Infrared Thermography as a Non-Invasive Tool in Musculoskeletal Disease Rehabilitation—The Control Variables in Applicability—A Systematic Review
Abstract
1. Introduction
2. Technical Fundamentals of IRT
3. Standardization in Thermographic Research
- Concerning the examination room where the thermovision measurements are carried out: it should allow for the convenient and correct placement of the measuring devices and the visualization of the entire examined area and it should not be smaller than 6 m2 and it should be without unnecessary equipment; it should have a stable temperature and a relative humidity system; air-conditioning equipment should be located so that draughts are not directed at the patient and that overall air speed is kept as low as possible; airflow should be inferior to 2 m/s and the illumination should have protections to avoid incident lightning over the subject; laboratory windows have to be shut (to prevent solar radiation).
- Concerning the environmental conditions: a range of temperatures from 18 °C to 25 °C should be adjustable; the selection of the ambient temperature in which the research is conducted depends on the type and purpose of the tests (recommendation for the examination of the extremities: a warmer ambient temperature of 22–24 °C; large body surfaces examinations within 25–27 °C; lower temperatures enable better diagnosis of inflammatory changes (20 °C)); for medical examinations, it is recommended to keep the room humidity at the level of 45–55%.
- Concerning the patients: the intrinsic factors that should be taken into account during the implementation and interpretation of the research results are sex, age, anthropometric measurements, body composition, circadian and infradian rhythms, hair density, skin emissivity, medical history, metabolic rate, skin blood flow, genetics, and emotions; those that should be avoided are factors that alter metabolism (smoking, drinking sparkling water and/or hot coffee and tea; a heavy meal a minimum of two hours prior to the investigation; alcohol or drug consumption; and physiotherapy or sports on the image-collection day); also on the day of the examination, the patient must not apply any cosmetics to the tested body surface; the patient should report any infections and any medications taken; the subject’s acclimatization or equilibration period should be 15–20 min, while during this process the body surface that will be recorded must be uncovered; to minimize thermal reflections, the person under testing should be as far as possible from all equipment and walls; physical activity during all testing procedures should be kept to a minimum; the analyzed area of the body must not be touched; the examined person should not have any jewelry and should avoid crossing legs or holding arms close to or on the body; seating should be abstained from, to prevent marks that can result in skin temperature changes.
- Concerning the technical determinants of measurements: in medicine, cameras with a tunable focal length (zoom) are most often used, with a minimum focusing distance of approx. 0.5 m, a row’s field of view 25° × 19°, and an angle of divergence equal to or less than 1 mrad; an optimized temperature range (approximately 20–50 °C) will maximize the sensitivity of the sensor; a larger number of pixels (resolution) means more thermal information; it is important to control the calibration; the distance between the camera and the analyzed object should not be less than 1–1.5 m; in the selection of the ROI, the body region must be perpendicular to the lens of the infrared camera; when setting an ROI manually, including random pixels from the background or from the borders to the ROI in the analysis should be avoided [11,19,21,22,23].
