Potential Risk of Consuming Vegetables Planted in Soil with Copper and Cadmium and the Influence on Vegetable Antioxidant Activity
Abstract
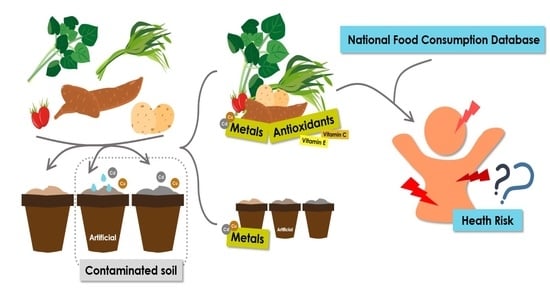
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Preparation of Soil Artificially Contaminated with Metals
2.3. Sample Collection and Pre-Treatment
2.4. Chemicals
2.5. Metals Analysis of Soils, Water, and Vegetables
2.6. Vitamin C Analysis in Vegetables
2.7. Vitamin E Analysis in Vegetables
2.8. Bioaccumulation Factor of Heavy Metals in Vegetables
2.9. Statistical Analysis
2.10. Health Risk Assessment
- ADD: average daily dose via ingestion pathway (mg/kg b.w./day)
- C: concentration of metal in the vegetable (mg/kg)
