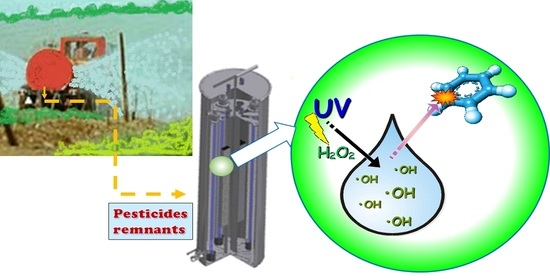
A Simple Device for the On-Site Photodegradation of Pesticide Mixes Remnants to Avoid Environmental Point Pollution
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. The Photodegradation Treatment
2.2. The Toxicity Assays
2.2.1. Bioluminescence Inhibition Assay
2.2.2. Algal Growth Inhibition Assay
3. Results
3.1. The Portable Photoreactor Prototype
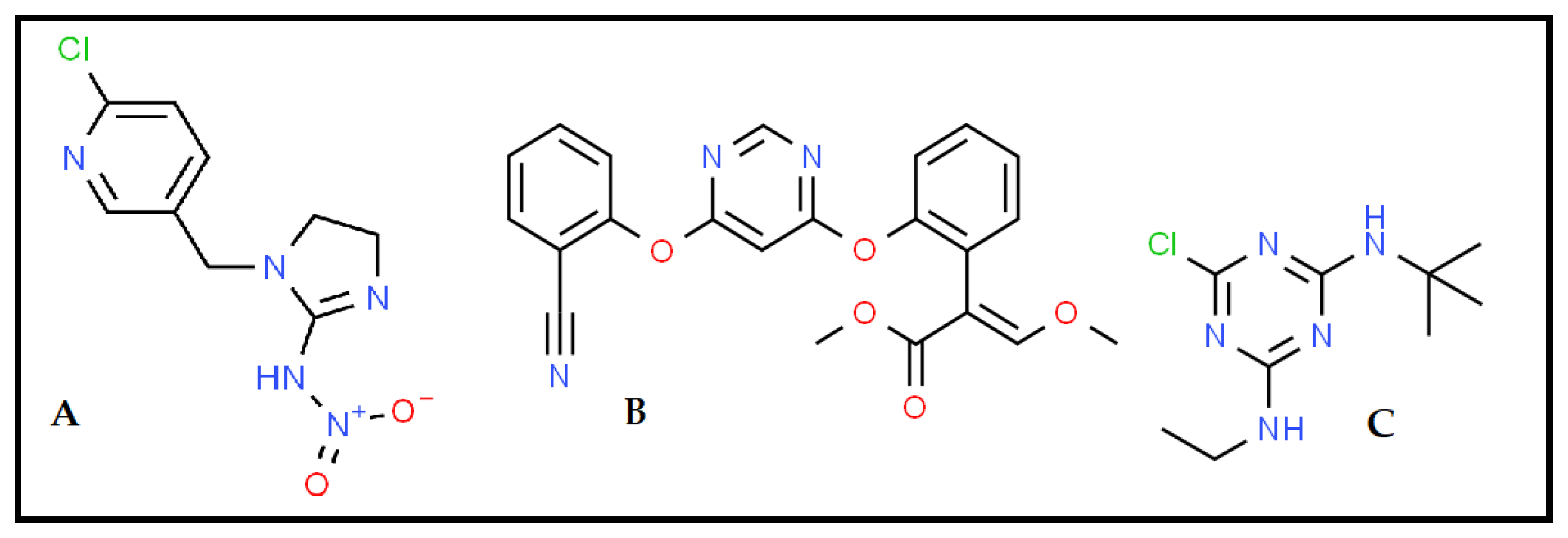
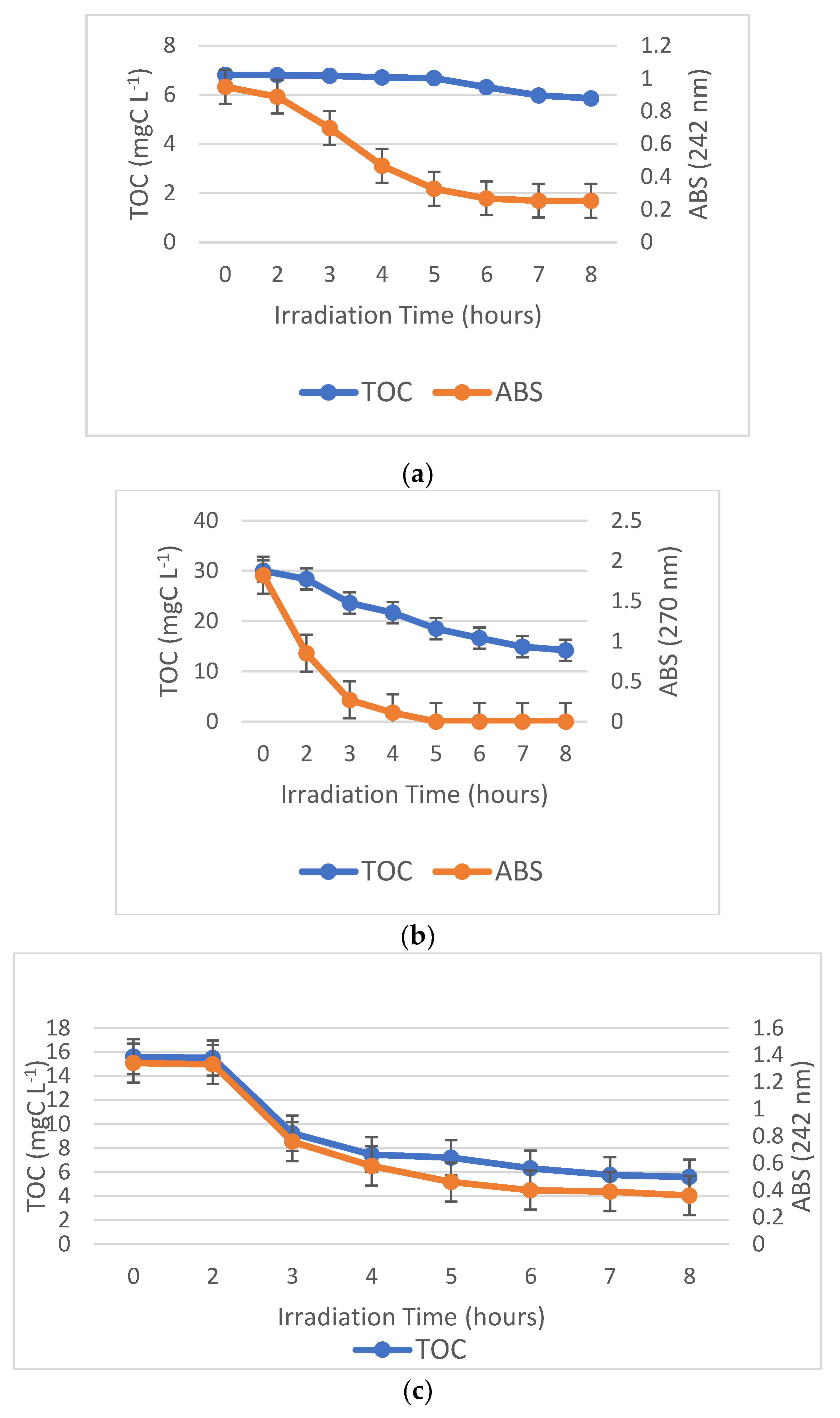
3.2. The Photodegradation and Mineralization Profiles
3.3. The Toxicity Assays
3.3.1. The Bacterial Bioluminescence Inhibition Test