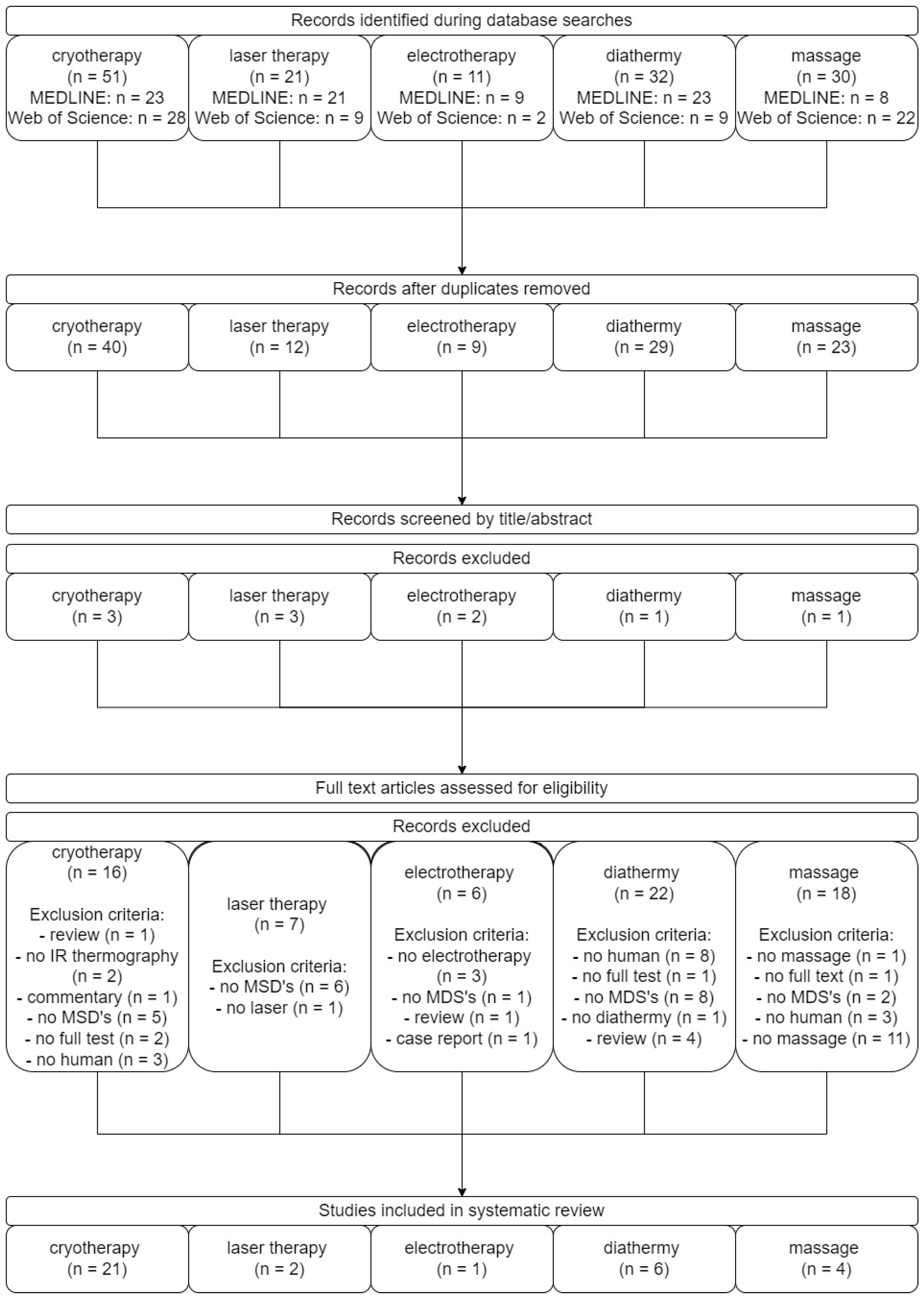
4. Materials and Methods
4.1. Study Design
4.2. Search Strategies
4.3. Article Protocol Selection
5. The Use of Thermovision to Assess the Effects of Chosen Physiotherapy Treatments
5.1. Cryotherapy
5.2. Laser Therapy
5.3. Electrotherapy
5.4. Diathermy
5.5. Massage
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zawawi, T.N.S.T.; Abdullah, T.; Sudriman, R.; Saad, N.M.; Too, J.; Shair, E.F. Classification of EMG signal for health screening task for musculoskeletal disorder. Int. J. Eng. Technol. 2019, 8, 219–226. [Google Scholar] [CrossRef]
- Albuquerque, N.F.; Lopes, B.S. Musculoskeletal applications of infrared thermography on back and neck syndromes: A systematic review. Eur. J. Phys. Rehabil. Med. 2021, 57, 386–396. [Google Scholar] [CrossRef]
- Wenham, C.Y.J.; Grainger, A.J.; Conaghan, P.G. The role of imaging modalities in the diagnosis, differential diagnosis and clinical assessment of peripheral joint osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1692–1702. [Google Scholar] [CrossRef]
- Bevan, S. Economic impact of musculoskeletal disorders (MSDs) on work in Europe. Best Pract. Res. Clin. Rheumatol. 2015, 29, 356–373. [Google Scholar] [CrossRef]
- Musculoskeletal Conditions. Available online: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 27 February 2022).
