. [], also explored the effects of a combination therapy of CIT and transresveratrol with phosphodiesterase 5 inhibitors (PDE5i) versus placebo for erectile dysfunction in men. They reported that the addition of CIT and transresveratrol was effective for erectile dysfunction in men and may be added to PDE5i treatment to increase its efficacy.
7.4. L-Citrulline and Cancer
Dietary antioxidants have an instrumental role in preventing many human diseases (cancer, atherosclerosis, stroke, neurodegeneration, and diabetes), as redox imbalance can be strongly associated to oxidative disorders []. Related to this, iNOS is one of three classes of NOS and the strongest producer of NO which contributes to immune responses in certain types of cancer []. However, numerous aspects may affect NO having anti- or pro-tumorigenic effects; these include how much NO is at a specific location for a particular time span, the tumor environment, and the chemical redox environment []. Low NO concentrations (in the peak to nanomolar range) enhance tumor growth by angiogenesis and anti-apoptotic effects [], while higher levels of NO (micro- to millimolar) contribute to increase the oxidative levels resulting in cell apoptosis [,]. Increased arginase activity reduces NO availability and uncoupled NOS, producing reactive nitrogen species (RNS) and reactive oxygen species (ROS), respectively, rather than NO, and thus carcinogenesis is promoted []. Therefore, arginase promotes cancer progressing via the polyamine pathway or NO downregulation []. According to this, myeloid-derived suppressor cells (MDSCs) express arginase I and NOS to produce urea and l-ornithine, and NO and CIT, respectively to suppress tumor-specific T cell proliferation and cytotoxicity []. Arg is essential for T cell proliferation, which recognize and kill infected or cancerous cells []. Treatments involving only arginase inhibitor or combined with other therapies decreased and controlled tumor growth, in vivo, in mice with Lewis lung carcinoma tumors to improve the immune system []. Recently, a small-molecule arginase inhibitor CB-1158, 2-amino-6-borono-2-(1-(2,4-dichlorobenzyl) piperidin-4-yl) hexanoic acid, has been authorized for Phase I clinical trials in patients with advanced or metastatic solid tumors []. By contrast, the iNOS gene over-expression and increased NO levels were associated with the start and progress of oral precancer and gastric carcinoma [,,]. Furthermore, ablation of arginase II expression in a murine model of prostate cancer led to more aggressive tumor growth and development, therefore alternate pathways of polyamine biosynthesis may improve in the cancer cells in the absence of arginase II expression []. Additionally, the use of oral NO donors could provide a strategy for enhancing chemotherapeutic delivery to malignant brain tumors []. The delivery of chemotherapeutics in brain tumors is limited by the blood–brain tumor barrier (BTB), but Arg or hydroxyurea administered orally significantly increased the BTB. Moreover, the levels of NO, CIT and cGMP were increased in the tumor tissue by Arg and/or hydroxyurea administered orally. These increased cGMP levels in tumors may activate cGMP-dependent protein kinase, stimulating KCa channels [] which are highly expressed in tumor micro-vessels. Activating KCa channels leads to an increased vesicular transport of the drug from blood to brain tumor tissue [].
8. Studies with Citrulline from Natural Sources: Watermelon
The global sports nutrition market was valued at 50.84 billion U.S. dollars in 2018 and is expected to increase in value to 81.5 billion U.S. dollars by 2023 []. The main incentive of sports drinks consumption is to rehydrate, their purpose being primarily to replace the water and salts lost through perspiration; they contain water, electrolytes, and carbohydrates []. For elite and regular athletes involved in prolonged, physically demanding activities, these types of drink can be very useful, but prepubescent youths do not lose electrolytes at the same rate as adult athletes [] and these sports drinks are also consumed for leisure []. The American Academy of Pediatrics and other scientific associations do not recommend the consumption of sports drinks by children and adolescents due to their deleterious effects on health, such as the development of dental caries, overweight, obesity and an increased risk of hypertension in adulthood []. In this context, this is an opportunity for the beverage industry to provide natural drinks with bioactive compounds such as CIT when there are specific fruits such as watermelon that are rich in CIT. Consumers are encouraged to snack on natural products such as fruit and vegetables, and watermelon has the potential of a fruit snack []. Watermelon sales are increasing, including a variety of options, from fresh-cut to personal size. The fresh-cut industry helps to promote the consumption of fruit and vegetables, providing a healthy and convenient way to ingest the recommended daily intake. However, this industry presents a problem in the large quantity of waste produced, i.e., non-edible portions such as rind, seeds or even damaged fruits, which are discarded. In general, fresh-cut yields vary between 50% and 70% of the initial whole product. In the case of fresh-cut watermelon, the inedible portion is between 31% and 40% of the initial fresh weight, depending on the cultivar []. However, the reuse of these under-utilized agricultural wastes (which contain functional compounds) could be an example of circular economy for the fresh-cut industry, a policy to reduce loss and waste in line with the United Nations aim declared in this International Year of Fruits and Vegetables []. As mentioned before, in the case of watermelon, the rind is a valuable source of CIT and the extraction of this amino acid from watermelon rind and other fruits of the Cucurbitaceae family is possible []. This CIT could have food, pharmaceutical, or cosmetic applications.
Watermelon belongs to the Cucurbitaceae family, which includes squash, melon, pumpkin, cucumber, etc. Hartman et al. [] analyzed the CIT content from different Cucurbitaceae fruits: Citrullus lanatus (watermelon), Cucumis melo (melon), Cucumis metuliferus (horned melon), Cucumis sativus (cucumber), Cucurbita pepo (squash), Melothria scabra (mouse melon), and Momordica charantia (bitter gourd). They found the highest CIT concentration in red-flesh seeded watermelon (2.85 to 2.43 g/kg) followed by casaba-type melon (0.86), mouse melon (0.64), and horned melon rind (0.45 g/kg fresh weight). According to Akashi et al. [], this increase in CIT in some Citrullus species is a response to stress situations such as high light intensity and drought during the growth of the plant in the field.