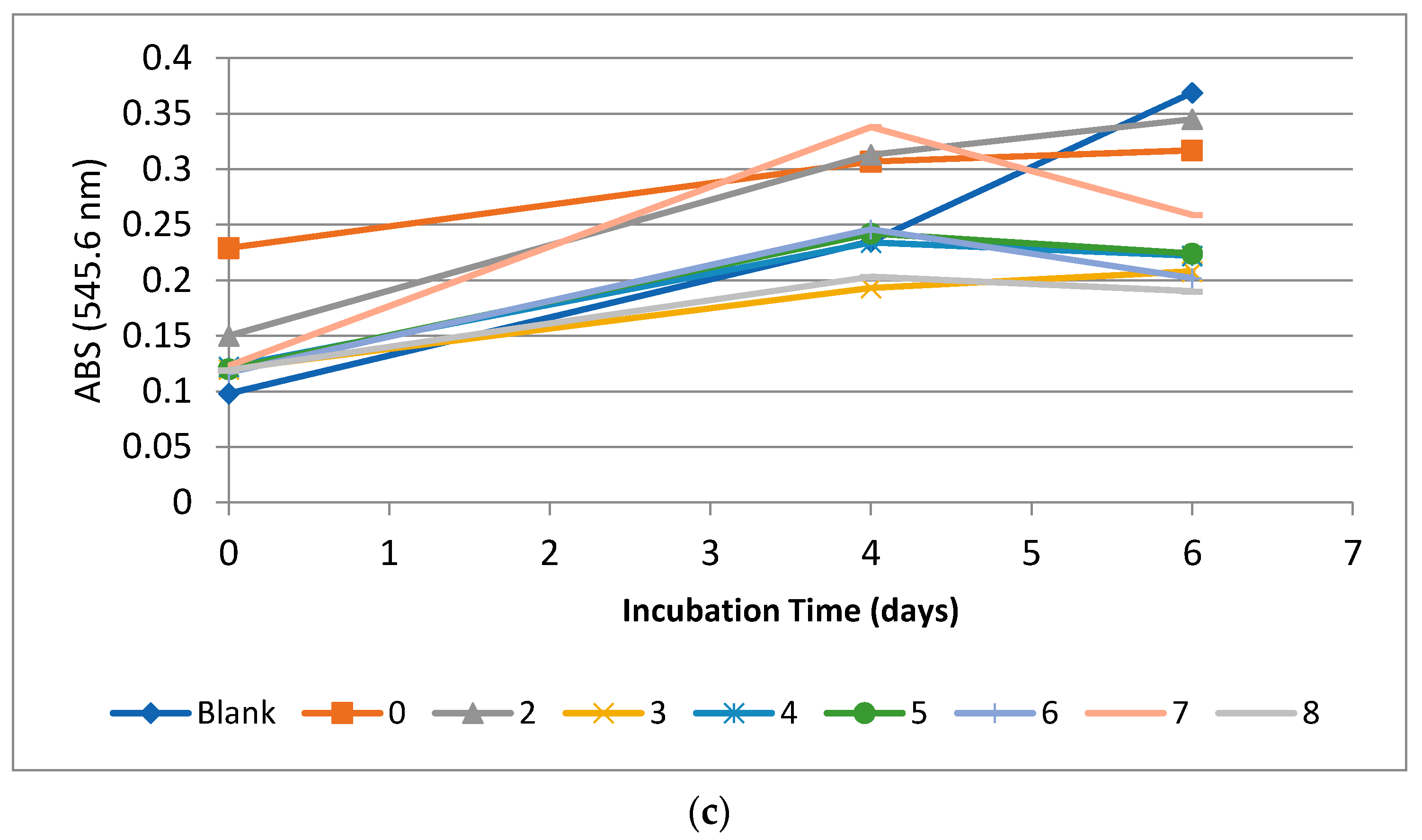
3.3.2. The Algal Growth Inhibition Assay
4. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- De Wilde, T.; Spanoghe, P.; Debae, C.; Ryckeboer, J.; Springael, D.; Jaeken, P. Overview of on-farm bioremediation systems to reduce the occurrence of point source contamination. Pest Manag. Sci. 2007, 63, 111–128. [Google Scholar] [CrossRef]
- Boyd, A.; Ridout, C.; O’Sullivan, D.M.; Leach, J.E.; Leung, H. Plant–pathogen interactions: Disease resistance in modern agriculture. Trends Genet. 2013, 29, 233–240. [Google Scholar] [CrossRef]
- Duru, M.; Therond, O.; Martin, G.; Martin-Clouaire, R.; Magne, M.A.; Justes, E.; Journet, E.P.; Aubertot, J.N.; Savary, S.; Bergez, J.E.; et al. How to implement biodiversity-based agriculture to enhance ecosystem services: A review. Agron. Sustain. Dev. 2015, 35, 1259–1281. [Google Scholar] [CrossRef]
- Sakadevan, K.; Nguyen, M.-L. Chapter Four-Livestock Production and Its Impact on Nutrient Pollution and Greenhouse Gas Emission. Adv. Agron. 2017, 141, 147–184. [Google Scholar]
- FAO. World Food and Agriculture—Statistical Pocketbook 2020. Rome. Available online: https://doi.org/10.4060/cb1521en (accessed on 16 March 2021).