- IR: daily intake of vegetable (kg/day)
- AF: absorption rate (%), set as 100%
- BW: average body weight (kg)
3. Results and Discussion
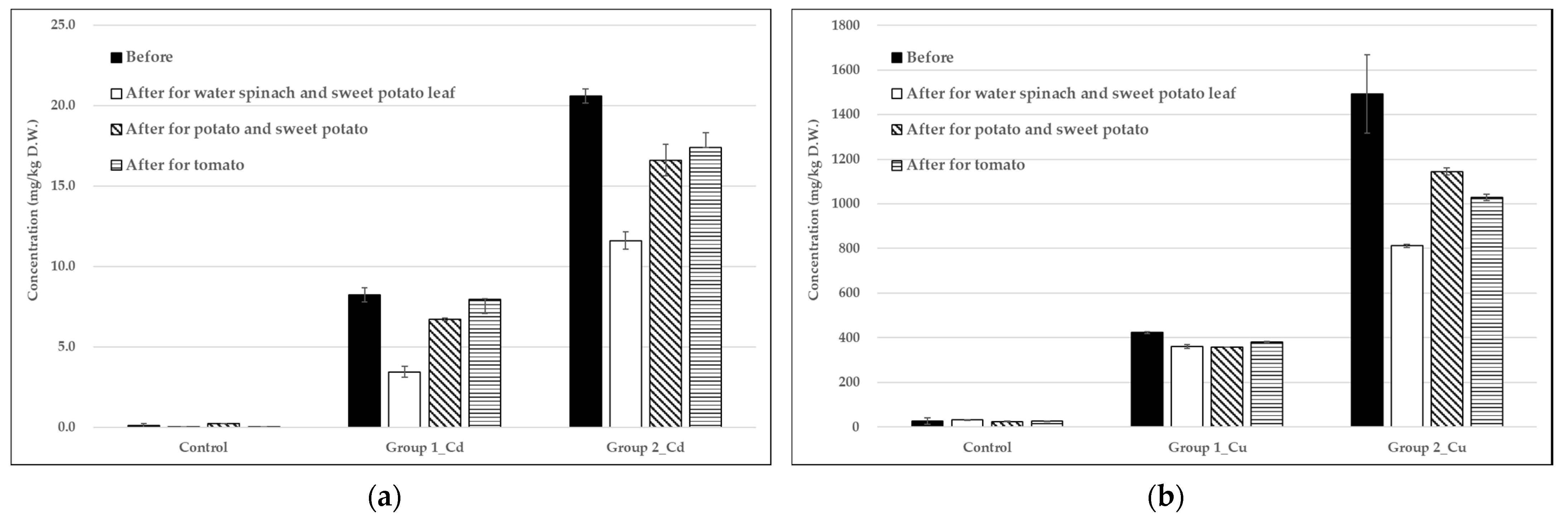
3.1. Heavy Metal Concentrations in Soil and Water
3.2. Vegetable Growth
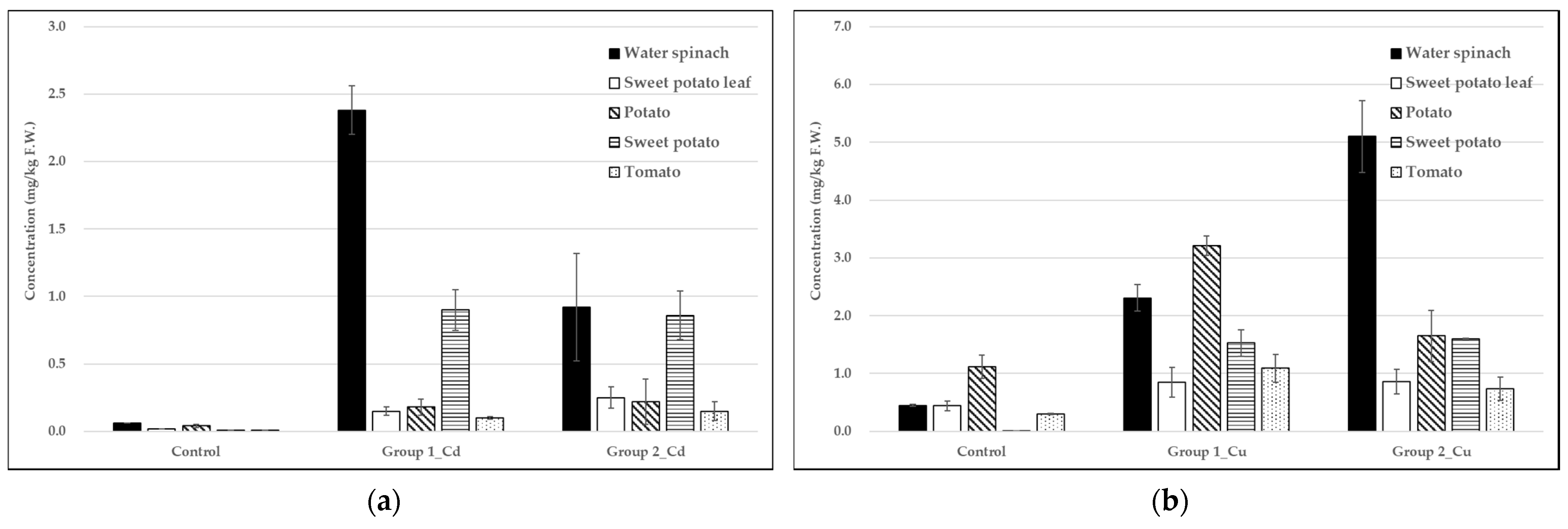
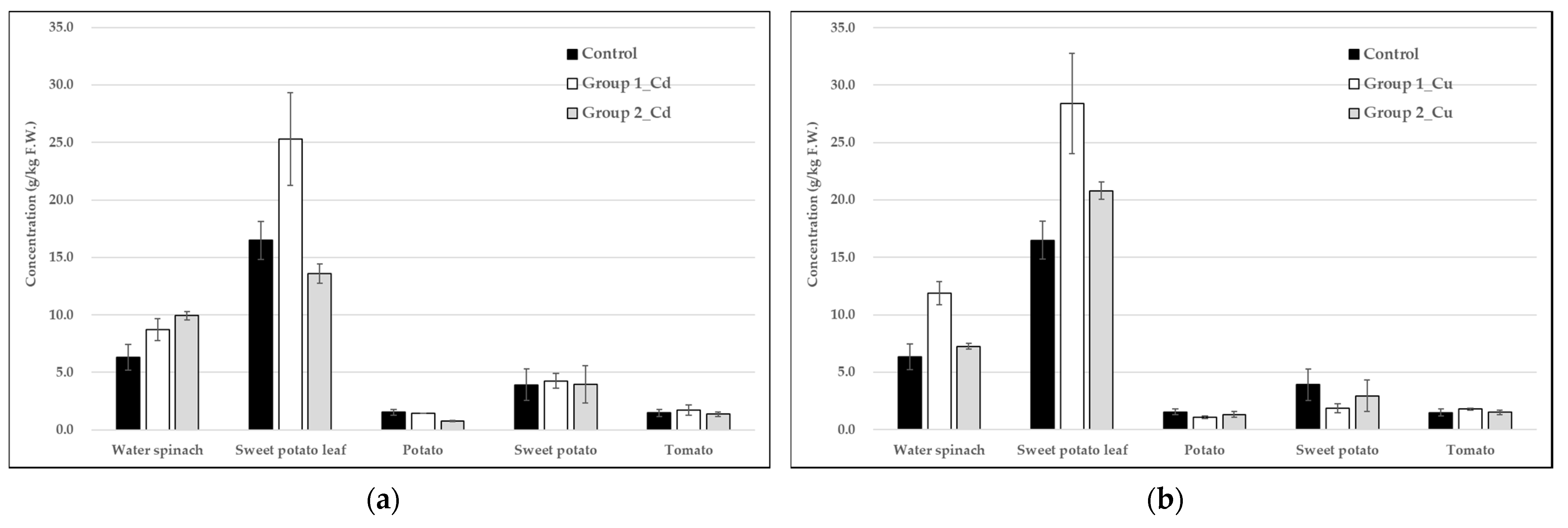
3.3. Analysis of Heavy Metals in Vegetables
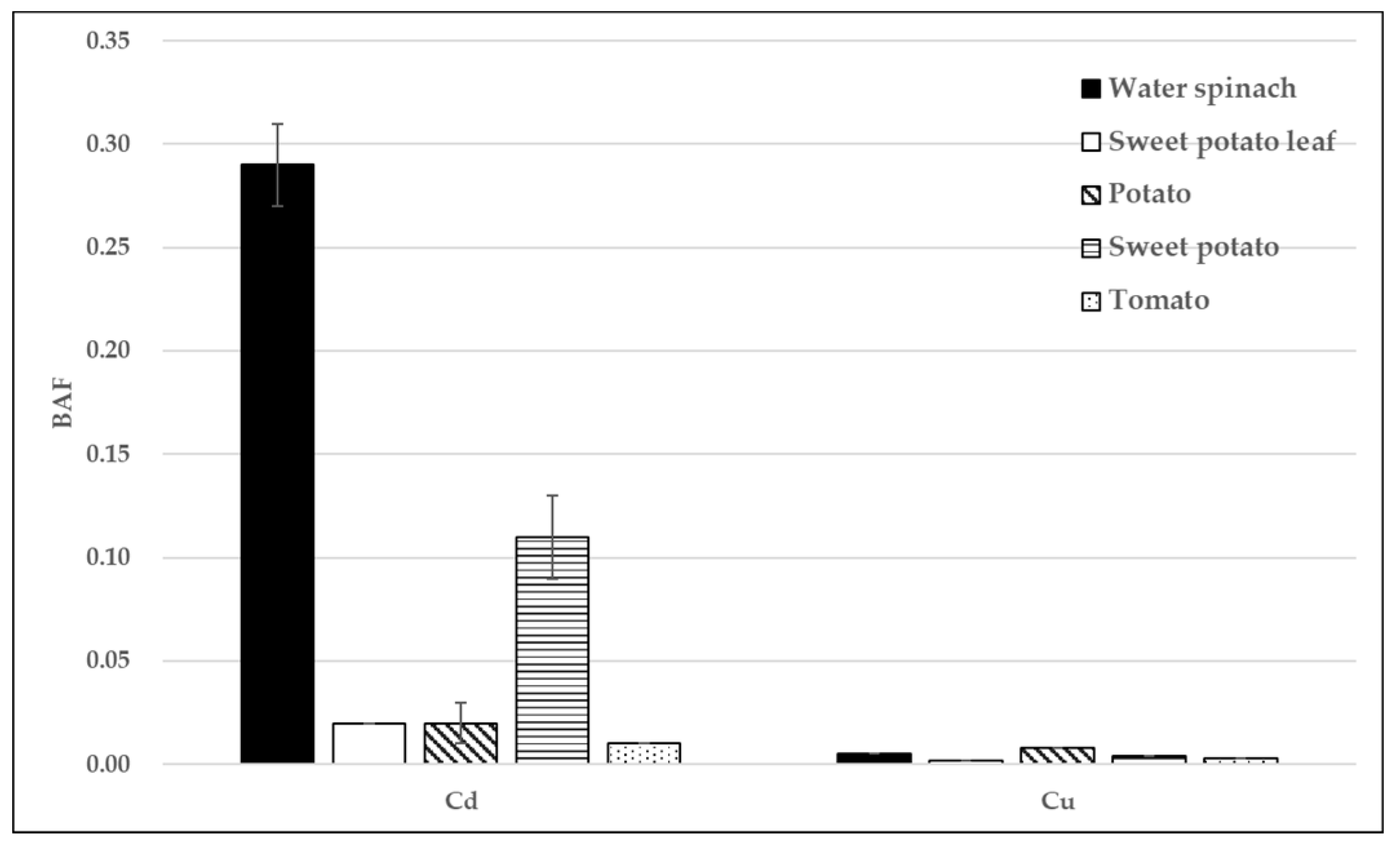
3.4. Bioaccumulation Factors of Heavy Metals in Vegetables
3.5. Antioxidant Analysis in the Vegetables
3.6. Exposure Dose of Heavy Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, P.; Wang, X.; Zhang, T.; Zhou, D.; He, Y. Effects of several amendments on rice growth and uptake of copper and cadmium from a contaminated soil. J. Environ. Sci. 2008, 20, 449–455. [Google Scholar] [CrossRef]
- Akpor, O.; Muchie, M. Remediation of heavy metals in drinking water and wastewater treatment systems: Processes and applications. Int. J. Phys. Sci. 2010, 5, 1807–1817. [Google Scholar]
- Xie, L.; Hao, P.; Cheng, Y.; Ahmed, I.M.; Cao, F. Effect of combined application of lead, cadmium, chromium and copper on grain, leaf and stem heavy metal contents at different growth stages in rice. Ecotoxicol. Environ. Saf. 2018, 162, 71–76. [Google Scholar] [CrossRef]
- EPA. 2018 Annual Report Soil and Groundwater Pollution Remediation; EPA: Washington, DC, USA, 2018.