- Mock, C.; Cherian, M.N. The global burden of musculoskeletal injuries: Challenges and solutions. Clin. Orthop. Relat. Res. 2008, 466, 2306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Sun, H.; Jiang, Y.; Lou, J.; He, X.; Fang, J. Infrared thermography in the diagnosis of musculoskeletal injuries: A protocol for a systematic review and meta-analysis. Medicine 2020, 99, e23529. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, C.; Zeilberger, K.; Francis, E.; Ring, J.; Raschner, C. The application of medical infrared thermography in sports medicine. In An International Perspective on Topics in Sports Medicine and Sports Injury; Zaslav, K.R., Ed.; Intech Open: London, UK, 2012. [Google Scholar] [CrossRef]
- Minkina, W. Theoretical basics of radiant heat transfer—Practical examples of calculation for the infrared (IR) used in infrared thermography measurements. Quant. Infrared Thermogr. J. 2021, 18, 269–282. [Google Scholar] [CrossRef]
- Speakman, J.R.; Ward, S. Infrared thermography: Principles and applications. Zoology 1998, 101, 224–232. [Google Scholar]
- Vardasca, R.; Vaz, L.; Mendes, J. Classification and decision making of medical infrared thermal images. In Lecture Notes in Computational Vision and Biomechanics; Springer: Berlin/Heidelberg, Germany, 2018; Volume 26, pp. 79–104. [Google Scholar]
- Batista-Leyva, A.J. Radiometry and photometry: Two visions of one phenomenon. Rev. Cub. Fis. 2019, 36, 66–72. [Google Scholar]
- Hildebrandt, C.; Raschner, C.; Ammer, K. An overview of recent application of medical infrared thermography in sports medicine in Austria. Sensors 2010, 10, 4700. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Diakides, N.A. Infrared Imaging in Medicine; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849390272. [Google Scholar]
- Pereira, C.B.; Yu, X.; Dahlmanns, S.; Blazek, V.; Leonhardt, S.; Teichmann, D. Infrared thermography. In Multi-Modality Imaging; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–30. ISBN 9783319989747. [Google Scholar]
- Steketee, J. Spectral emissivity of skin and pericardium. Phys. Med. Biol. 1973, 18, 686–694. [Google Scholar] [CrossRef]
- Merla, A.; Romani, G.L. Functional infrared imaging in medicine: A quantitative diagnostic approach. In Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 224–227. [Google Scholar]
- Guyton, A.; Hall, J. Textbook of Medical Physiology, 11th ed.; Elsevier Saunders: Amsterdam, The Netherlands, 2006; ISBN 9780323597128. [Google Scholar]
- Shterenshis, M. Challenges to global implementation of infrared thermography technology: Current perspective. Cent. Asian J. Glob. Health 2017, 6, 289. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.E.; Costa, C.M.A.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I.; et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consensus statement on the measurement of human skin temperature. J. Therm. Biol. 2017, 69, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Fujimasa, I. Standardization of techniques for thermal imaging testing: The current situation. Part 1. Basic information. Biomed. Thermol. J. Jpn. Soc. Thermorogy 1995, 15, 63–68. [Google Scholar]
- Al-Nakhli, H.H.; Petrofsky, J.S.; Laymon, M.S.; Berk, L.S. The use of thermal infra-red imaging to detect delayed onset muscle soreness. J. Vis. Exp. 2012, 59, e3551. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cuevas, I.; Bouzas Marins, J.C.; Arnáiz Lastras, J.; Gómez Carmona, P.M.; Piñonosa Cano, S.; García-Concepción, M.Á.; Sillero-Quintana, M. Classification of factors influencing the use of infrared thermography in humans: A review. Infrared Phys. Technol. 2015, 71, 28–55. [Google Scholar] [CrossRef]
- ISO TR13154; Medical Electrical Equipment—Deployment, Implementation and Operational Guidelines for Identifying Febrile Humans Using a Screening Thermograph. International Organization for Standardization: Geneva, Switzerland, 2009.