- Viessman, W., Jr.; Hammer, M.J. Water Supply and Pollution Control, 6th ed.; Addison Wesley Longman Inc: Boston, CA, USA, 1998; pp. 876–885. [Google Scholar]
- Breach, R.A. UK experiences in developing a catchment scale, risk based, approach to mitigating all routes for pesticide loss to water. In Proceeding of the International Advances in Pesticide Application, Robinson College, Cambridge, UK, 9–11 January 2008; Alexander, L.S., Carpenter, P.I., Cooper, S.E., Glass, C.R., Gummer Andersen, P., Magri, B., Robinson, T.H., Stock, D., Taylor, W.A., Thornhill, E.W., Eds.; Association of Applied Biologists: Wellesbourne, UK, 2008; pp. 353–356. [Google Scholar]
- Shen, C.; Yang, X.; Wang, Y.; Zhou, J.; Chen, C. Complexation of capsaicin with β-cyclodextrins to improve pesticide formulations: Effect on aqueous solubility, dissolution rate, stability and soil adsorption. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 263–274. [Google Scholar] [CrossRef]
- Briggs, J.A.; Riley, M.B.; Whitwell, T. Quantification and remediation of pesticides in runoff water from containerized plant production. J. Environ. Qual. 1998, 27, 814–820. [Google Scholar] [CrossRef]
- Vischetti, C.; Capri, E.; Trevisan, M.; Casucci, C.; Perucci, P. Biomassbed: A biological system to reduce pesticide point contamination at farm level. Chemosphere 2004, 55, 823–828. [Google Scholar] [CrossRef]
- Grella, M.; Miranda-Fuentes, A.; Marucco, P.; Balsari, P. Field assessment of a newly-designed pneumatic spout to contain spray drift in vineyards: Evaluation of canopy distribution and off-target losses. Pest Manag. Sci. 2020, 76, 4173–4191. [Google Scholar] [CrossRef]
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides (Text with EEA relevance). OJ L 24.11. 2009, 309, 71–86. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009L0128 (accessed on 16 March 2021).
- Vischetti, C.; Monaci, E.; Coppola, L.; Marinozzi, M.; Casucci, C. Evaluation of BiomassBed system in bio-cleaning water contaminated by fungicides applied in vineyard. Int. J. Environ. Anal. Chem. 2012, 92, 949–962. [Google Scholar] [CrossRef]
- Özkara, A.; Akyıl, D.; Konuk, M. Pesticides, environmental pollution, and health. In Environmental Health Risk-Hazardous Factors to Living Species; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Jouy, L.; Moquet, M.; Yème, P.-V. Rinçage et lavage du pulvérisateur: Au champ ou à la ferme, bien gérer ses effluents phytosanitaires. Perspect. Agric. 2009, 354, 34–42. [Google Scholar]
- Fait, G.; Nicelli, M.; Fragoulis, G.; Trevisan, M.; Capri, E. Reduction of point contamination sources of pesticide from a vineyard farm. Environ. Sci. Technol. 2007, 41, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Vagi, M.C.; Petsas, A.S. Recent advances on the removal of priority organochlorine and organophosphorous biorecalcitrant pesticides defined by Directive 2013/39/EU from environmental matrices by using advanced oxidation processes: An overview (2007–2018). J. Environ. Chem. Eng. 2020, 8, 102940. [Google Scholar] [CrossRef]