Consumer interest in watermelon has increased in recent years, especially due to its beneficial effects on human health []. It is important to highlight the most important phytochemicals or bioactive compounds in watermelon; as has been mentioned before, watermelon is the richest known natural source of CIT, [,] and it is an effective precursor of Arg. Since watermelon was recognized as a significant and natural source of CIT with numerous therapeutic properties, many reports have been published in this area. This fruit is a good source of potassium (126 mg/100 g) and contains magnesium (10 mg/100 g), calcium (5.6 mg/100 g), sodium (0.81 mg/100 g), iron (0.26 mg/100 g), copper (0.042 mg/100 g), and zinc (0.031 mg/100 g) []. The sugar composition in watermelon includes, in decreasing order, fructose (3.45%), followed by sucrose (2.83%) and glucose (2.45%) []. The main vitamin in watermelon is vitamin C (8.1 mg/100 g), providing a strong antioxidant activity against free radicals [,]. This fruit is also rich in carotenoid such as lycopene and β-carotene. Watermelon lycopene extracts also have the capability to scavenge free radicals, acting as a natural antioxidant []. Many epidemiological studies have demonstrated that tomato lycopene could help to lower the risk of developing the incidence of some cancers []. The amounts of lycopene in watermelon fruits range from 35 to 112 mg/kg fresh weight, depending on the watermelon cultivar and ripening stages []. Lycopene from watermelon does not need to be heated, like tomato, for improved bioavailability, with watermelon being one of the few sources with readily available cis-isomeric lycopene []. Watermelon is also a rich source of β-carotene, which acts as an antioxidant and precursor of vitamin A. This fruit has a significant source of total phenolic and flavonoid []. All these functional compounds explain the health benefits of watermelon consumption. However, this review is focused on CIT benefits and we will discuss this section from this point of view but with the knowledge that other bioactive compounds present in watermelon could contribute to a specific health effect.
Based on the richness of bioactive compounds, the food industry is researching different natural foods, offering healthy properties or positive effects in a particular population (such as sportspeople) as substitutes for pharmacological products []. Athletes frequently employ legal ergogenic aids to enhance exercise performance, with the elimination of fatigue symptoms being particularly important. Eccentric exercise produces delayed-onset muscle soreness that often persists for several days. Such muscle damage causes muscle fatigue, thus limiting performance, and negatively affecting force, peak power, and/or velocity []. The number of studies focusing on CIT describing enhanced exercise performance has increased recently []. Watermelon is a principal food source of CIT [] and its consumption raises plasma Arg concentrations in adults []. Furthermore, in vitro CIT bioavailability tests have reported that a natural source of CIT (watermelon juice) could be more bioavailable than a synthetic source [].
Recent studies have reported that diminished muscle soreness perception with just one dose of watermelon juice enriched in CIT can enhance exercise performance [,]. In addition, plasma lactate levels were lower in watermelon juice enriched in CIT (3.45 g per 500 mL) than placebo, after a half-marathon race in trained runners []. Additionally, Martínez-Sánchez et al. [] demonstrated the functionality of two different watermelon juices, to test a single dose of two different concentrations of CIT (0.5 and 3.3 g per 200 mL) in watermelon juice matrix and the positive effect of ellagitannins (22 mg per 200 mL) on submaximal resistance exercise performance to exhaustion in trained resistance athletes. They observed an increase in mean peak force of about 3% and a five-fold fall in the decrease in peak torque. Subjects that took the watermelon and pomegranate juice mix enriched with CIT reported the lowest muscle soreness perception values 1 h after eight repetition maximum (RM) exercises and 24 h after exercise, showing around 44–60% decrease in muscle soreness compared to placebo. In another previous study, acute watermelon juice consumption (providing 1.17 g of CIT per 500 mL) and enriched watermelon juice (6 g of CIT per 500 mL) attenuated moderate muscle soreness after 24 h of exercise in untrained healthy subjects participating in high-intensity exercise intervals, although performance was unimproved []. In another study, with healthy non-athletic adults, no significant differences were found among different sport supplements in blood lactate, heart rate recovery or post-exercise muscle soreness. However, when females had acute watermelon juice supplementation (355 mL) it prevented increased diastolic blood pressure and post-exercise systolic pressure; such responses were not obtained in men [].
On the other hand, chronic supplementation of watermelon juice (16 days with 300 mL/day of watermelon juice concentrate, providing a daily CIT dose of ~3.4 g/day) with respect to placebo showed a higher skeletal muscle oxygenation index in watermelon juice conditions than in placebo and control conditions, although time-to-exhaustion during the severe-intensity exercise test did not differ significantly between conditions, with systolic blood pressure being higher in watermelon juice conditions []. Moreover, another study of chronic supplementation of frozen watermelon puree (two weeks ingesting 980 mL/day 0.2 g/kg carbohydrate with 1.47 g CIT, 0.465 g Arg versus a 6% carbohydrate beverage every 15 min throughout the time trials) in 20 trained cyclists, found that exercise performance was similar under watermelon and placebo conditions, and that the highest RPE was observed under watermelon conditions []. In line with this, Cutrufello et al. [] did not find improved exercise performance following one dose of CIT (6 g) or watermelon juice (~1.0 g CIT) taken 1–2 h before exercise in healthy male subjects. A systematic review [] to evaluate the effect of CIT watermelon supplements (1.17–6 g of CIT obtained from watermelon) on the post-exercise RPE and muscle soreness has recently been published. In that review, the supplementation time frame was limited to 2 h before exercise. The types and number of participants, types of exercise tests performed, supplementation protocols, and primary (RPE and muscle soreness) and secondary (blood lactate level) study outcomes were extracted from the identified studies. The study involved 13 eligible articles (10 of them using CM and 3 using watermelon supplementation) and a total of 206 participants. The most frequent dosage used in the studies was 8 g of CM. CIT watermelon supplementation significantly reduced RPE (n = 7, p = 0.03) and muscle soreness 24-h and 48-h after post-exercise (n = 7, p = 0.04; n = 6, p = 0.03, respectively). However, CIT from watermelon supplementation did not significantly reduce muscle soreness 72-h post-exercise (n = 4, p = 0.62) or lower blood lactate levels (n = 8, p = 0.17). As the main conclusion, CIT supplements from watermelon significantly reduced post-exercise RPE and muscle soreness, without affecting blood lactate levels. This is especially recommended for power and strength athletes to adequately recover and subsequently train at their desired intensity level. Different factors probably influence the ergogenic effect of CIT on perceived exercise improvement, which makes the results in studies analyzing CIT responses somewhat controversial and so warrants further study in the future.