- Ring, E.F.J.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33. [Google Scholar] [CrossRef] [PubMed]
- ASTM E 1213; Standard Practice for Minimum Resolvable Temperature Difference for Thermal Imaging Systems. ASTM International: West Conshohocken, PA, USA, 2009.
- ASTM E 1256; Standard Test Methods for Radiation Thermometers (Single Waveband Type). ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM E 1311; Standard Test Method for Minimum Detectable Temperature Difference for Thermal Imaging Systems. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM E 1543; Standard Test Method for Noise Equivalent Temperature Difference of Thermal Imaging Systems. ASTM International: West Conshohocken, PA, USA, 2006.
- ASTM E 1862; Standard Test Methods for Measuring and Compensating for Reflected Temperature Using Infrared Imaging Radiometers. ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM E 1933; Standard Test Methods for Measuring and Compensating for Emissivity Using Infrared Imaging Radiometers. ASTM International: West Conshohocken, PA, USA, 2005.
- Jasti, N.; Bista, S.; Bhargav, H.; Sinha, S.; Gupta, S.; Chaturvedi, S.K.; Gangadhar, B.N. Medical applications of infrared thermography: A narrative review. In Stem Cells in Disease Pathogenesis; Nova Science Publishers: Hauppauge, NY, USA, 2021; pp. 415–453. ISBN 9781536196580. [Google Scholar]
- Brenner, M.; Braun, C.; Oster, M.; Gulko, P.S. Thermal signature analysis as a novel method for evaluating inflammatory arthritis activity. Ann. Rheum. Dis. 2006, 65, 306. [Google Scholar] [CrossRef]
- Lasanen, R.; Piippo-Savolainen, E.; Remes-Pakarinen, T.; Kröger, L.; Heikkilä, A.; Julkunen, P.; Karhu, J.; Töyrös, J. Thermal imaging in screening of joint inflammation and rheumatoid arthritis in children. Physiol. Meas. 2015, 36, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Denoble, A.E.; Hall, N.; Pieper, C.F.; Kraus, V.B. Patellar skin surface temperature by thermography reflects knee osteoarthritis severity. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2010, 3, 69–75. [Google Scholar] [CrossRef]
- Zuzda, J.G.; Topczewska, M.; Borkowski, P.; Latosiewicz, R. The influence of rotational training on muscle activity of young adults in thermographic imaging. Stud. Logic Gramm. Rhetor. 2018, 56, 91–105. [Google Scholar] [CrossRef]
- Choi, E.; Lee, P.B.; Nahm, F.S. Interexaminer reliability of infrared thermography for the diagnosis of complex regional pain syndrome. Skin Res. Technol. 2013, 19, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Conwell, T.D.; Hobbins, W.B.; Giordano, J. Sensitivity, specificity and predictive value of infrared cold water autonomic functional stress testing as compared with modified IASP criteria for CRPS. Thermol. Int. 2010, 20, 60–68. [Google Scholar]
- Mitani, Y.; Fukunaga, M.; Kanbara, K.; Takebayashi, N.; Ishino, S.; Nakai, Y. Evaluation of psychophysiological asymmetry in patients with fibromyalgia syndrome. Appl. Psychophysiol. Biofeedback 2006, 31, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zaproudina, N. Methodological Aspects of Use of Infrared Thermography in Healthy Individuals and Patients with Non-Specific Musculoskeletal Disorders. Doctoral Dissertation, University of Eastern Finland, Kuopio, Finland, 2011. [Google Scholar]
- Schmitt, M.; Guillot, Y. Thermography and muscular injuries in sports medicine. In Recent Advances in Medical Thermology; Springer: Boston, MA, USA, 1984; pp. 439–445. [Google Scholar] [CrossRef]