- Chmielnicka, J.; Cherian, M.G. Environmental exposure to cadmium and factors affecting trace-element metabolism and metal toxicity. Biol. Trace Element Res. 1986, 10, 243–262. [Google Scholar] [CrossRef]
- Levenson, C.; Janghorbani, M. Long-Term Measurement of Organ Copper Turnover in Rats by Continuous Feeding of a Stable Isotope. Anal. Biochem. 1994, 221, 243–249. [Google Scholar] [CrossRef]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced Cancers in Animals and in Humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andujar, P.; Bensefa-Colas, L.; Descatha, A. Acute and chronic cadmium poisoning. La Rev. Méd. Interne 2010, 31, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matovic, V.; Buha, A.; Bulat, Z.; Dukic-Cosic, D. Cadmium toxicity revisited: Focus on oxidative stress induction and interactions with zinc and magnesium. Arch. Ind. Hyg. Toxicol. 2011, 62, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, A.P.; Mota, A.; De Varennes, A.; Pinto, F. Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci. Total. Environ. 2004, 326, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Asakura, K.; Sasaki, S.; Hirota, N.; Notsu, A.; Todoriki, H.; Miura, A.; Fukui, M.; Date, C. Estimation of intakes of copper, zinc, and manganese in Japanese adults using 16-day semi-weighed diet records. Asia Pac. J. Clin. Nutr. 2014, 23, 465–472. [Google Scholar] [PubMed]
- Kendall, N.R.; Holmes-Pavord, H.R.; Bone, P.A.; Ander, E.L.; Young, S.D. Liver copper concentrations in cull cattle in the UK: Are cattle being copper loaded? Vet. Rec. 2015, 177, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Han, F.; Gong, W.; Feng, L.; Han, J. The effect of copper from water and food: Changes of serum nonceruloplasmin copper and brain’s amyloid-beta in mice. Food Funct. 2016, 7, 3740–3747. [Google Scholar] [CrossRef] [PubMed]
- Sipter, E.; Rózsa, E.; Gruiz, K.; Tátrai, E.; Morvai, V. Site-specific risk assessment in contaminated vegetable gardens. Chemosphere 2008, 71, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Wichelns, D.; Raschid-Sally, L.; McCornick, P.; Drechsel, P.; Bahri, A.; Minhas, P. The challenges of wastewater irrigation in developing countries. Agric. Water Manag. 2010, 97, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Intawongse, M.; Dean, J.R. Uptake of heavy metals by vegetable plants grown on contaminated soil and their bioavailability in the human gastrointestinal tract. Food Addit. Contam. 2006, 23, 36–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, S.; Nawaz, M.F.; Gul, S.; Rizwan, M.; Ali, S.; Kareem, A. Uptake and distribution of minerals and heavy metals in commonly grown leafy vegetable species irrigated with sewage water. Environ. Monit. Assess. 2016, 188, 541. [Google Scholar] [CrossRef] [PubMed]
- Balkhair, K.S.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Alamzeb, S.; Begum, S. Accumulation of heavy metals in edible parts of vegetables irrigated with waste water and their daily intake to adults and children, District Mardan, Pakistan. Food Chem. 2013, 136, 1515–1523. [Google Scholar]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total. Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- Monsen, E.R. Dietary Reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J. Am. Diet. Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef]