Other benefits of watermelon consumption have been related to cardiovascular risk factors. Massa et al. [] found a reduction in low-density lipoprotein and plasma total cholesterol when volunteers with dyslipidemia were supplemented daily with 6 g of watermelon extract for 42 days. Figueroa et al. [] went further and demonstrated that the consumption of watermelon extract (4:2 CIT/Arg, 6 g per day divided into three doses for 6 weeks) in obese hypertension postmenopausal women could reduce arterial stiffness and aortic systolic blood pressure by reducing the pressure wave reflection amplitude, providing cardio protection by lessening cold-induced aortic hemodynamic responses []. Figueroa et al. [] suggested in another study that watermelon extract (6 g of CIT/Arg (2/1) per day for 6 weeks separated by a 2-week washout period) improved arterial function in obese middle-aged adults with prehypertension or stage 1 hypertension. Shanely et al. [] recommended daily watermelon consumption (710 mL 100% watermelon puree per day, containing natural 1.88 g CIT and 0.40 g Arg) for 6 weeks, because it helped to decrease sVCAM-1 levels, a marker connected with atherogenesis, in overweight and obese postmenopausal women, without changing glycemic homeostasis. Lum et al. [] also demonstrated the capacity of watermelon (consumption of 2 cups daily for 4 weeks) to generate satiety responses, decreasing body mass index and weight in overweight and obese adults. In blood, the watermelon consumption increased the total antioxidant capacity, reduced the systolic blood pressure, and improved the blood lipid profile.
Studies performed in rats have shown that watermelon improved risk factors for cardiovascular disease by improving lipid profiles and antioxidant capacity, reducing inflammation, by altering gene expression for lipid metabolism and improved colitis by maintaining normal colonic crypt morphology and modulating the homeostasis of cell proliferation and apoptosis in rats [,,].
9. Conclusion, Perspectives and Limitations
The intake of CIT can help to prevent important diseases such as cardiovascular diseases and the effects of diabetes, reduce erectile dysfunction and improve exercise performance. However, some results are controversial, perhaps due to the different doses applied and the different origin of CIT (natural source or chemical origin). This non-essential amino acid has important roles in human health, and it ought to be further studied in large-scale clinical trials. The consumption of watermelon is recommended since it is the fruit that is richest in CIT. The limitation of this review lies in the coexistence of studies carried out with both humans and animals; this fact should be considered before using the results in the clinical field and in sports.
Author Contributions
Writing-original draft preparation, E.A., A.M.-S. and B.F.-L. Writing-review and editing, E.A. and F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to AMC Juice and Drink company and Fashion Watermelon Association (AGF) for supporting the experiments performed in watermelon.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bescós, R.; Sureda, A.; Tur, J.A.; Pons, A. The Effect of Nitric-Oxide-Related Supplements on Human Performance. Sports Med. 2012, 42, 99–117. [Google Scholar] [CrossRef]
- Mandel, H.; Levy, N.; Izkovitch, S.; Korman, S.H. Elevated plasma citrulline and arginine due to consumption of Citrullus vulgaris (watermelon). J. Inherit. Metab. Dis. 2005, 28, 467–472. [Google Scholar] [CrossRef]
- Windmueller, H.G.; Spaeth, A.E. Source and fate of circulating citrulline. Am. J. Physiol. Endocrinol. Metab. 1981, 241, E473–E480. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.P.; Viegas, J.; Moldao Martins, M.; Aguayo, E. Bio-active compounds of different cultivars from flesh and by-product of fresh-cut watermelons. J. Sci. Food Agric. 2011, 91, 805–812. [Google Scholar] [CrossRef]
- Joshi, V.; Joshi, M.; Silwal, D.; Noonan, K.; Rodriguez, S.; Penalosa, A. Systematized biosynthesis and catabolism regulate citrulline accumulation in watermelon. Phytochemistry 2019, 162, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, E.; Tarazona-Díaz, M.P. National Patent: Procedimiento Para la Obtención de un Extracto de L-Citrulina a Partir de Plantas Cucurbitáceas. ES2394250 A1. Available online: http://www.oepm.es/pdf/ES/0000/000/02/39/42/ES-2394250_A1.pdf. (accessed on 12 November 2020).
- FAOSTAT Agriculture Data. 2021. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 12 November 2020).
- FAO. Fruit and Vegetables–Your Dietary Essentials. The International Year of Fruits and Vegetables, 2021, Background Paper. Rome. 2020. Available online: http://www.fao.org/documents/card/en/c/cb2395en (accessed on 15 November 2020).