- Park, J.Y.; Hyun, J.K.; Seo, J.B. The effectiveness of digital infrared thermographic imaging in patients with shoulder impingement syndrome. J. Shoulder Elb. Surg. 2007, 16, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Lubkowska, A.; Gajewska, E. Temperature distribution of selected body surfaces in scoliosis based on static infrared thermography. Int. J. Environ. Res. Public Health 2020, 17, 8913. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.G.; Park, H.J.; Chae, H.Y.; Kim, B.G.; Lim, H.S.; Park, J.I.; Kim, J.H. Comparison of changes in facial skin temperature caused by ethyl chloride spraying, ice block rubbing and cold gel packing in healthy subjects. J. Oral Rehabil. 2012, 39, 931–940. [Google Scholar] [CrossRef]
- Korman, P.; Straburzyńska-Lupa, A.; Romanowski, W.; Trafarski, A. Temperature changes in rheumatoid hand treated with nitrogen vapors and cold air. Rheumatol. Int. 2012, 32, 2987–2992. [Google Scholar] [CrossRef][Green Version]
- Cholewka, A.; Stanek, A.; Sieroń, A.; Drzazga, Z. Thermography study of skin response due to whole-body cryotherapy. Ski. Res. Technol. 2012, 18, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo de Carvalho, A.; Lazzeri de Medeiros, D.; Tibes de Souza, F.; Francine de Paula, G.; Mantovani Barbosa, P.; Olegini Vasconcellos, P.R.; Rosângela Buzanello, M.; Flor Bertolini, G.R. Temperature variation of the quadriceps femoris muscle exposed to two forms of cryotherapy by means of thermography. Rev. Bras. Med. Esporte 2012, 18, 109–111. [Google Scholar]
- Zalewski, P.; Klawe, J.J.; Pawlak, J.; Tafil-Klawe, M.; Newton, J. Thermal and hemodynamic response to whole-body cryostimulation in healthy subjects. Cryobiology 2013, 66, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Dȩbiec-Ba̧k, A.; Skrzek, A.; Podbielska, H. Application of thermovision for estimation of the optimal and safe parameters of the whole body cryotherapy. J. Therm. Anal. Calorim. 2013, 111, 1853–1859. [Google Scholar] [CrossRef]
- Keramidas, M.E.; Kölegård, R.; Mekjavic, I.B.; Eiken, O. Acute effects of normobaric hypoxia on hand-temperature responses during and after local cold stress. High Alt. Med. Biol. 2014, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Chudecka, M.; Zaborski, D.; Lubkowska, A.; Grzesiak, W.; Klimek, A.; Modrzejewski, A. Temperature changes in selected areas of body surface induced by systemic cryostimulation. Aviat. Space. Environ. Med. 2014, 85, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Dębiec-Bąk, A.; Pawik, Ł.; Skrzek, A. Thermoregulation of football players after cryotherapy in thermography. J. Therm. Anal. Calorim. 2016, 126, 1633–1644. [Google Scholar] [CrossRef]
- Vellard, M.; Arfaoui, A. Detection by infrared thermography of the effect of local cryotherapy exposure on thermal spreadin skin. J. Imaging 2016, 2, 20. [Google Scholar] [CrossRef]
- Adamczyk, J.G.; Krasowska, I.; Boguszewski, D.; Reaburn, P. The use of thermal imaging to assess the effectiveness of ice massage and cold-water immersion as methods for supporting post-exercise recovery. J. Therm. Biol. 2016, 60, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Bouzigon, R.; Arfaoui, A.; Grappe, F.; Ravier, G.; Jarlot, B.; Dugue, B. Validation of a new whole-body cryotherapy chamber based on forced convection. J. Therm. Biol. 2017, 65, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Cholewka, A.; Stanek, A.; Wójcik, M.; Sieroń-Stołtny, K.; Drzazga, Z. Does local cryotherapy improve thermal diagnosis similar to whole-body cryotherapy in spinal diseases? J. Therm. Anal. Calorim. 2017, 127, 1155–1162. [Google Scholar] [CrossRef]
- Polidori, G.; Taiar, R.; Legrand, F.; Beaumont, F.; Murer, S.; Bogard, F.; Boyer, F.C. Infrared thermography for assessing skin temperature differences between partial body cryotherapy and whole body cryotherapy devices at −140 °C. Infrared Phys. Technol. 2018, 93, 158–161. [Google Scholar] [CrossRef]