- Acero, J.L.; Real, F.J.; Benitez, F.J.; Matamoros, E. Degradation of neonicotinoids by UV irradiation: Kinetics and effect of real water constituents. Sep. Purif. Technol. 2019, 211, 218–226. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, L.; Chen, B.; Chen, Y.; Ma, Y. Understanding and modeling the formation and trasformation of hydrogen peroxide in water irradiated by 254 nm ultraviolet (UV) and 185 nm vacuum UV (VUV): Effects of pH and oxygen. Chemosphere 2020, 244, 125483. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.L.; Kim, K.-H. A review of photochemical approaches for the treatment of a wide range of pesticides. J. Hazard. Mater. 2015, 285, 325–335. [Google Scholar] [CrossRef]
- Rozas, O.; Vidal, C.; Baeza, C.; Jardim, W.F.; Rossner, A.; Mansilla, H.D. Organic micropollutants (OMPs) in natural waters: Oxidation by UV/H2O2 treatment and toxicity assessment. Water Res. 2016, 98, 109–118. [Google Scholar] [CrossRef]
- Fenoll, J.; Garrido, I.; Flores, P.; Hellín, P.; Vela, N.; Navarro, G.; García-García, J.; Navarro, S. Implementation of a new modular facility to detoxify agro-wastewater polluted with neonicotinoid insecticides in farms by solar photocatalysis. Energy 2019, 175, 722–729. [Google Scholar] [CrossRef]
- Díez, A.M.; Sanromán, M.A.; Pazos, M. New approaches on the agrochemical degradation by UV oxidation processes. Chem. Eng. J. 2019, 376, 120026. [Google Scholar] [CrossRef]
- Vela, N.; Fenoll, J.; Garrido, I.; Pérez-Lucas, G.; Flores, P.; Hellín, P.; Navarro, S. Reclamation of agro-wastewater polluted with pesticide residues using sunlight activated persulfate for agricultural reuse. Sci. Tot. Env. 2019, 660, 923–930. [Google Scholar] [CrossRef]
- Kitsiou, V.; Filippidis, N.; Matzavinos, D.; Poulios, I. Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in acqueous solutions. Appl. Catal. B Environ. 2009, 86, 27–35. [Google Scholar] [CrossRef]
- Sanlaville, Y.; Guittonneau, S.; Mansour, M.; Feicht, E.A.; Meallier, P.; Kettrup, A. Photosensitized degradation of terbuthylazine in water. Chemosphere 1996, 33, 353–362. [Google Scholar] [CrossRef]
- Wamhoff, H.; Schneider, V. Photodegradation of imidacloprid. J. Agric. Food Chem. 1999, 47, 1730–1734. [Google Scholar] [CrossRef]
- Lányi, K.; Dinya, Z. Photodegradation study for assessing the environmental fate of some triazine-, urea-, and thiolcarbamate-type herbicides. Microchem. J. 2005, 80, 79–87. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Evans, A.; DeWinne, D.; White, P.; Mitchell, F. Modeling photodegradation kinetics of three systemic neonicotinoids-dinotefuran, imidacloprid, and thiamethoxam-in aqueous and soil environment. Environ. Toxicol. Chem. 2016, 35, 1718–1726. [Google Scholar] [CrossRef]
- Boudina, A.; Emmelin, C.; Baaliouamer, A.; Païssé, O.; Chovelon, J.M. Photochemical transformation of azoxystrobin in aqueous solutions. Chemosphere 2007, 68, 1280–1288. [Google Scholar] [CrossRef]
- Katagi, T. Direct photolysis-mechanism of pesticides in water. J. Pestic. Sci. 2018, 43, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Emmi, S.S.; Caminati, S.; Castro, C.; Esposito, B.; Saracino, M.; Sconziano, S. Comparison between Ionizing and Non-Ionizing Radiation Technologies for Wastewater Remediation. Proceeding of the 1st Research Coordination Meeting on “Radiation Treatment of Wastewater for Reuse with Particular Focus on Wastewaters Containing Organic Pollutants", Vienna, Austria, 2–6 May 2011; pp. 82–91. Available online: http://www-naweb.iaea.org/napc/iachem/working_materials/RC-1188-1_report_complete.pdf (accessed on 16 March 2021).
