Football Juggling Learning Alters the Working Memory and White Matter Integrity in Early Adulthood: A Randomized Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design and Procedure
2.3. Football Juggling Learning
2.4. Assessment of Working Memory
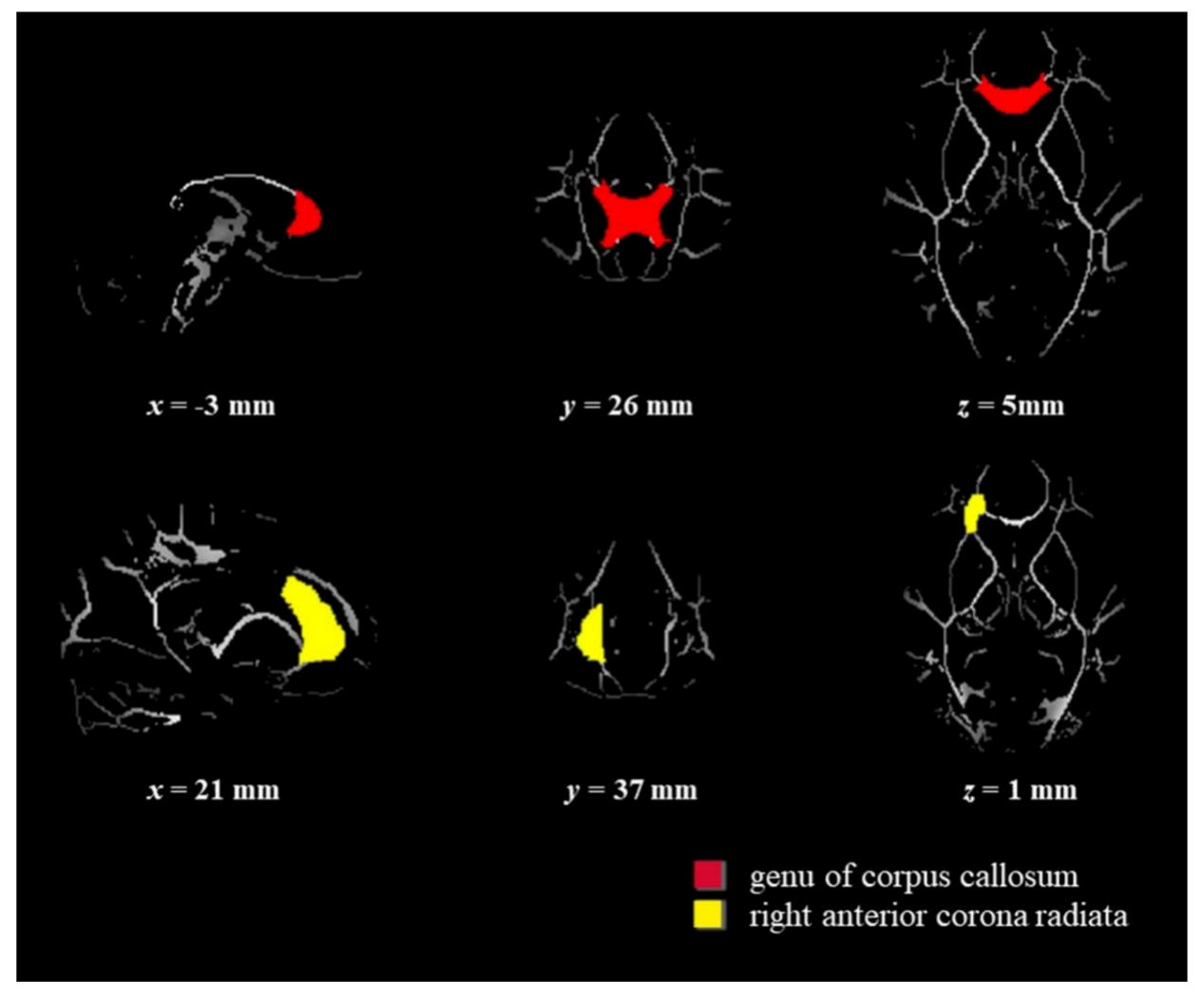
2.5. DTI Imaging Acquisition and Analysis
2.6. Statistical Analyses
3. Results
3.1. Working Memory Data
3.2. White Matter Structure Data
3.3. Mediation Model
4. Discussion
4.1. Behavior
4.2. White Matter Integrity
4.3. The Relationship between Football Juggling Learning, WM, and WMI
4.4. Strengths and Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dishman, R.K.; Berthoud, H.R.; Booth, F.W.; Cotman, C.W.; Edgerton, V.R.; Fleshner, M.R.; Gandevia, S.C.; Gomez-Pinilla, F.; Greenwood, B.N.; Hillman, C.H.; et al. Neurobiology of exercise. Obesity 2006, 14, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Seidler, R.D.; Bo, J.; Anguera, J.A. Neurocognitive Contributions to Motor Skill Learning: The Role of Working Memory. J. Mot. Behav. 2012, 44, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Dayan, E.; Cohen, L.G. Neuroplasticity subserving motor skill learning. Neuron 2011, 72, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willingham, D.B. A neuropsychological theory of motor skill learning. Psychol. Rev. 1998, 105, 558–584. [Google Scholar] [CrossRef] [Green Version]
- Chaddock-Heyman, L.; Erickson, K.I.; Voss, M.W.; Powers, J.P.; Knecht, A.M.; Pontifex, M.B.; Drollette, E.S.; Moore, R.D.; Raine, L.B.; Scudder, M.R. White matter microstructure is associated with cognitive control in children. Biol. Psychol. 2013. [Google Scholar] [CrossRef] [Green Version]
- Stroth, S.; Kubesch, S.; Dieterle, K.; Ruchsow, M.; Heim, R.; Kiefer, M. Physical fitness, but not acute exercise modulates event-related potential indices for executive control in healthy adolescents. Brain Res. 2009, 1269, 114–124. [Google Scholar] [CrossRef]
- Anguera, J.A.; Reuter-Lorenz, P.A.; Willingham, D.T.; Seidler, R.D. Contributions of Spatial Working Memory to Visuomotor Learning. J. Cogn. Neurosci. 2010, 22, 1917–1930. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, E.; Vaezmosavi, M.; Gerber, M.; Puhse, U.; Brand, S. Dual-task training on cognition and resistance training improved both balance and working memory in older people. Phys. Sportsmed. 2019, 47, 471–478. [Google Scholar] [CrossRef]
- Niederer, I.; Kriemler, S.; Gut, J.; Hartmann, T.; Puder, J.J. Relationship of aerobic fitness and motor skills with memory and attention in preschoolers (Ballabeina): A cross-sectional and longitudinal study. BMC Pediatr. 2011, 11. [Google Scholar] [CrossRef] [Green Version]
- Pereira, T.; Cipriano, I.; Costa, T.; Saraiva, M.; Martins, A.; Consortium, A.G.l. Exercise, ageing and cognitive function—Effects of a personalized physical exercise program in the cognitive function of older adults. Physiol. Behav. 2019, 202, 8–13. [Google Scholar] [CrossRef]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Nozawa, T.; Kambara, T.; Sekiguchi, A.; Miyauchi, C.M.; Kotozaki, Y.; Nouchi, H. Brain Training Game Boosts Executive Functions, Working Memory and Processing Speed in the Young Adults: A Randomized Controlled Trial. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Rogge, A.K.; Röder, B.; Zech, A.; Nagel, V.; Hötting, K. Balance training improves memory and spatial cognition in healthy adults. Sci. Rep. 2017, 7, 572. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, T.; Sugasawa, S.; Matsuda, Y.; Mizuno, M. Relationship of tennis play to executive function in children and adolescents. Eur. J. Sport Sci. 2017, 17, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Scharfen, H.E.; Memmert, D. The Relationship Between Cognitive Functions and Sport-Specific Motor Skills in Elite Youth Soccer Players. Front. Psychol. 2019, 10, 817. [Google Scholar] [CrossRef]
- Grassi, M.; Meneghetti, C.; Toffalini, E.; Borella, E. Auditory and cognitive performance in elderly musicians and nonmusicians. PLoS ONE 2017, 12, e0192918. [Google Scholar] [CrossRef] [Green Version]
- Geertsen, S.S.; Thomas, R.; Larsen, M.N.; Dahn, I.M.; Andersen, J.N.; Krause-Jensen, M.; Korup, V.; Nielsen, C.M.; Wienecke, J.; Ritz, C.; et al. Motor Skills and Exercise Capacity Are Associated with Objective Measures of Cognitive Functions and Academic Performance in Preadolescent Children. PLoS ONE 2016, 11, e0161960. [Google Scholar] [CrossRef]
- Verburgh, L.; Scherder, E.J.; Van Lange, P.A.; Oosterlaan, J. Do Elite and Amateur Soccer Players Outperform Non-Athletes on Neurocognitive Functioning? A Study Among 8–12 Year Old Children. PLoS ONE 2016, 11, e0165741. [Google Scholar] [CrossRef] [Green Version]
- Wouters, H.; Aalbers, T.; Maessen, M.; Verbeek, A.; Olde Rikkert, M.; Kessels, R.; Hopman, M.; Eijsvogels, T. Physical activity and cognitive function of long distance walkers: Studying four days marches participants. Rejuvenation Res. 2017, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar, M. Working Memory: How Important Is White Matter? Neuroscientist 2017, 23, 197–210. [Google Scholar] [CrossRef] [PubMed]
- D’Esposito, M.; Postle, B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015, 66, 115–142. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.K.; Lundqvist, M.; Bastos, A.M. Working Memory 2.0. Neuron 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaio-Baptista, C.; Khrapitchev, A.A.; Foxley, S.; Schlagheck, T.; Scholz, J.; Jbabdi, S.; DeLuca, G.C.; Miller, K.L.; Taylor, A.; Thomas, N.; et al. Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci. 2013, 33, 19499–19503. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Klein, M.C.; Behrens, T.E.; Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009, 12, 1370–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badea, A.; Ng, K.L.; Anderson, R.J.; Zhang, J.; Miller, M.I.; O’Brien, R.J. Magnetic resonance imaging of mouse brain networks plasticity following motor learning. PLoS ONE 2019, 14, e0216596. [Google Scholar] [CrossRef] [Green Version]
- Draganski, B.; Gaser, C.; Busch, V.; Schuierer, G.; Bogdahn, U.; May, A. Neuroplasticity: Changes in grey matter induced by training. Nature 2004, 427, 311–312. [Google Scholar] [CrossRef]