- Kuhnlein, H.; Barthet, V.; Farren, A.; Falahi, E.; Leggee, D.; Receveur, O.; Berti, P. Vitamins A, D, and E in Canadian Arctic traditional food and adult diets. J. Food Compos. Anal. 2006, 19, 495–506. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; De Klerk, N.H.; Fritschi, L.; Mackerras, D.; Musk, B. Fruit, vegetable, vitamin A intakes, and prostate cancer risk. Prostate Cancer Prostatic Dis. 2007, 11, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haard, N.F. Postharvest physiology and biochemistry of fruits and vegetables. J. Chem. Educ. 1984, 61, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Orsavová, J.; Hlaváčová, I.; Mlček, J.; Snopek, L.; Mišurcová, L. Contribution of phenolic compounds, ascorbic acid and vitamin E to antioxidant activity of currant (Ribes L.) and gooseberry (Ribes uva-crispa L.) fruits. Food Chem. 2019, 284, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Collin, V.C.; Eymery, F.; Genty, B.; Rey, P.; Havaux, M. Vitamin E is essential for the tolerance of Arabidopsis thaliana to metal-induced oxidative stress. Plant Cell Environ. 2007, 31, 244–257. [Google Scholar] [CrossRef]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X. Effects of cadmium and copper mixtures to carrot and pakchoi under greenhouse cultivation condition. Ecotoxicol. Environ. Saf. 2018, 159, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-Y.; Chen, B.-C. The Dynamic Growth Exhibition and Accumulation of Cadmium of Pak Choi (Brassica campestris L. ssp. chinensis) Grown in Contaminated Soils. Int. J. Environ. Res. Public Health 2013, 10, 5284–5298. [Google Scholar] [CrossRef] [Green Version]
- Sipter, E.; Auerbach, R.; Gruiz, K.; Mathe-Gaspar, G. Change of Bioaccumulation of Toxic Metals in Vegetables. Commun. Soil Sci. Plant Anal. 2009, 40, 285–293. [Google Scholar] [CrossRef]
- Chang, W.H.; Liu, S.C.; Chen, H.L.; Lee, C.C. Dietary intake of 4-nonylphenol and bisphenol A in Taiwanese population: Integrated risk assessment based on probabilistic and sensitive approach. Environ. Pollut. 2019, 244, 143–152. [Google Scholar] [CrossRef]
- Lee, C.C.; Chang, W.H.; Chen, H.L. Dietary exposure and risk assessment of exposure to hexabromocyclododecanes in a Taiwan population. Environ. Pollut. 2019, 249, 728–734. [Google Scholar] [CrossRef]
- Zeng, X.-B.; Li, L.-F.; Mei, X.-R. Heavy Metal Content in Chinese Vegetable Plantation Land Soils and Related Source Analysis. Agric. Sci. China 2008, 7, 1115–1126. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Liu, T.-S.; Guo, H.-Y.; Chiang, C.-M.; Tang, H.-J.; Chen, H.-T.; Chen, J.-H. Relationships between Cd concentrations in different vegetables and those in arable soils, and food safety evaluation of vegetables in Taiwan. Soil Sci. Plant Nutr. 2015, 61, 983–998. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhou, Q.; Diao, C. Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Bioresour. Technol. 2008, 99, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- EPA. 2016 Annual Report Soil and Groundwater Pollution Remediation; EPA: Washington, DC, USA, 2016.
- Ahada, C.; Patel, A. Effects of Heavy Metals (Cu and Cd) on Growth of Leafy Vegetables Spinacia oleracea and Amaranthus caudatus. Int. Res. J. Environ. Sci. 2015, 4, 63–69. [Google Scholar]
- Peralta, J.R.; Gardea-Torresdey, J.L.; Tiemann, K.J.; Gomez, E.; Arteaga, S.; Rascon, E.; Parsons, J.G. Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (Medicago sativa L.). Bull. Environ. Contam. Toxicol. 2001, 66, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Schneider, R.L.; Morreale, S.J.; Xie, Y.; Li, C.; Li, J. Woody organic amendments for retaining soil water, improving soil properties and enhancing plant growth in desertified soils of Ningxia, China. Geoderma 2018, 310, 143–152. [Google Scholar] [CrossRef]
- Islam, M.S.; Khanam, M.S.; Sarker, N.I. Health risk assessment of metals transfer from soil to the edible part of some vegetables grown in Patuakhali province of Bangladesh. Arch. Agric. Environ. Sci. 2018, 3, 187–197. [Google Scholar] [CrossRef]