- Gruszka, K.; Szczuka, E.; Całkosiński, I.; Sobiech, K.A.; Chwałczyńska, A. Thermovision analysis of surface body temperature changes after thermal stimulation treatments in healthy men. Acta Bioeng. Biomech. 2018, 20, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Vargas e Silva, N.C.O.; Rubio, A.L.; Alfieri, F.M. Pain tolerance: The influence of cold or heat therapy. J. Chiropr. Med. 2019, 18, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Rhodes, D.D.; Birdsall, D.; Selfe, P.J. Comparison of cryotherapy modality application over the anterior thigh across rugby union positions; a crossover randomized controlled trial. Int. J. Sports Phys. Ther. 2020, 15, 210–220. [Google Scholar] [CrossRef] [PubMed]
- De Estéfani, D.; Ruschel, C.; Benincá, I.L.; dos Santos Haupenthal, D.P.; de Avelar, N.C.P.; Haupenthal, A. Volume of water added to crushed ice affects the efficacy of cryotherapy: A randomised, single-blind, crossover trial. Physiotherapy 2020, 107, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Alcamí, M.; Priego-Quesada, J.I.; Gimeno Raga, M.; Durán Lozano, Á.; Gil-Calvo, M. Effect of fatigue strength exercise on anterior thigh skin temperature rewarming after cold stress test. J. Therm. Biol. 2021, 101, 103098. [Google Scholar] [CrossRef] [PubMed]
- Priego-Quesada, J.I.; Gandia-Soriano, A.; Pellicer-Chenoll, M.T.; Catalá-Vilaplana, I.; Bermejo-Ruiz, J.L.; Encarnación-Martínez, A.; Salvador-Palmer, R.; Cibrián Ortiz de Anda, R. Reproducibility of skin temperature response after cold stress test using the game ready system: Preliminary study. Int. J. Environ. Res. Public Health 2021, 18, 8295. [Google Scholar] [CrossRef] [PubMed]
- Radecka, A.; Pluta, W.; Lubkowska, A. Assessment of the dynamics of temperature changes in the knee joint area in response to selected cooling agents in thermographic tests. Int. J. Environ. Res. Public Health 2021, 18, 5326. [Google Scholar] [CrossRef] [PubMed]
- Bilska, A.; Stangret, A.; Pyzlak, M.; Wojdasiewicz, P.; Szukiewicz, D. Skin surface infrared thermography in pressure ulcer outcome prognosis. J. Wound Care 2020, 29, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Stamborowski, S.F.; de Oliveira Spinelli, B.M.; Lima, F.P.S.; Costa, D.R.; de Silveira Souza, G.A.; Lima, M.O.; Lopes Martins, R.A.B. The influence of photobiomodulation on the temperature of the brachial biceps during muscle fatigue protocol. Lasers Med. Sci. 2021, 36, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Benito-Martínez, E.; Senovilla-Herguedas, D.; de la Torre-Montero, J.C.; Martínez-Beltrán, M.J.; Reguera-García, M.M.; Alonso-Cortés, B. Local and contralateral effects after the application of neuromuscular electrostimulation in lower limbs. Int. J. Environ. Res. Public Health 2020, 17, 9028. [Google Scholar] [CrossRef]
- Dymarek, R.; Taradaj, J.; Rosińczuk, J. The effect of radial extracorporeal shock wave stimulation on upper limb spasticity in chronic stroke patients: A single-blind, randomized, placebo-controlled study. Ultrasound Med. Biol. 2016, 42, 1862–1875. [Google Scholar] [CrossRef]
- De Sousa, N.T.A.; Guirro, E.C.D.O.; Calió, J.G.; de Queluz, M.C.; Guirro, R.R.D.J. Application of shortwave diathermy to lower limb increases arterial blood flow velocity and skin temperature in women: A randomized controlled trial. Braz. J. Phys. Ther. 2017, 21, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Clijsen, R.; Leoni, D.; Schneebeli, A.; Cescon, C.; Soldini, E.; Li, L.; Barbero, M. Does the application of tecar therapy affect temperature and perfusion of skin and muscle microcirculation? A pilot feasibility study on healthy subjects. J. Altern. Complement. Med. 2020, 26, 147–153. [Google Scholar] [CrossRef]