- Pi, Y.L.; Wu, X.H.; Wang, F.J.; Liu, K.; Wu, Y.; Zhu, H.; Zhang, J. Motor skill learning induces brain network plasticity: A diffusion-tensor imaging study. PLoS ONE 2019, 14, e0210015. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, N.; Tolentino-Castro, J.W.; Kaminski, E.; Ragert, P.; Taubert, M. Interindividual differences in gray and white matter properties are associated with early complex motor skill acquisition. Hum. Brain Mapp. 2019, 40. [Google Scholar] [CrossRef]
- Deng, F.; Zhao, L.; Liu, C.; Lu, M.; Zhang, S.; Huang, H.; Chen, L.; Wu, X.; Niu, C.; He, Y. Plasticity in deep and superficial white matter: A DTI study in world class gymnasts. Brain Struct. Funct. 2018, 223, 1849–1862. [Google Scholar] [CrossRef]
- Schmithorst, V.J.; Wilke, M. Differences in white matter architecture between musicians and non-musicians: A diffusion tensor imaging study. Neurosci. Lett. 2002, 321, 57–60. [Google Scholar] [CrossRef]
- Claudia, P.; Ben-Soussan, T.D.; Federica, M.; Mallio, C.A.; Yuri, E.; Quattrocchi, C.C.; Filippo, C. White Matter Microstructural Changes Following Quadrato Motor Training: A Longitudinal Study. Front. Hum. Neurosci. 2017, 11, 590. [Google Scholar] [CrossRef] [Green Version]
- Hofstetter, S.; Tavor, I.; Moryosef, S.T.; Assaf, Y. Short-Term Learning Induces White Matter Plasticity in the Fornix. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 12844–12850. [Google Scholar] [CrossRef]
- Weber, B.; Koschutnig, K.; Schwerdtfeger, A.; Rominger, C.; Papousek, I.; Weiss, E.M.; Tilp, M.; Fink, A. Learning Unicycling Evokes Manifold Changes in Gray and White Matter Networks Related to Motor and Cognitive Functions. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Bai, X.; Shao, M.; Liu, T.; Yin, J.; Jin, H. Altered structural plasticity in early adulthood after badminton training. Acta Psychol. Sin. 2019, 52, 173–183. [Google Scholar] [CrossRef]
- Reid, L.B.; Sale, M.V.; Cunnington, R.; Mattingley, J.B.; Rose, S.E. Brain changes following four weeks of unimanual motor training: Evidence from fMRI-guided diffusion MRI tractography. Hum. Brain Mapp. 2017, 38, 4302–4312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, B.J.; Jones, R.M.; Hare, T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008, 1124, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Herting, M.M.; Colby, J.B.; Sowell, E.R.; Nagel, B.J. White matter connectivity and aerobic fitness in male adolescents. Dev. Cogn. Neurosci. 2014, 7, 65–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.K.; Sun, S.W.; Ju, W.K.; Lin, S.J.; Cross, A.H.; Neufeld, A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003, 20, 1714–1722. [Google Scholar] [CrossRef]
- Warbrick, T.; Rosenberg, J.; Shah, N.J. The relationship between BOLD fMRI response and the underlying white matter as measured by fractional anisotropy (FA): A systematic review. Neuroimage 2017, 153, 369–381. [Google Scholar] [CrossRef]
- Shahab, S.; Stefanik, L.; Foussias, G.; Lai, M.C.; Anderson, K.K.; Voineskos, A.N. Sex and Diffusion Tensor Imaging of White Matter in Schizophrenia: A Systematic Review Plus Meta-analysis of the Corpus Callosum. Schizophr. Bull. 2018, 44, 203–221. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tian, L.; Zhang, L.; Cheng, R.; Wei, R.; He, F.; Li, J.; Luo, B.; Ye, X. Relationship between white matter integrity and post-traumatic cognitive deficits: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 98–107. [Google Scholar] [CrossRef]