- Crenn, P.; Messing, B.; Cynober, L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin. Nutr. 2008, 27, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Breuillard, C.; Cynober, L.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.C.; Cynober, L.; Sfar, S. Citrulline and nitrogen homeostasis: An overview. Amino Acids 2015, 47, 685–691. [Google Scholar] [CrossRef]
- Bahri, S.; Zerrouk, N.; Aussel, C.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.C.; Cynober, L.; Sfar, S. Citrulline: From metabolism to therapeutic use. Nutrition 2013, 29, 479–484. [Google Scholar] [CrossRef]
- Marini, J.C.; Didelija, I.C.; Castillo, L.; Lee, B. Plasma Arginine and Ornithine Are the Main Citrulline Precursors in Mice Infused with Arginine-Free Diets. J. Nutr. 2010, 140, 1432–1437. [Google Scholar] [CrossRef]
- Tomlinson, C.; Rafii, M.; Ball, R.O.; Pencharz, P.B. Arginine Can Be Synthesized from Enteral Proline in Healthy Adult Humans. J. Nutr. 2011, 141, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Intestinal Mucosal Amino Acid Catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Barzał, J.A.; Szczylik, C.; Rzepecki, P.; Jaworska, M.; Anuszewska, E. Plasma citrulline level as a biomarker for cancer therapy-induced small bowel mucosal damage. Acta Biochim. Pol. 2014, 61, 615–631. [Google Scholar] [CrossRef]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Ten Have, G.A.M.; Wolfe, R.R.; Deutz, N.E.P. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef]
- Morris, S.M. Regulation of arginine availability and its impact on NO synthesis. In Nitric Oxide: Biology and Pathobiology; Academic Press: Cambridge, MA, USA, 2020; pp. 187–197. [Google Scholar]
- Van De Poll, M.C.G.; Soeters, P.B.; Deutz, N.E.P.; Fearon, K.C.H.; DeJong, C.H.C. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Gotoh, T. Arginine Metabolic Enzymes, Nitric Oxide and Infection. J. Nutr. 2004, 134, 2820S–2825S; discussion 2853S. [Google Scholar] [CrossRef] [PubMed]
- Durante, W.; Johnson, F.K.; Johnson, R.A. Arginase: A Critical Regulator of Nitric Oxide Synthesis and Vascular Function. Clin. Exp. Pharmacol. Physiol. 2007, 34, 906–911. [Google Scholar] [CrossRef]
- Pernow, J.; Jung, C. Arginase as a potential target in the treatment of cardiovascular disease: Reversal of arginine steal? Cardiovasc. Res. 2013, 98, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease-A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Böger, R.H.; Galland, A.; Tsikas, D.; Frölich, J.C. L-arginine-induced vasodilation in healthy humans: Pharmacokinetic-pharmacodynamic relationship. Br. J. Clin. Pharmacol. 1998, 46, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Husson, A.; Brasse-Lagnel, C.; Fairand, A.; Renouf, S.; Lavoinne, A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur. J. Biochem.. 2003, 270, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Foörstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Morita, M.; Kobayashi, Y.; Kamimura, A. Oral L-citrulline supplementation enhances cycling time trial performance in healthy trained men: Double-blind randomized placebo-controlled 2-way crossover study. J. Int. Soc. Sports Nutr. 2016, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Hayashi, T.; Ochiai, M.; Maeda, M.; Yamaguchi, T.; Ina, K.; Kuzuya, M. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem. Biophys. Res. Commun. 2014, 454, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef]
- Böger, R.H.; Sullivan, L.M.; Schwedhelm, E.; Wang, T.J.; Maas, R.; Benjamin, E.J.; Schulze, F.; Xanthakis, V.; Benndorf, R.A.; Vasan, R.S. Plasma Asymmetric Dimethylarginine and Incidence of Cardiovascular Disease and Death in the Community. Circulation 2009, 119, 1592–1600. [Google Scholar] [CrossRef]
- Kaore, S.N.; Amane, H.S.; Kaore, N.M. Citrulline: Pharmacological perspectives and its role as an emerging biomarker in future. Fundam. Clin. Pharmacol. 2013, 27, 35–50. [Google Scholar] [CrossRef]
- Cynober, L.; de Bandt, J.P.; Moinard, C. Leucine and citrulline: Two major regulators of protein turnover. In Nutrition in Intensive Care Medicine: Beyond Physiology; Singer, P., Ed.; Karger Medical and Scientific Publishers: Basel, Switzerland, 2012; Volume 105, pp. 97–105. [Google Scholar]
- Le Plénier, S.; Walrand, S.; Noirt, R.; Cynober, L.; Moinard, C. Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: A common activation pathway? Amino Acids 2012, 43, 1171–1178. [Google Scholar] [CrossRef]
- Faure, C.; Raynaud-Simon, A.; Ferry, A.; Daugé, V.; Cynober, L.; Aussel, C.; Moinard, C. Leucine and citrulline modulate muscle function in malnourished aged rats. Amino Acids 2011, 42, 1425–1433. [Google Scholar] [CrossRef]
- Jourdan, M.; Nair, K.S.; Carter, R.E.; Schimke, J.; Ford, G.C.; Marc, J.; Aussel, C.; Cynober, L. Citrulline stimulates muscle protein synthesis in the post-absorptive state in healthy people fed a low-protein diet–A pilot study. Clin. Nutr. 2015, 34, 449–456. [Google Scholar] [CrossRef]
- Goron, A.; Lamarche, F.; Cunin, V.; Dubouchaud, H.; Hourdé, C.; Noirez, P.; Corne, C.; Couturier, K.; Sève, M.; Fontaine, E.; et al. Synergistic effects of citrulline supplementation and exercise on performance in male rats: Evidence for implication of protein and energy metabolisms. Clin. Sci. 2017, 131, 775–790. [Google Scholar] [CrossRef]
- Ventura, G.; Noirez, P.; Breuillé, D.; Godin, J.P.; Pinaud, S.; Cleroux, M.; Choisy, C.; Le Plénier, S.; Bastic, V.; Neveux, N.; et al. Effect of citrulline on muscle functions during moderate dietary restriction in healthy adult rats. Amino Acids 2013, 45, 1123–1131. [Google Scholar] [CrossRef]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Benazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef]
- Romero, M.J.; Platt, D.H.; Caldwell, R.W. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar]
- Rougé, C.; Des Robert, C.; Robins, A.; Le Bacquer, O.; Volteau, C.; De La Cochetière, M.F.; Darmaun, D. Manipulation of citrulline availability in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Moinard, C.; Maccario, J.; Walrand, S.; Lasserre, V.; Marc, J.; Boirie, Y.; Cynober, L. Arginine behaviour after arginine or citrulline administration in older subjects. Br. J. Nutr. 2016, 115, 399–404. [Google Scholar] [CrossRef][Green Version]