- Khan, S.; Aijun, L.; Zhang, S.; Hu, Q.; Zhu, Y.-G. Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. J. Hazard. Mater. 2008, 152, 506–515. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Z.; Xie, X.; Li, Y.; Zhang, Y.; Xu, X. Effects of Dissolved Organic Matter on Uptake and Translocation of Lead in Brassica chinensis and Potential Health Risk of Pb. Int. J. Environ. Res. Public Health 2016, 13, 687. [Google Scholar] [CrossRef] [Green Version]
- Sengar, R.S.; Gautam, M.; Garg, S.K.; Sengar, K.; Chaudhary, R. Lead Stress Effects on Physiobiochemical Activities of Higher Plants. Rev. Environ. Contam. Toxicol. 2008, 196, 73–93. [Google Scholar] [CrossRef]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; Reeves, R.D. Soil pH Effects on Uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 2006, 281, 325–337. [Google Scholar] [CrossRef]
- Bhatti, S.S.; Kumar, V.; Sambyal, V.; Singh, J.; Nagpal, A.K. Comparative analysis of tissue compartmentalized heavy metal uptake by common forage crop: A field experiment. Catena 2018, 160, 185–193. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Li, H.; Cheng, F. Evaluating heavy metal accumulation and potential risks in soil-plant systems applied with magnesium slag-based fertilizer. Chemosphere 2018, 197, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Yari, M.; Rahimi, G.; Ebrahimi, E.; Sadeghi, S.; Fallah, M.; Ghesmatpoor, E. Effect of Three Types of Organic Fertilizers on the Heavy Metals Transfer Factor and Maize Biomass. Waste Biomass Valoriz. 2016, 8, 2681–2691. [Google Scholar] [CrossRef]
- Antonious, G.F.; Kochhar, T.S.; Coolong, T. Yield, quality, and concentration of seven heavy metals in cabbage and broccoli grown in sewage sludge and chicken manure amended soil. J. Environ. Sci. Health Part A 2012, 47, 1955–1965. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, M.; Liu, Y.; Zhu, Y. Health risk assessment of heavy metals in soils and vegetables from wastewater irrigated area, Beijing-Tianjin city cluster, China. J. Environ. Sci. 2012, 24, 690–698. [Google Scholar] [CrossRef]
- Bi, C.; Zhou, Y.; Chen, Z.; Jia, J.; Bao, X. Heavy metals and lead isotopes in soils, road dust and leafy vegetables and health risks via vegetable consumption in the industrial areas of Shanghai, China. Sci. Total. Environ. 2018, 619, 1349–1357. [Google Scholar] [CrossRef]
- Alam, M.; Snow, E.; Tanaka, A. Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci. Total. Environ. 2003, 308, 83–96. [Google Scholar] [CrossRef]
- Katrien, D. Voluntary risk assessment of copper, copper ii sulphate pentahydrate, copper(i)oxide, copper(ii)oxide, dicopper chloride trihydroxide Euro. In Union Risk Assessment Report; JRC: Brussels, Belgium, 2008. [Google Scholar]
- Manciulea, A.; Ramsey, M.H. Effect of scale of Cd heterogeneity and timing of exposure on the Cd uptake and shoot biomass, of plants with a contrasting root morphology. Sci. Total. Environ. 2006, 367, 958–967. [Google Scholar] [CrossRef]
- Anderson, P.R.; Christensen, T.H. Distribution coefficients of Cd, Co, Ni, and Zn in soils. J. Soil Sci. 1988, 39, 15–22. [Google Scholar] [CrossRef]
- Mayer, L.M.; Xing, B. Organic Matter-Surface Area Relationships in Acid Soils. Soil Sci. Soc. Am. J. 2001, 65, 250–258. [Google Scholar] [CrossRef]
- Kongkachuichai, R.; Charoensiri, R.; Yakoh, K.; Kringkasemsee, A.; Insung, P. Nutrients value and antioxidant content of indigenous vegetables from Southern Thailand. Food Chem. 2015, 173, 838–846. [Google Scholar] [CrossRef]