- Golestani, A.M.; Miles, L.; Babb, J.; Castellanos, F.X.; Lazar, M. Constrained by Our Connections: White Matter’s Key Role in Interindividual Variability in Visual Working Memory Capacity. J. Neurosci. 2014, 34, 14913–14918. [Google Scholar] [CrossRef] [Green Version]
- Nagy, Z.; Westerberg, H.; Klingberg, T.; Nagy, Z.; Westerberg, H.; Klingberg, T. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci. 2004, 16, 1227–1233. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Fieremans, E.; Kucukboyaci, N.E.; Wang, X.; Morton, C.J.; Novikov, D.S.; Rath, J.F.; Lui, Y.W. Working Memory And Brain Tissue Microstructure: White Matter Tract Integrity Based On Multi-Shell Diffusion MRI. Sci. Rep. 2018, 8, 3175. [Google Scholar] [CrossRef] [Green Version]
- Alesi, M.; Bianco, A.; Luppina, G.; Palma, A.; Pepi, A. Improving Children’s Coordinative Skills and Executive Functions: The Effects of a Football Exercise Program. Percept. Mot. Ski. 2016, 122, 27. [Google Scholar] [CrossRef]
- Marianna, A.; Antonino, B.; Johnny, P.; Giorgio, L.; Marco, P.; Antonio, P.; Antonio, P.; Annamaria, P. Motor and cognitive growth following a Football Training Program. Front. Psychol. 2015, 6, 1627. [Google Scholar] [CrossRef] [Green Version]
- Lind, R.R.; Geertsen, S.S.; Ørntoft, C.; Madsen, M.; Larsen, M.N.; Dvorak, J.; Ritz, C.; Krustrup, P. Improved cognitive performance in preadolescent Danish children after the school-based physical activity programme “FIFA 11 for Health” for Europe—A cluster-randomised controlled trial. Eur. J. Sport Sci. 2018. [Google Scholar] [CrossRef] [Green Version]
- Chia-Liang, T.; Pan, C.Y.; Chen, F.C.; Yu-Ting, T. Open- and Closed-Skill Exercise Interventions Produce Different Neurocognitive Effects on Executive Functions in the Elderly: A 6-Month Randomized, Controlled Trial. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Chen, A.; Guo, W.; Zhu, F.; Wang, B. Which Type of Exercise Is More Beneficial for Cognitive Function? A Meta-Analysis of the Effects of Open-Skill Exercise versus Closed-Skill Exercise among Children, Adults, and Elderly Populations. Appl. Sci. 2020, 10, 2737. [Google Scholar] [CrossRef] [Green Version]
- Kai, P.; Hkkinen, K.; Santtila, M.; Raitanen, J.; Kyrlinen, H. Differences in Training Adaptations of Endurance Performance during Combined Strength and Endurance Training in a 6-Month Crisis Management Operation. Int. J. Environ. Res. Public Health 2020, 17, 1688. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.B.; Artero, E.G.; Ruiz, J.R.; Vicente-Rodriguez, G.; Bergman, P.; Hagströmer, M.; Ottevaere, C.; Nagy, E.; Konsta, O.; Rey-López, J.P. Reliability of health-related physical fitness tests in European adolescents. The HELENA Study. Int. J. Obes. 2008, 32 (Suppl. 5), S49. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.C.; Gu, M.; Lee, T.S.; Lu, C.J. The Effects of Daily Sleep Condition on Performances of Physical Fitness among Taiwanese Adults: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 1907. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Raven, J.; Court, J.; Raven, J. Raven’s Standard Progressive Matrices; Oxford Pyschologists Press Oxford: Oxford, UK, 1988. [Google Scholar]