- Closs, E.I.; Simon, A.; Vékony, N.; Rotmann, A. Plasma Membrane Transporters for Arginine. J. Nutr. 2004, 134, 2752S–2759S. [Google Scholar] [CrossRef]
- Vadgama, J.V.; Evered, D.F. Characteristics of L-Citrulline Transport across Rat Small Intestine In Vitro. Pediatr. Res. 1992, 32, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. L-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Grimble, G.K. Adverse Gastrointestinal Effects of Arginine and Related Amino Acids. J. Nutr. 2007, 137, 1693S–1701S. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.K.; Wu, G.; Perkins-Veazie, P.; Spears, K.; Claypool, P.L.; Baker, R.A.; Clevidence, B.A. Watermelon consumption increases plasma arginine concentrations in adults. Nutrients 2007, 23, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Flet, L.; Vavasseur, F.; Lemerle, M.; Ferchaud-Roucher, V.; Picot, D.; Darmaun, D. Oral citrulline does not affect whole body protein metabolism in healthy human volunteers: Results of a prospective, randomized, double-blind, cross-over study. Clin. Nutr. 2011, 30, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Cutrufello, P.T.; Gadomski, S.J.; Zavorsky, G.S. The effect of L-citrulline and watermelon juice supplementation on anaerobic and aerobic exercise performance. J. Sports Sci. 2015, 33, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. l-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Alacid, F.; Rubio-Arias, J.A.; Fernández-Lobato, B.; Ramos-Campo, D.J.; Aguayo, E. Consumption of Watermelon Juice Enriched in l -Citrulline and Pomegranate Ellagitannins Enhanced Metabolism during Physical Exercise. J. Agric. Food Chem. 2017, 65, 4395–4404. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Ramos-Campo, D.J.; Fernández-Lobato, B.; Rubio-Arias, J.A.; Alacid, F.; Aguayo, E. Biochemical, physiological, and performance response of a functional watermelon juice enriched in L-citrulline during a half-marathon race. Food Nutr. Res. 2017, 61, 1330098. [Google Scholar] [CrossRef]
- Buckinx, F.; Gouspillou, G.; Carvalho, L.P.; Marcangeli, V.; Boutros, G.E.H.; Dulac, M.; Noirez, P.; Morais, J.A.; Gaudreau, P.; Aubertin-Leheudre, M. Effect of High-Intensity Interval Training Combined with L-Citrulline Supplementation on Functional Capacities and Muscle Function in Dynapenic-Obese Older Adults. J. Clin. Med. 2018, 7, 561. [Google Scholar] [CrossRef]
- Stanelle, S.T.; McLaughlin, K.L.; Crouse, S.F. One Week of L-Citrulline Supplementation Improves Performance in Trained Cyclists. J. Strength Cond. Res. 2020, 34, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.; Kim, Y.; Gonzales, J.U. Impact of l-citrulline supplementation on oxygen uptake kinetics during walking. Appl. Physiol. Nutr. Metab. 2018, 43, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Platt, D.H.; Tawfik, H.E.; Labazi, M.; El-Remessy, A.B.; Bartoli, M.; Caldwell, R.B.; Caldwell, R.W. Diabetes-induced Coronary Vascular Dysfunction Involves Increased Arginase Activity. Circ. Res. 2008, 102, 95–102. [Google Scholar] [CrossRef]
- McKinley-Barnard, S.; Andre, T.; Morita, M.; Willoughby, D.S. Combined L-citrulline and glutathione supplementation increases the concentration of markers indicative of nitric oxide synthesis. J. Int. Soc. Sports Nutr. 2015, 12, 27. [Google Scholar] [CrossRef]
- Sureda, A.; Córdova, A.; Ferrer, M.D.; Pérez, G.; Tur, J.A.; Pons, A. l-Citrulline-malate influence over branched chain amino acid utilization during exercise. Eur. J. Appl. Physiol. 2010, 110, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Machida, M.; Kohara, A.; Omi, N.; Takemasa, T. Effects of citrulline supplementation on fatigue and exercise performance in mice. J. Nutr. Sci. Vitaminol. 2011, 57, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.U.; Raymond, A.; Ashley, J.; Kim, Y. Doesl-citrulline supplementation improve exercise blood flow in older adults? Exp. Physiol. 2017, 102, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Meneguello, M.O.; Mendonça, J.R.; Lancha, A.H.; Costa Rosa, L.F.B.P. Effect of arginine, omithine and citrulline supplementation upon performance and metabolism of trained rats. Cell Biochem. Funct. 2013, 21, 85–91. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Blomstrand, E. Branched-Chain Amino Acids and Central Fatigue. J. Nutr. 2006, 136, 274S–276S. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Branched-Chain Amino Acids and Brain Function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef]
- MacLean, D.A.; Graham, T.E.; Saltin, B. Stimulation of muscle ammonia production during exercise following branched-chain amino acid supplementation in humans. J. Physiol. 1996, 493 Pt 3, 909–922. [Google Scholar] [CrossRef]
- Nybo, L.; Dalsgaard, M.K.; Steensberg, A.; Møller, K.; Secher, N.H. Cerebral ammonia uptake and accumulation during prolonged exercise in humans. J. Physiol. 2005, 563, 285–290. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Smeeton, N.J.; Watt, P.W. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog. Neurobiol. 2010, 91, 200–219. [Google Scholar] [CrossRef]
- Chang, C.-K.; Chien, K.-M.C.; Chang, J.-H.; Huang, M.-H.; Liang, Y.-C.; Liu, T.-H. Branched-Chain Amino Acids and Arginine Improve Performance in Two Consecutive Days of Simulated Handball Games in Male and Female Athletes: A Randomized Trial. PLoS ONE 2015, 10, e0121866. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-F.; Wu, H.-J.; Chen, C.-Y.; Chou, K.-M.; Chang, C.-K. Branched-chain amino acids, arginine, citrulline alleviate central fatigue after 3 simulated matches in taekwondo athletes: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2016, 13, 28. [Google Scholar] [CrossRef]