- Gupta, S.; Prakash, J. Studies on Indian Green Leafy Vegetables for Their Antioxidant Activity. Plant Foods Hum. Nutr. 2008, 64, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Y.; Jiang, Y.; Li, G.; Cui, J.; Wang, Y.; Zhang, H.; Wang, S.; Xu, S.; Wang, R. The accumulation and health risk of heavy metals in vegetables around a zinc smelter in northeastern China. Environ. Sci. Pollut. Res. 2016, 23, 25114–25126. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.-T.; Zhou, X.; Liu, L.; Gu, J.-F.; Wang, W.-L.; Zou, J.-L.; Tian, T.; Peng, P.-Q.; Liao, B.-H. Accumulation of Heavy Metals in Vegetable Species Planted in Contaminated Soils and the Health Risk Assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Planting Time (Week) | Water Spinach | Sweet Potato Leaf | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1_Cd | Group 2_Cd | Control | p-Value | Group 1_Cd | Group 2_Cd | Control | p-Value | |||||||||||||
| Planting height (cm) | 1st | 0.00 ± 0.00 | 1.33 ± 1.53 | 1.33 ± 1.15 | 0.234 | 7.67 ± 1.15 | 7.67 ± 1.53 | 8.00 ± 2.65 | 0.870 | |||||||||||
| 2nd | 3.67 ± 1.15 | 3.67 ± 2.08 | 3.67 ± 1.53 | 0.988 | 11.0 ± 1.00 | 16.3 ± 1.15 | 17.3 ± 2.08 | 0.054 | ||||||||||||
| 3rd | 8.67 ± 1.15 | 9.67 ± 1.15 | 13.7 ± 1.53 | 0.048 * | 21.7 ± 1.15 | 26.3 ± 2.52 | 33.3 ± 7.64 | 0.050 | ||||||||||||
| 4th | 27.0 ± 1.00 | 23.0 ± 2.00 | 30.7 ± 5.51 | 0.820 | 29.0 ± 5.29 | 42.7 ± 5.51 | 47.7 ± 8.08 | 0.051 | ||||||||||||
| 5th | 34.0 ± 2.65 | 30.0 ± 2.65 | 41.0 ± 5.29 | 0.047 * | 34.0 ± 9.64 | 56.0 ± 10.8 | 64.0 ± 12.2 | 0.079 | ||||||||||||
| Harvest weight (g) | 44.2 ± 5.10 | 56.0 ± 25.5 | 52.8 ± 6.88 | 0.393 | 51.0 ± 6.63 | 62.0 ± 15.1 | 55.1 ± 3.27 | 0.491 | ||||||||||||
| Planting time (month) | Potato | Sweet potato | Tomato | |||||||||||||||||
| Group 1_Cd | Group 2_Cd | Control | p-value | Group 1_Cd | Group 2_Cd | Control | p-value | Group 1_Cd | Group 2_Cd | Control | p-value | |||||||||
| Harvest weight (g) | 15.5 ± 5.72 | 27.2 ± 2.19 | 82.8 ± 20.4 | 0.027 * | 62.0 ± 12.1 | 25.0 ± 21.1 | 143 ± 9.61 | 0.032 * | 39.5 ± 11.2 | 31.8 ± 16.2 | 60.4 ± 13.2 | 0.184 | ||||||||
| Number of harvested † | 9 | 12 | 15 | 0.074 | 12 | 5 | 23 | 0.025 * | 15 | 14 | 28 | 0.084 | ||||||||
| Planting Time (Week) | Water Spinach | Sweet Potato Leaf | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1_Cu | Group 2_Cu | Control | p-Value | Group 1_Cu | Group 2_Cu | Control | p-Value | |||||||||||||
| Planting height (cm) | 1st | 0.00 ± 0.00 | 1.00 ± 1.00 | 1.33 ± 1.15 | 0.211 | 6.33 ± 1.53 | 9.67 ± 1.53 | 8.00 ± 2.65 | 0.194 | |||||||||||
| 2nd | 2.67 ± 1.15 | 2.67 ± 1.53 | 3.67 ± 1.53 | 0.565 | 9.67 ± 2.08 | 15.3 ± 2.52 | 17.3 ± 2.08 | 0.048 * | ||||||||||||
| 3rd | 7.67 ± 2.08 | 10.7 ± 1.53 | 13.7 ± 1.53 | 0.047 * | 23.3 ± 4.16 | 28.0 ± 4.00 | 33.3 ± 7.64 | 0.172 | ||||||||||||
| 4th | 25.3 ± 2.52 | 20.0 ± 3.61 | 30.7 ± 5.51 | 0.054 | 30.0 ± 8.89 | 40.0 ± 4.58 | 47.7 ± 8.08 | 0.172 | ||||||||||||
| 5th | 29.3 ± 2.52 | 27.0 ± 1.73 | 41.0 ± 5.29 | 0.050 | 41.0 ± 12.8 | 55.0 ± 8.00 | 64.0 ± 12.2 | 0.172 | ||||||||||||
| Harvest weight (g) | 52.0 ± 2.95 | 72.4 ± 10.5 | 52.8 ± 6.88 | 0.063 | 44.2 ± 15.3 | 72.5 ± 32.0 | 55.1 ± 3.27 | 0.329 | ||||||||||||
| Planting time (month) | Potato | Sweet potato | Tomato | |||||||||||||||||
| Group 1_Cu | Group 2_Cu | Control | p-value | Group 1_Cu | Group 2_Cu | Control | p-value | Group 1_Cu | Group 2_Cu | Control | p-value | |||||||||
| Harvest weight (g) | 77.6 ± 39.1 | 28.1 ± 3.82 | 82.8 ± 20.4 | 0.066 | 107 ± 24.6 | 18.2 ± 3.83 | 143 ± 9.61 | 0.027 * | 30.7 ± 12.2 | 33.5 ± 19.7 | 60.4 ± 13.2 | 0.118 | ||||||||
| Number of harvested † | 10 | 12 | 15 | 0.463 | 21 | 4 | 23 | 0.048 * | 17 | 15 | 28 | 0.146 | ||||||||