- Arrindell, W.A.; Ettema, J.H.M. SCL-90: Handleiding bij een Multidimensionele Psychopathologieindicator; Swets en Zeitlinger: Lisse, The Netherlands, 1986. [Google Scholar]
- Notice of the General Office of the Ministry of Education on the Issuance of the “National Youth Campus Football Teaching Guide (Trial)” and the “Students Football Sports Skilled Grade Assessment Standard (Trial)”. Available online: http://www.moe.gov.cn/srcsite/A17/s7059/201607/t20160718_272137.html (accessed on 20 December 2019).
- Chen, A.G.; Yan, J.; Yin, H.C.; Pan, C.Y.; Chang, Y.K. Effects of acute aerobic exercise on multiple aspects of executive function in preadolescent children. Psychol. Sport Exerc. 2014, 15, 627–636. [Google Scholar] [CrossRef]
- Chen, A.G.; Zhu, L.N.; Yan, J.; Yin, H.C. Neural Basis of Working Memory Enhancement after Acute Aerobic Exercise: fMRI Study of Preadolescent Children. Front. Psychol. 2016, 7, 1804. [Google Scholar] [CrossRef] [Green Version]
- Pelegrina, S.; Lechuga, M.T.; Garcia-Madruga, J.A.; Elosua, M.R.; Macizo, P.; Carreiras, M.; Fuentes, L.J.; Bajo, M.T. Normative data on the n-back task for children and young adolescents. Front. Psychol. 2015, 6, 1544. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, S.; Cui, J.; Chen, L.Z.; Wang, X.; Fan, M.; Wei, G.X. Fitness-Dependent Effect of Acute Aerobic Exercise on Executive Function. Front. Psychol. 2019, 10, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Lang, S.; Zheng, Y.; Qin, X.; Chen, H.; You, Y.; Ou, H. The Effects of Transcranial Direct Current Stimulation Versus Electroacupuncture on Working Memory in Healthy Subjects. J. Altern. Complement. Med. 2019, 25, 637–642. [Google Scholar] [CrossRef]
- Cui, Z.; Zhong, S.; Xu, P.; He, Y.; Gong, G. PANDA: A pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Hua, K.; Zhang, J.; Wakana, S.; Jiang, H.; Li, X.; Reich, D.S.; Calabresi, P.A.; Pekar, J.J.; Zijl, P.C.M.V.; Mori, S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008, 39, 336–347. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Oishi, K.; Jiang, H.; Jiang, L.; Li, X.; Akhter, K.; Hua, K.; Faria, A.V.; Mahmood, A.; Woods, R. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008, 40, 570–582. [Google Scholar] [CrossRef] [Green Version]
- Neurosurgery; Radiology. MRI Atlas of Human White Matter; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Wakana, S.; Caprihan, A.; Panzenboeck, M.M.; Fallon, J.H.; Perry, M.; Gollub, R.L.; Hua, K.; Zhang, J.; Jiang, H.; Dubey, P. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007, 36, 630–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackey, S.; Chaarani, B.; Kan, K.J.; Spechler, P.A.; Orr, C.; Banaschewski, T.; Barker, G.; Bokde, A.L.W.; Bromberg, U.; Büchel, C.; et al. Brain Regions Related to Impulsivity Mediate the Effects of Early Adversity on Antisocial Behavior. Biol. Psychiatry 2017, 82, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opel, N.; Redlich, R.; Kaehler, C.; Grotegerd, D.; Dohm, K.; Heindel, W.; Kugel, H.; Thalamuthu, A.; Koutsouleris, N.; Arolt, V.; et al. Prefrontal gray matter volume mediates genetic risks for obesity. Mol. Psychiatry 2017, 22, 703–710. [Google Scholar] [CrossRef]