- Emmi, S.S.; Caminati, S.; Esposito, B.; Saracino, M. About the OH yield in the radiolysis of an aqueous/H2O2 system. Its optimisation for water treatment. Radiat. Phys. Chem. 2012, 81, 1430–1433. [Google Scholar] [CrossRef]
- Esposito, B.R.; Capobianco, M.L.; Martelli, A.; Navacchia, M.L.; Pretali, L.; Saracino, M.; Zanelli, A.; Emmi, S.S. Advanced water remediation from ofloxacin by ionizing radiation. Radiat. Phys. Chem. 2017, 141, 118–124. [Google Scholar] [CrossRef]
- Tasca, A.; Puccini, M.; Clematis, D.; Panizza, M. Electrochemical removal of Terbuthylazine: Boron-doped diamond anode coupled with solid polymer electrolyte. Environ. Pollut. 2019, 251, 285–291. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sánchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Bryden, J.; Gill, R.J.; Mitton, R.A.A.; Raine, N.E.; Jansen, V.A.A. Chronic sublethal stress causes bee colony failure. Ecol. Lett. 2013, 16, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are bee diseases linked to pesticides?—A brief review. Environ. Int. 2016, 89–99, 7–11. [Google Scholar] [CrossRef]
- Hocinat, A.; Boudemagh, A. Biodegradation of commercial Ortiva fungicide by isolated actinomycetes from the acti-vated sludge. Desalination Water Treat. 2015, 57, 6091–6097. [Google Scholar] [CrossRef]
- Banić, N.D.; Šojić, D.V.; Krstić, J.B.; Abramović, B.F. Photodegradation of neonicotinoid active ingredients and their commercial formulations in water by different advanced oxidation processes. Water Air Soil Pollut. 2014, 225, 1954. [Google Scholar] [CrossRef]
- ISO 11348-3:2007. Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test). Available online: https://www.iso.org/standard/40518.html (accessed on 16 March 2021).
- ISO 14442:2006. Water Quality—Guidelines for Algal Growth Inhibition Tests with Poorly Soluble Materials, Volatile Compounds, Metals, and Wastewater. Available online: https://www.iso.org/standard/34814.html (accessed on 16 March 2021).
- ISO 8692:2004. Water Quality–Freshwater Algal Growth Inhibition Test with Unicellular Green Algae. (Revised by ISO 8692:2012). Available online: https://www.iso.org/standard/29924.html (accessed on 16 March 2021).
- Lányi, K.; Dinya, Z. Photodegradation study of some triazine–type herbicides. Microchem. J. 2003, 75, 1–14. [Google Scholar] [CrossRef]
- Navarro, S.; Vela, N.; Giménez, M.J.; Navarro, G. Persistence of four s-triazine herbicides in river, sea and groundwater samples exposed to sunlight and darkness under laboratory conditions. Sci. Total Env. 2004, 329, 87–97. [Google Scholar] [CrossRef]
- Žabar, R.; Dolenc, D.; Jerman, T.; Franko, M.; Trebe, P. Photolytic and photocatalytic degradation of 6-chloronicotinic acid. Chemosphere 2011, 85, 861–868. [Google Scholar] [CrossRef]
- Han, B.; Kim, J.K.; Kim, Y.; Zommer, N. Treatment of wastewater for reuse with mobile electron beam plant. In Proceedings of the 1st Research Coordination Meeting on “Radiation Treatment of Wastewater for Reuse with Particular Focus on Wastewaters containing Organic Pollutants”, Vienna, Austria, 2–6 May 2011; Available online: http://www-naweb.iaea.org/napc/iachem/working_materials/RC-1188-1_report_complete.pdf (accessed on 16 March 2021).








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, B.; Riminucci, F.; Di Marco, S.; Metruccio, E.G.; Osti, F.; Sangiorgi, S.; Ferri, E.N. A Simple Device for the On-Site Photodegradation of Pesticide Mixes Remnants to Avoid Environmental Point Pollution. Appl. Sci. 2021, 11, 3593. https://doi.org/10.3390/app11083593
Esposito B, Riminucci F, Di Marco S, Metruccio EG, Osti F, Sangiorgi S, Ferri EN. A Simple Device for the On-Site Photodegradation of Pesticide Mixes Remnants to Avoid Environmental Point Pollution. Applied Sciences. 2021; 11(8):3593. https://doi.org/10.3390/app11083593
Chicago/Turabian StyleEsposito, Biagio, Francesco Riminucci, Stefano Di Marco, Elisa Giorgia Metruccio, Fabio Osti, Stefano Sangiorgi, and Elida Nora Ferri. 2021. "A Simple Device for the On-Site Photodegradation of Pesticide Mixes Remnants to Avoid Environmental Point Pollution" Applied Sciences 11, no. 8: 3593. https://doi.org/10.3390/app11083593
APA StyleEsposito, B., Riminucci, F., Di Marco, S., Metruccio, E. G., Osti, F., Sangiorgi, S., & Ferri, E. N. (2021). A Simple Device for the On-Site Photodegradation of Pesticide Mixes Remnants to Avoid Environmental Point Pollution. Applied Sciences, 11(8), 3593. https://doi.org/10.3390/app11083593