- Pérez-Guisado, J.; Jakeman, P.M. Citrulline Malate Enhances Athletic Anaerobic Performance and Relieves Muscle Soreness. J. Strength Cond. Res. 2010, 24, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Bendahan, D.; Mattei, J.P.; Ghattas, B.; Confort-Gouny, S.; Le Guern, M.E.; Cozzone, P.J. Citrulline/malate promotes aerobic energy production in human exercising muscle. Br. J. Sports Med. 2002, 36, 282–289. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 92–98. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Wethington, L.N.; Stone, M.S.; Stewart, R.W.; Moyen, N.E. Acute citrulline malate supplementation improves upper- and lower-body submaximal weightlifting exercise performance in resistance-trained females. Eur. J. Nutr. 2015, 56, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Kiyici, F.; Eroglu, H.; Kishali, N.F.; Burmaoglu, G. The Effect of Citrulline/Malate on Blood Lactate Levels in Intensive Exercise. Biochem. Genet. 2017, 55, 387–394. [Google Scholar] [CrossRef]
- López-Cabral, J.A.; Rivera-Cisneros, A.; Rodríguez-Camacho, H.; Sánchez-González, J.M.; Serna-Sánchez, I.; Trejo-Trejo, M. Modification of fatigue indicators using citrulline malate for high performance endurance athletes. Rev. Mex. Patol. Clin. Med. Lab. 2012, 59, 194–201. [Google Scholar]
- Giannesini, B.; Le Fur, Y.; Cozzone, P.J.; Verleye, M.; Le Guern, M.E.; Bendahan, D. Citrulline malate supplementation increases muscle efficiency in rat skeletal muscle. Eur. J. Pharmacol. 2011, 667, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Wax, B.; Kavazis, A.N.; Weldon, K.; Sperlak, J. Effects of Supplemental Citrulline Malate Ingestion During Repeated Bouts of Lower-Body Exercise in Advanced Weightlifters. J. Strength Cond. Res. 2015, 29, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, B.; Papageorgiou, M.; O’Brien, B.; Davies, N.A.; Grimble, G.K.; Cardinale, M. Acute Citrulline-Malate Supplementation and High-Intensity Cycling Performance. J. Strength Cond. Res. 2016, 30, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.K.; Jacinto, J.L.; de Andrade, W.B.; Roveratti, M.C.; Estoche, J.M.; Balvedi, M.C.W.; de Oliveira, D.B.; da Silva, R.A.; Aguiar, A.F. Citrulline malate does not improve muscle recovery after resistance exercise in untrained young adult men. Nutrients 2017, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Casonatto, J.; Cavalaria, J.V.; Goessler, K.F.; Christofaro, D.G.D.; Polito, M.D.; Enokida, D.M.; Grandolfi, K. Citrulline malate supplementation might potentiate post-exercise hypotension in hypertensives: A 24-hour analysis. Sci. Sports 2019, 34, 261.1–261.9. [Google Scholar] [CrossRef]
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelial Dysfunction as a Target for Prevention of Cardiovascular Disease. Diabetes Care 2009, 32, S314–S321. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karıncaoglu, D.; Gülseren, G.; Şenol, E.; Demircan, E.; et al. Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef]
- Le Roux-Mallouf, T.; Pelen, F.; Vallejo, A.; Halimaoui, I.; Doutreleau, S.; Verges, S. Effect of chronic nitrate and citrulline supplementation on vascular function and exercise performance in older individuals. Aging 2019, 11, 3315–3332. [Google Scholar] [CrossRef]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Juliet, P.A.R.; Matsui-Hirai, H.; Miyazaki, A.; Fukatsu, A.; Funami, J.; Iguchi, A.; Ignarro, L.J. L-citrulline and L-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Proc. Natl. Acad. Sci. USA 2005, 102, 13681–13686. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Sakurada, M.; Watanabe, F.; Yamasaki, T.; Doi, H.; Ezaki, H.; Morishita, K.; Miyake, T. Effects of Oral L-Citrulline Supplementation on Lipoprotein Oxidation and Endothelial Dysfunction in Humans with Vasospastic Angina. Immunol. Endocr. Metab. Agents Med. Chem. 2013, 13, 214–220. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Schutzler, S.E.; Schrader, A.; Spencer, H.J.; Azhar, G.; Deutz, N.E.P.; Wolfe, R.R. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am. J. Physiol. Metab. 2015, 309, E915–E924. [Google Scholar] [CrossRef]
- Wong, A.; Chernykh, O.; Figueroa, A. Chronic l-citrulline supplementation improves cardiac sympathovagal balance in obese postmenopausal women: A preliminary report. Auton. Neurosci. 2016, 198, 50–53. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; El-Fawal, R.; Fahmy, A. Arginase inhibition alleviates hypertension associated with diabetes: Effect on endothelial dependent relaxation and NO production. Vasc. Pharmacol. 2012, 57, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Maeda, M.; Hayashi, T. Administration of L-arginine plus L-citrulline or L-citrulline alone successfully retarded endothelial senescence. PLoS ONE 2018, 13, e0192252. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Collins, J.K.; Perkins-Veazie, P.; Siddiq, M.; Dolan, K.D.; Kelly, K.A.; Heaps, C.L.; Meininger, C.J. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J. Nutr. 2007, 137, 2680–2685. [Google Scholar] [CrossRef]
- Kövamees, O.; Shemyakin, A.; Checa, A.; Wheelock, C.E.; Lundberg, J.O.; Östenson, C.-G.; Pernow, J. Arginase inhibition improves microvascular endothelial function in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2016, 101, 3952–3958. [Google Scholar] [CrossRef] [PubMed]
- El-Bassossy, H.M.; El-Fawal, R.; Fahmy, A.; Watson, M.L. Arginase inhibition alleviates hypertension in the metabolic syndrome. Br. J. Pharmacol. 2013, 169, 693–703. [Google Scholar] [CrossRef]
- Wijnands, K.A.; Meesters, D.M.; Van Barneveld, K.W.Y.; Visschers, R.G.J.; Briedé, J.J.; VandenDriessche, B.; Van Eijk, H.M.H.; Bessems, B.A.F.M.; Hoven, N.V.D.; Von Wintersdorff, C.J.H.; et al. Citrulline Supplementation Improves Organ Perfusion and Arginine Availability under Conditions with Enhanced Arginase Activity. Nutrients 2015, 7, 5217–5238. [Google Scholar] [CrossRef]
- You, H.; Gao, T.; Cooper, T.K.; Morris, S.M.; Awad, A.S. Diabetic nephropathy is resistant to oral l-arginine or l-citrulline supplementation. Am. J. Physiol. Renal Physiol. 2014, 307, F1292–F1301. [Google Scholar] [CrossRef]
- Azizi, S.; Mahdavi, R.; Vaghef-Mehrabany, E.; Maleki, V.; Karamzad, N.; Ebrahimi-Mameghani, M. Potential roles of Citrulline and watermelon extract on metabolic and inflammatory variables in diabetes mellitus, current evidence and future directions: A systematic review. Clin. Exp. Pharmacol. Physiol. 2019, 47, 187–198. [Google Scholar] [CrossRef]
- Cormio, L.; De Siati, M.; Lorusso, F.; Selvaggio, O.; Mirabella, L.; Sanguedolce, F.; Carrieri, G. Oral L-Citrulline Supplementation Improves Erection Hardness in Men With Mild Erectile Dysfunction. Urology 2011, 77, 119–122. [Google Scholar] [CrossRef]