- Opel, N.; Redlich, R.; Dohm, K.; Zaremba, D.; Goltermann, J.; Repple, J.; Kaehler, C.; Grotegerd, D.; Leehr, E.J.; Böhnlein, J. Mediation of the influence of childhood maltreatment on depression relapse by cortical structure: A 2-year longitudinal observational study. Lancet Psychiatry 2019, 6, 318–326. [Google Scholar] [CrossRef]
- Best, J.R.; Dao, E.; Churchill, R.; Cosco, T.D. Associations Between Physical Fitness and Brain Structure in Young Adulthood. Front. Psychol. 2020, 11, 608049. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Correa, M.G.; Santos, M.A.R.D.; Almeida, A.P.C.P.S.C.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. The Effects of Moderate Physical Exercise on Adult Cognition: A Systematic Review. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Williams, R.A.; Cooper, S.B.; Dring, K.J.; Hatch, L.; Nevill, M.E. Effect of football activity and physical fitness on information processing, inhibitory control and working memory in adolescents. BMC Public Health 2020, 20, 1398. [Google Scholar] [CrossRef]
- Al-Thaqib, A.; Al-Sultan, F.; Al-Zahrani, A.; Al-Kahtani, F.; Al-Regaiey, K.; Iqbal, M.; Bashir, S. Brain Training Games Enhance Cognitive Function in Healthy Subjects. Med Sci. Monit. Basic Res. 2018, 24, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Korz, V.; Frey, J.U. Emotional and cognitive reinforcement of rat hippocampal long-term potentiation by different learning paradigms. Neuron Glia Biol. 2004, 1, 253–261. [Google Scholar] [CrossRef]
- Covey, T.J.; Zivadinov, R.; Shucard, J.L.; Shucard, D.W. Information processing speed, neural efficiency, and working memory performance in multiple sclerosis: Differential relationships with structural magnetic resonance imaging. J. Clin. Exp. Neuropsychol. 2011, 33, 1129–1145. [Google Scholar] [CrossRef]
- Ebaid, D.; Crewther, S.G. Temporal Aspects of Memory: A Comparison of Memory Performance, Processing Speed and Time Estimation Between Young and Older Adults. Front. Aging Neurosci. 2018, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Akitsuki, Y.; Shigemune, Y.; Sekiguchi, A.; Kotozaki, Y.; Tsukiura, T.; Yomogida, Y.; et al. Brain training game improves executive functions and processing speed in the elderly: A randomized controlled trial. PLoS ONE 2012, 7, e29676. [Google Scholar] [CrossRef]
- Suarez-Manzano, S.; Ruiz-Ariza, A.; De La Torre-Cruz, M.; Martinez-Lopez, E.J. Acute and chronic effect of physical activity on cognition and behaviour in young people with ADHD: A systematic review of intervention studies. Res. Dev. Disabil. 2018, 77, 12–23. [Google Scholar] [CrossRef]
- Pellis, S.M.; Pellis, V.C. Rough-and-tumble play and the development of the social brain. Curr. Dir. Psychol. Sci. 2007, 16, 95–98. [Google Scholar] [CrossRef]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [Green Version]
- Ruotsalainen, I.; Gorbach, T.; Perkola, J.; Renvall, V.; Parviainen, T. Physical activity, aerobic fitness, and brain white matter: Their role for executive functions in adolescence. Dev. Cogn. Neurosci. 2020. [Google Scholar] [CrossRef]