| Age | Water Spinach | Sweet Potato Leaf | Potato | Sweet Potato | Tomato |
|---|---|---|---|---|---|
| 0–3 | 0.0014–0.0043 | 0.0001–0.0003 | 0.0001–0.0003 | 0.0027–0.0086 | 0.0001–0.0005 |
| 3–6 | 0.0014–0.0042 | 0.0001–0.0003 | 0.0001–0.0002 | 0.0005–0.0014 | 0.0002–0.0006 |
| 6–12 | 0.0018–0.0058 | 0.0001–0.0004 | 0.0002–0.0006 | 0.0007–0.0022 | 0.0001–0.0004 |
| 12–16 | 0.0009–0.0028 | 0.0001–0.0002 | 0.0001–0.0004 | 0.0003–0.0008 | 0.0001–0.0003 |
| 16–18 | 0.0013–0.0039 | 0.0001–0.0003 | 0.0001–0.0003 | 0.0003–0.0008 | 0.0001–0.0002 |
| 19–65 | 0.0024–0.0070 | 0.0002–0.0005 | 0.0000–0.0002 | 0.0004–0.0011 | 0.0001–0.0004 |
| >65 | 0.0033–0.0097 | 0.0002–0.0006 | 0.0001–0.0002 | 0.0005–0.0016 | 0.0001–0.0003 |
| Age | Water Spinach | Sweet Potato Leaf | Potato | Sweet Potato | Tomato |
|---|---|---|---|---|---|
| 0–3 | 0.0013–0.0042 | 0.0005–0.0017 | 0.0016–0.0051 | 0.0043–0.0137 | 0.0015–0.0049 |
| 3–6 | 0.0013–0.0040 | 0.0005–0.0016 | 0.0011–0.0032 | 0.0008–0.0023 | 0.0020–0.0061 |
| 6–12 | 0.0017–0.0057 | 0.0007–0.0022 | 0.0035–0.0113 | 0.0011–0.0037 | 0.0013–0.0042 |
| 12–16 | 0.0008–0.0026 | 0.0003–0.0011 | 0.0024–0.0077 | 0.0004–0.0013 | 0.0010–0.0031 |
| 16–18 | 0.0012–0.0038 | 0.0005–0.0015 | 0.0017–0.0052 | 0.0004–0.0014 | 0.0007–0.0022 |
| 19–65 | 0.0023–0.0067 | 0.0009–0.0027 | 0.0009–0.0026 | 0.0006–0.0017 | 0.0015–0.0046 |
| >65 | 0.0031–0.0093 | 0.0012–0.0037 | 0.0011–0.0034 | 0.0008–0.0024 | 0.0011–0.0034 |
| Age | Water Spinach | Sweet Potato Leaf | Potato | Sweet Potato | Tomato | HI |
|---|---|---|---|---|---|---|
| 0–3 | 1.40–4.36 | 0.09–0.28 | 0.09–0.29 | 2.70–8.69 | 0.15–0.45 | 4.80–11.6 |
| 3–6 | 1.42–4.19 | 0.09–0.27 | 0.06–0.19 | 0.47–1.41 | 0.19–0.57 | 2.40–5.44 |
| 6–12 | 1.81–5.82 | 0.12–0.38 | 0.19–0.65 | 0.68–2.26 | 0.12–0.39 | 3.18–7.77 |
| 12–16 | 0.87–2.75 | 0.06–0.18 | 0.13–0.44 | 0.25–0.82 | 0.09–0.29 | 1.52–3.65 |
| 16–18 | 1.27–3.96 | 0.08–0.26 | 0.09–0.29 | 0.27–0.85 | 0.07–0.21 | 1.88–4.74 |
| 19–65 | 2.38–7.02 | 0.15–0.45 | 0.05–0.15 | 0.36–1.08 | 0.14–0.42 | 3.20–7.97 |
| >65 | 3.27–9.63 | 0.21–0.62 | 0.06–0.19 | 0.52–1.54 | 0.10–0.31 | 4.36–10.8 |
| Age | Water Spinach | Sweet Potato Leaf | Potato | Sweet Potato | Tomato | HI |
|---|---|---|---|---|---|---|
| 0–3 | 0.03–0.11 | 0.01–0.04 | 0.04–0.13 | 0.11–0.34 | 0.04–0.12 | 0.26–0.56 |
| 3–6 | 0.03–0.10 | 0.01–0.04 | 0.03–0.08 | 0.02–0.06 | 0.05–0.15 | 0.16–0.31 |
| 6–12 | 0.04–0.14 | 0.02–0.06 | 0.09–0.28 | 0.03–0.09 | 0.03–0.11 | 0.23–0.52 |
| 12–16 | 0.02–0.07 | 0.01–0.03 | 0.06–0.19 | 0.01–0.03 | 0.02–0.08 | 0.14–0.30 |
| 16–18 | 0.03–0.09 | 0.01–0.04 | 0.04–0.13 | 0.01–0.03 | 0.02–0.06 | 0.13–0.26 |
| 19–65 | 0.06–0.17 | 0.02–0.07 | 0.02–0.07 | 0.01–0.04 | 0.04–0.11 | 0.17–0.33 |
| >65 | 0.08–0.23 | 0.03–0.09 | 0.03–0.09 | 0.02–0.06 | 0.03–0.08 | 0.21–0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-L.; Chang, W.-H.; Cheng, S.-F.; Li, H.-Y.; Chen, H.-L. Potential Risk of Consuming Vegetables Planted in Soil with Copper and Cadmium and the Influence on Vegetable Antioxidant Activity. Appl. Sci. 2021, 11, 3761. https://doi.org/10.3390/app11093761
Huang W-L, Chang W-H, Cheng S-F, Li H-Y, Chen H-L. Potential Risk of Consuming Vegetables Planted in Soil with Copper and Cadmium and the Influence on Vegetable Antioxidant Activity. Applied Sciences. 2021; 11(9):3761. https://doi.org/10.3390/app11093761
Chicago/Turabian StyleHuang, Wen-Lii, Wei-Hsiang Chang, Shu-Fen Cheng, Huai-Yuan Li, and Hsiu-Ling Chen. 2021. "Potential Risk of Consuming Vegetables Planted in Soil with Copper and Cadmium and the Influence on Vegetable Antioxidant Activity" Applied Sciences 11, no. 9: 3761. https://doi.org/10.3390/app11093761