- Barassi, A.; Romanelli, M.M.C.; Pezzilli, R.; Damele, C.A.L.; Vaccalluzzo, L.; Goi, G.; Papini, N.; Colpi, G.M.; Massaccesi, L.; D’Eril, G.V.M. Levels of l -arginine and l -citrulline in patients with erectile dysfunction of different etiology. Andrology 2017, 5, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Lacchini, R.; Tanus-Santos, J.E. Pharmacogenetics of erectile dysfunction: Navigating into uncharted waters. Pharmacogenomics 2014, 15, 1519–1538. [Google Scholar] [CrossRef]
- Shiota, A.; Hotta, Y.; Kataoka, T.; Morita, M.; Maeda, Y.; Kimura, K. Oral l-Citrulline Supplementation Improves Erectile Function in Rats with Acute Arteriogenic Erectile Dysfunction. J. Sex. Med. 2013, 10, 2423–2429. [Google Scholar] [CrossRef]
- Shirai, M.; Hiramatsu, I.; Aoki, Y.; Shimoyama, H.; Mizuno, T.; Nozaki, T.; Fukuhara, S.; Iwasa, A.; Kageyama, S.; Tsujimura, A. Oral L-citrulline and Transresveratrol Supplementation Improves Erectile Function in Men With Phosphodiesterase 5 Inhibitors: A Randomized, Double-Blind, Placebo-Controlled Crossover Pilot Study. Sex. Med. 2018, 6, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Azzini, E.; Zucca, P.; Varoni, E.M.; Kumar, N.V.A.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Burke, A.J.; Sullivan, F.J.; Giles, F.J.; Glynn, S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis 2013, 34, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.M.; Girotti, A.W. Accelerated migration and invasion of prostate cancer cells after a photodynamic therapy-like challenge: Role of nitric oxide. Nitric Oxide-Biol. Chem. 2015, 49, 47–55. [Google Scholar] [CrossRef]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef]
- Seabra, A.B. Nitric Oxide Donors: Novel Biomedical Applications and Perspective, 1st ed.; Elsevier Inc.: London, UK, 2017. [Google Scholar]
- Timosenko, E.; Hadjinicolaou, A.V.; Cerundolo, V. Modulation of cancer-specific immune responses by amino acid degrading enzymes. Immunotherapy 2017, 9, 83–97. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Munder, M. Arginase: An emerging key player in the mammalian immune system: REVIEW. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.-N.; Liagre, B.; Girard-Thernier, C.; Demougeot, C. Research of novel anticancer agents targeting arginase inhibition. Drug Discov. Today 2018, 23, 871–878. [Google Scholar] [CrossRef]
- Ambe, K.; Watanabe, H.; Takahashi, S.; Nakagawa, T.; Sasaki, J. Production and physiological role of NO in the oral cavity. Jpn. Dent. Sci. Rev. 2016, 52, 14–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajendran, R.; Varkey, S. Inducible nitric oxide synthase expression is upregulated in oral submucous fibrosis. Indian J. Dent. Res. 2007, 18, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Saito, H.; Oro, S.; Tatebe, S.; Ikeguchi, M.; Tsujitani, S. Expression of Inducible Nitric Oxide Synthase Is Significantly Correlated with Expression of Vascular Endothelial Growth Factor and Dendritic Cell Infiltration in Patients with Advanced Gastric Carcinoma. Oncology 2005, 68, 471–478. [Google Scholar] [CrossRef]
- Mumenthaler, S.M.; Rozengurt, N.; Livesay, J.C.; Sabaghian, A.; Cederbaum, S.D.; Grody, W.W. Disruption of arginase II alters prostate tumor formation in TRAMP mice. Prostate 2008, 68, 1561–1569. [Google Scholar] [CrossRef]
- Yin, D.; Wang, X.; Konda, B.M.; Ong, J.M.; Hu, J.; Sacapano, M.R.; Ko, M.K.; Espinoza, A.J.; Irvin, D.K.; Shu, Y.; et al. Increase in Brain Tumor Permeability in Glioma-Bearing Rats with Nitric Oxide Donors. Clin. Cancer Res. 2008, 14, 4002–4009. [Google Scholar] [CrossRef] [PubMed]
- Onoue, H.; Katusic, Z.S. Role of potassium channels in relaxations of canine middle cerebral arteries induced by nitric oxide donors. Stroke 1997, 28, 1264–1271. [Google Scholar] [CrossRef]
- Ningaraj, N.S.; Rao, M.; Hashizume, K.; Asotra, K.; Black, K.L. Regulation of Blood-Brain Tumor Barrier Permeability by Calcium-Activated Potassium Channels. J. Pharmacol. Exp. Ther. 2002, 301, 838–851. [Google Scholar] [CrossRef]
- Statista. Global Sports Nutrition Market 2018–2023. Available online: https://www.statista.com/study/37001/sports-drinks-statista-dossier (accessed on 8 January 2021).
- Fields, S.K.; Macdonald, J.; Joseph, A.M.; Wold, L.E.; Collins, C.L.; Comstock, R.D. Consumption of Sports and Energy Drinks by High School Athletes in the United States: A Pilot Study. Beverages 2015, 1, 218–224. [Google Scholar] [CrossRef]
- Cordrey, K.; Keim, S.A.; Milanaik, R.; Adesman, A. Adolescent Consumption of Sports Drinks. Pediatrics 2018, 141, e20172784. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Muñoz, V.; Urquizu-Rovira, M.; Valls-Ibañez, V.; Manresa-Domínguez, J.M.; Ruiz-Blanco, G.; Urquizu-Rovira, M.; Toran, P. Consumo de bebidas refrescantes, deportivas y energéticas en adolescentes. Estudio BEENIS. In Anales de Pediatría; Elsevier Doyma: Amsterdam, The Netherlands, 2020; Volume 93, pp. 242–250. [Google Scholar]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef]
- Maoto, M.M.; Beswa, D.; Jideani, A.I.O. Watermelon as a potential fruit snack. Int. J. Food Prop. 2019, 22, 355–370. [Google Scholar] [CrossRef]
- Hartman, J.L.; Wehner, T.C.; Ma, G.; Perkins-Veazie, P. Citrulline and Arginine Content of Taxa of Cucurbitaceae. Horticulturae 2019, 5, 22. [Google Scholar] [CrossRef]
- Akashi, K.; Miyake, C.; Yokota, A. Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 2001, 508, 438–442. [Google Scholar] [CrossRef]
- Hdider, C.; Tlili, I.; Ilahy, R. Chapter 32–Watermelon. Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 515–531. ISBN 9780128127803. [Google Scholar]
- Naz, A.; Butt, M.S.; Pasha, I.; Nawaz, H. Antioxidant Indices of Watermelon Juice and Lycopene Extract. Pak. J. Nutr. 2013, 12, 255–260. [Google Scholar] [CrossRef]
- Candir, E.; Yetisir, H.; Karaca, F.; Ustun, D. Phytochemical characteristics of grafted watermelon on different bottle gourds (Lagenaria siceraria) collected from Mediterranean region of Turkey. Turk. J. Agric.-Food Sci. Technol. 2013, 37, 443–456. [Google Scholar]
- USDA. USDA National Nutrient Database for Standard Reference Release 23; Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2020.