- Xuan, X.; Zhu, L.N.; Dong, X.X.; Wei, W.; Yan, J.; Chen, A.G. Aerobic Exercise Intervention Alters Executive Function and White Matter Integrity in Deaf Children: A Randomized Controlled Study. Neural Plast. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Casadio, M.; Weber, K.A.; Mussa-Ivaldi, F.A.; Parrish, T.B. White matter microstructure changes induced by motor skill learning utilizing a body machine interface. Neuroimage 2014, 88, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, B. White Matter Development in Adolescence: A DTI Study. Cereb. Cortex 2010, 20, 2122–2131. [Google Scholar] [CrossRef]
- Fjell, A.M. Life-Span Changes of the Human Brain White Matter: Diffusion Tensor Imaging (DTI) and Volumetry. Cereb. Cortex 2010, 20, 2055–2068. [Google Scholar] [CrossRef] [Green Version]
- Lebel, C.; Treit, S.; Beaulieu, C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2017, e3778. [Google Scholar] [CrossRef]
- Opel, N.; Martin, S.; Meinert, S.; Redlich, R.; Enneking, V.; Richter, M.; Goltermann, J.; Johnen, A.; Dannlowski, U.; Repple, J. White matter microstructure mediates the association between physical fitness and cognition in healthy, young adults. Sci. Rep. 2019, 9, 12885. [Google Scholar] [CrossRef] [Green Version]
- D’Esposito, M.; Grafman, J.H. Preface. Handb. Clin. Neurol. 2019, 163, ix. [Google Scholar] [CrossRef]
- Yaple, Z.A.; Stevens, W.D.; Arsalidou, M. Meta-analyses of the n-back working memory task: fMRI evidence of age-related changes in prefrontal cortex involvement across the adult lifespan. Neuroimage 2019, 196, 16–31. [Google Scholar] [CrossRef]
- Luders, E.; Narr, K.L.; Bilder, R.M.; Thompson, P.M.; Szeszko, P.R.; Hamilton, L.; Toga, A.W. Positive correlations between corpus callosum thickness and intelligence. Neuroimage 2007, 37, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J.M.; Bohan, T.P.; Brandt, M.E.; Brookshire, B.L.; Beaver, S.R.; Francis, D.J.; Davidson, K.C.; Thompson, N.M.; Miner, M.E. Cerebral white matter and cognition in hydrocephalic children. Arch. Neurol. 1992, 49, 818–824. [Google Scholar] [CrossRef]
- Haasz, J.; Westlye, E.T.; Fjaer, S.; Espeseth, T.; Lundervold, A.; Lundervold, A.J. General fluid-type intelligence is related to indices of white matter structure in middle-aged and old adults. Neuroimage 2013, 83, 372–383. [Google Scholar] [CrossRef]
- Kuznetsova, K.A.; Maniega, S.M.; Ritchie, S.J.; Cox, S.R.; Storkey, A.J.; Starr, J.M.; Wardlaw, J.M.; Deary, I.J.; Bastin, M.E. Brain white matter structure and information processing speed in healthy older age. Brain Struct. Funct. 2016, 221, 3223–3235. [Google Scholar] [CrossRef] [Green Version]
- Oberlin, L.E.; Verstynen, T.D.; Burzynska, A.Z.; Voss, M.W.; Prakash, R.S.; Chaddock-Heyman, L.; Wong, C.; Fanning, J.; Awick, E.; Gothe, N.; et al. White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. Neuroimage 2016, 131, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.F.; Kim, C.; Gold, B.T. Socioeconomic status is positively correlated with frontal white matter integrity in aging. Age 2013, 35, 2045–2056. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, K.M.; Raz, N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia 2009, 47, 916–927. [Google Scholar] [CrossRef] [Green Version]