- Yeste-Fabregat, M.; Baraja-Vegas, L.; Vicente-Mampel, J.; Pérez-Bermejo, M.; Bautista González, I.J.; Barrios, C. Acute effects of tecar therapy on skin temperature, ankle mobility and hyperalgesia in myofascial pain syndrome in professional basketball players: A pilot study. Int. J. Environ. Res. Public Health 2021, 18, 8756. [Google Scholar] [CrossRef]
- Kaźmierska, B.; Sobiech, K.A.; Demczuk-Włodarczyk, E.; Chwałczyńska, A. Thermovision assessment of temperature changes in selected body areas after short-wave diathermy treatment. J. Therm. Anal. Calorim. 2021, 1–8. [Google Scholar] [CrossRef]
- Benincá, I.L.; de Estéfani, D.; Pereira de Avelar, N.C.; Pacheco dos Santos Haupenthal, D.; Lock Silveira, P.C.; Haupenthal, A. Coplanar arrangement of shortwave diathermy is the most effective in skin temperature change: A randomized crossover trial. J. Bodyw. Mov. Ther. 2021, 26, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Wälchli, C.; Saltzwedel, G.; Krüerke, D.; Kaufmann, C.; Schnorr, B.; Rist, L.; Eberhard, J.; Decker, M.; Simões-Wüst, A.P. Physiologic effects of rhythmical massage: A prospective exploratory cohort study. J. Altern. Complement. Med. 2014, 20, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Boguszewski, D.; Adamczyk, J.G.; Urbańska, N.; Mrozek, N.; Piejko, K.; Janicka, M.; Białoszewski, D. Using thermal imaging to assess the effect of classical massage on selected physiological parameters of upper limbs. Biomed. Hum. Kinet. 2014, 60, 20–25. [Google Scholar] [CrossRef]
- Boguszewski, D.; Adamczyk, J.G.; Hadamus, A.; Mosiołek, A.; Ochal, A.; Białoszewski, D. Evaluation of the effect of isometric and classic massage on selected physiological and biomechanical parameters of the lower extremities. Acta Kinesiol. 2020, 14, 109–114. [Google Scholar]
- Roszkowska, K.; Witkowska-Pilaszewicz, O.; Przewozny, M.; Cywinska, A. Whole body and partial body cryotherapies—Lessons from human practice and possible application for horses. BMC Vet. Res. 2018, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Savic, M.; Fonda, B.; Sarabon, N. Actual temperature during and thermal response after whole-body cryotherapy in cryo-cabin. J. Therm. Biol. 2013, 38, 186–191. [Google Scholar] [CrossRef]
- Hirvonen, H.; Kautiainen, H.; Moilanen, E.; Mikkelsson, M.; Leirisalo-Repo, M. The effect of cryotherapy on total antioxidative capacity in patients with active seropositive rheumatoid arthritis. Rheumatol. Int. 2017, 37, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Mussttaf, R.A.; Jenkins, D.F.L.; Jha, A.N. Assessing the impact of low level laser therapy (LLLT) on biological systems: A review. Int. J. Radiat. Biol. 2019, 95, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological effects of low level laser therapy. J. Lasers Med. Sci. 2014, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Baroni, B.M.; Rodrigues, R.; Freire, B.B.; de Azevedo Franke, R.; Geremia, J.M.; Vaz, M.A. Effect of low-level laser therapy on muscle adaptation to knee extensor eccentric training. Eur. J. Appl. Physiol. 2015, 115, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, J. Basic concepts in electricity and electrotherapy. In Electrical Stimulation for Pelvic Floor Disorders; Springer: Cham, Switzerland, 2015; pp. 61–74. ISBN 9783319069470. [Google Scholar]
- Putowski, M.; Piróg, M.; Podgórniak, M.; Padała, O.; Sadowska, M.; Bazylevycz, A.; Wdowiak, A. The use of electromagnetic radiation in the physiotherapy. Eur. J. Med. Technol. 2016, 2, 53–58. [Google Scholar]