- Tlili, I.; Hdider, C.; Lenucci, M.S.; Ilahy, R.; Jebari, H.; Dalessandro, G. Bioactive compounds and antioxidant activities during fruit ripening of watermelon cultivars. J. Food Compos. Anal. 2011, 24, 923–928. [Google Scholar] [CrossRef]
- Kim, C.-H.; Park, M.-K.; Kim, S.-K.; Cho, Y.-H. Antioxidant capacity and anti-inflammatory activity of lycopene in watermelon. Int. J. Food Sci. Technol. 2014, 49, 2083–2091. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.; Mahomoodally, F.M.; Keum, Y.-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef] [PubMed]
- Perkins-Veazie, P.; Collins, J.K.; Davis, A.R.; Roberts, W. Carotenoid Content of 50 Watermelon Cultivars. J. Agric. Food Chem. 2006, 54, 2593–2597. [Google Scholar] [CrossRef]
- Edwards, A.J.; Vinyard, B.T.; Wiley, E.R.; Brown, E.D.; Collins, J.K.; Perkins-Veazie, P.; Baker, R.A.; Clevidence, B.A. Consumption of watermelon juice increases plasma concentrations of lycopene and beta-carotene in humans. J. Nutr. 2003, 133, 1043–1050. [Google Scholar] [CrossRef]
- Naderi, A.; Rezaei, S.; Moussa, A.; Levers, K.; Earnest, C.P. Fruit for sport. Trends Food Sci. Technol. 2018, 74, 85–98. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.P.; Alacid, F.; Carrasco, M.; Martínez, I.; Aguayo, E. Watermelon Juice: Potential Functional Drink for Sore Muscle Relief in Athletes. J. Agric. Food Chem. 2013, 61, 7522–7528. [Google Scholar] [CrossRef]
- Blohm, K.; Beidler, J.; Rosen, P.; Kressler, J.; Hong, M.Y. Effect of acute watermelon juice supplementation on post-submaximal exercise heart rate recovery, blood lactate, blood pressure, blood glucose and muscle soreness in healthy non-athletic men and women. Int. J. Food Sci. Nutr. 2019, 71, 482–489. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef]
- Shanely, R.A.; Nieman, D.C.; Perkins-Veazie, P.; Henson, D.A.; Meaney, M.P.; Knab, A.M.; Cialdell-Kam, L. Comparison of Watermelon and Carbohydrate Beverage on Exercise-Induced Alterations in Systemic Inflammation, Immune Dysfunction, and Plasma Antioxidant Capacity. Nutrients 2016, 8, 518. [Google Scholar] [CrossRef] [PubMed]
- Rhim, H.C.; Kim, S.J.; Park, J.; Jang, K.-M. Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: A systematic review and meta-analysis. J. Sport Heal. Sci. 2020, 9, 553–561. [Google Scholar] [CrossRef]
- Massa, N.M.L.; Silva, A.S.; de Oliveira, C.V.C.; Costa, M.J.C.; Persuhn, D.C.; Barbosa, C.V.S.; Goncalves, M.C.R. Supplementation with Watermelon Extract Reduces Total Cholesterol and LDL Cholesterol in Adults with Dyslipidaemia under the Influence of the MTHFR C677T Polymorphism. J. Am. Coll. Nutr. 2016, 35, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Wong, A.; Hooshmand, S.; Sanchez-Gonzalez, M.A. Effects of watermelon supplementation on arterial stiffness and wave reflection amplitude in postmenopausal women. Menopause 2013, 20, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Wong, A.; Kalfon, R. Effects of Watermelon Supplementation on Aortic Hemodynamic Responses to the Cold Pressor Test in Obese Hypertensive Adults. Am. J. Hypertens. 2014, 27, 899–906. [Google Scholar] [CrossRef]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Wong, A.; Arjmandi, B.H. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese adults with prehypertension or hypertension. Am. J. Hypertens. 2012, 25, 640–643. [Google Scholar] [CrossRef]
- Shanely, R.A.; Zwetsloot, J.J.; Jurrissen, T.J.; Hannan, L.C.; Zwetsloot, K.A.; Needle, A.R.; Bishop, A.E.; Wu, G.; Perkins-Veazie, P. Daily watermelon consumption decreases plasma sVCAM-1 levels in overweight and obese postmenopausal women. Nutr. Res. 2020, 76, 9–19. [Google Scholar] [CrossRef]
- Lum, T.; Connolly, M.; Marx, A.; Beidler, J.; Hooshmand, S.; Kern, M.; Liu, C.; Hong, M.Y. Effects of Fresh Watermelon Consumption on the Acute Satiety Response and Cardiometabolic Risk Factors in Overweight and Obese Adults. Nutrients 2019, 11, 595. [Google Scholar] [CrossRef]
- Hong, M.Y.; Hartig, N.; Kaufman, K.; Hooshmand, S.; Figueroa, A.; Kern, M. Watermelon consumption improves inflammation and antioxidant capacity in rats fed an atherogenic diet. Nutr. Res. 2015, 35, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Beidler, J.; Hooshmand, S.; Figueroa, A.; Kern, M. Watermelon and l-arginine consumption improve serum lipid profile and reduce inflammation and oxidative stress by altering gene expression in rats fed an atherogenic diet. Nutr. Res. 2018, 58, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Tseng, Y.-T.; Kalaba, M.; Beidler, J. Effects of watermelon powder supplementation on colitis in high-fat diet-fed and dextran sodium sulfate-treated rats. J. Funct. Foods 2019, 54, 520–528. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).