- Krogsrud, S.K.; Fjell, A.M.; Tamnes, C.K.; Grydeland, H.; Due-Tonnessen, P.; Bjornerud, A.; Sampaio-Baptista, C.; Andersson, J.; Johansen-Berg, H.; Walhovd, K.B. Development of white matter microstructure in relation to verbal and visuospatial working memory-A longitudinal study. PLoS ONE 2018, 13, e0195540. [Google Scholar] [CrossRef] [Green Version]
- Ostby, Y.; Tamnes, C.K.; Fjell, A.M.; Walhovd, K.B. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia 2011, 49, 3854–3862. [Google Scholar] [CrossRef]
- Vestergaard, M.; Madsen, K.S.; Baare, W.F.; Skimminge, A.; Ejersbo, L.R.; Ramsoy, T.Z.; Gerlach, C.; Akeson, P.; Paulson, O.B.; Jernigan, T.L. White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. J. Cogn. Neurosci. 2011, 23, 2135–2146. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.; Montojo, C.A.; Sheu, Y.S.; Marchette, S.A.; Harrison, D.M.; Newsome, S.D.; Zhou, F.; Shelton, A.L.; Courtney, S.M. Object working memory performance depends on microstructure of the frontal-occipital fasciculus. Brain Connect. 2011, 1, 317–329. [Google Scholar] [CrossRef]

| Variables | Control Group | Experimental Group |
|---|---|---|
| N | 41 | 53 |
| Gender (male/female) | 24/17 | 38/15 |
| Age (years) | 18.49 ± 0.746 | 18.26 ± 0.524 |
| BMI (height/weight2) | 21.81 ± 3.145 | 20.61 ± 2.737 |
| 4 × 10-m shuttle run (s) | 11.47 ± 1.042 | 11.71 ± 0.860 |
| Standing broad jump (m) | 1.99 ± 0.331 | 1.98 ± 0.283 |
| Sit-and-reach (cm) | 13.64 ± 8.185 | 12.37 ± 6.018 |
| Variables | Control Group | Experimental Group | ||
|---|---|---|---|---|
| Baseline | Posttest | Baseline | Posttest | |
| 1-back task | ||||
| RT | 680.954 ± 182.830 | 669.432 ± 120.663 | ||
| ACC | 0.948 ± 0.034 | 0.959 ± 0.044 | ||
| 2-back task | ||||
| RT | 1109.885 ± 179.623 | 1069.991 ± 215.187 | 1101.474 ± 160.020 | 911.799 ± 184.143 |
| ACC | 0.740 ± 0.167 | 0.815 ± 0.119 | 0.791 ± 0.171 | 0.894 ± 0.114 |
| FA | ||||
| R.ACR | 0.419 ± 0.029 | 0.411 ± 0.023 | 0.419 ± 0.023 | 0.427 ± 0.027 |
| GOCC | 0.618 ± 0.022 | 0.611 ± 0.018 | 0.617 ± 0.023 | 0.623 ± 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Cai, K.; Zhu, H.; Dong, X.; Xiong, X.; Zhu, L.; Sun, Z.; Chen, A. Football Juggling Learning Alters the Working Memory and White Matter Integrity in Early Adulthood: A Randomized Controlled Study. Appl. Sci. 2021, 11, 3843. https://doi.org/10.3390/app11093843
Shi Y, Cai K, Zhu H, Dong X, Xiong X, Zhu L, Sun Z, Chen A. Football Juggling Learning Alters the Working Memory and White Matter Integrity in Early Adulthood: A Randomized Controlled Study. Applied Sciences. 2021; 11(9):3843. https://doi.org/10.3390/app11093843
Chicago/Turabian StyleShi, Yifan, Kelong Cai, Hao Zhu, Xiaoxiao Dong, Xuan Xiong, Lina Zhu, Zhiyuan Sun, and Aiguo Chen. 2021. "Football Juggling Learning Alters the Working Memory and White Matter Integrity in Early Adulthood: A Randomized Controlled Study" Applied Sciences 11, no. 9: 3843. https://doi.org/10.3390/app11093843
APA StyleShi, Y., Cai, K., Zhu, H., Dong, X., Xiong, X., Zhu, L., Sun, Z., & Chen, A. (2021). Football Juggling Learning Alters the Working Memory and White Matter Integrity in Early Adulthood: A Randomized Controlled Study. Applied Sciences, 11(9), 3843. https://doi.org/10.3390/app11093843