- Benincá, I.L.; de Estéfani, D.; Pereira de Souza, S.; Weisshahn, N.K.; Haupenthal, A. Tissue heating in different short wave diathermy methods: A systematic review and narrative synthesis. J. Bodyw. Mov. Ther. 2021, 28, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Lineaweaver, W.C.; Zhang, F.; Zhang, J. Role of shortwave and microwave diathermy in peripheral neuropathy. J. Int. Med. Res. 2019, 47, 3569–3579. [Google Scholar] [CrossRef]
- Farahat, A.E.; Kahil, H.M.; Hussein, F.A. Microwave diathermy for deep heating therapy of knee joint. Prog. Electromagn. Res. C 2020, 99, 15–33. [Google Scholar] [CrossRef]
- Brosseau, L.; Wells, G.A.; Poitras, S.; Tugwell, P.; Casimiro, L.; Novikov, M.; Loew, L.; Sredic, D.; Clément, S.; Gravelle, A.; et al. Ottawa panel evidence-based clinical practice guidelines on therapeutic massage for low back pain. J. Bodyw. Mov. Ther. 2012, 16, 424–455. [Google Scholar] [CrossRef]
- Ernst, E. Massage therapy for cancer palliation and supportive care: A systematic review of randomised clinical trials. Support. Care Cancer 2009, 17, 333–337. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Sample (F/M) | Procedure | Aim of the Study | Treatment Procedure | IR Observation Period | ROI | Camera Type and Specyfication |
|---|---|---|---|---|---|---|---|
| Cryotherapy | |||||||
| [44] | n = 30 (15/15) | LC: 1. ethyl chloride spraying 2. ice block rubbing 3. cold pack | Comparing the effects of 3 cryotherapeutic modalities on facial skin temperature | Temperature/time 1. nd/nd 2. −20 °C/nd 3. −6 °C/10 min | 20 and 5 min before LC, every 30 s from the end of LC up to 10 min thereafter; and every 5 min from 10 to 60 min after LC | center of the muscle belly of the right masseter | HotShot LT IR thermometer resolution: 0.1 °C accuracy: ±2% |
| [45] | n = 47 (39/8) | LC: 1. liquid nitrogen vapors 2. cold air | Thermovisual comparison of mean temperature of hand surface changes after local cryotherapy | Temperature/time 1. −160 °C/3 min 2. −30 °C/3 min | 1, 5, 15, 30, 45, 60, 120, and 180 min after LC | hand | Flir ThermaCAM SC 2000 nd |
| [46] | n = 22 (5/17) | WBC | Studying skin temperature response, taking into account individual features of the patients and external factors | Temperature/time 1. −60 °C/30 s 2. −120 °C/3 min | pre-, post-WBC | all body | AGEMA Type 470, Flir Thermovision Camera A40 emissivity: 0.97–0.98 |
| [47] | n = 18 (nd) | LC: ice bag or bags containing a mixture of ice and water (760 g of ice and 240 g of water) | Comparing variations in surface temperature of the quadriceps muscle at three different times during exposure to two forms of cryotherapy | Temperature/time nd/15 min | pre-, post-LC and 15, 30 min after | right and left quadriceps | Therma CAME 320; detector size: 320 × 240 pixels sensitivity: −0.10 °C to 25 °C precision: ±2 °C |
| [48] | n = 30 (0/30) | WBC | Evaluating the complex hemodynamic physiological reactions that occur in response to WBC exposure in healthy subjects | Temperature/time −120 °C/3 min | pre-, post-WBC and 3, 6 h after | all body | Flir P640 nd |
| [49] | n = 480 (240/240) | WBC | Examining the temperature, blood pressure, and heart rate changes after whole-body cryotherapy in healthy subjects to determine the safety conditions of the treatment | Temperature/time −60 °C/1–3 min −100 °C/1–3 min −120 °C/1–3 min −140 °C/1–3 min | pre-WBC and 5, 30 min after | upper and lower extremities | ThermoVision A20 M nd |
| [50] | n = 15 (0/15) | LC: cold water immersion | Investigating acute effects of normobaric hypoxia on hand-temperature responses during and after cold-water hand immersion test | Temperature/time 8 °C/30 min | pre-, post- LC and 1, 2, 3, 4, 5, 10, and 15 min after | hand | Flir T365 spatial resolution: 1.36 mrad spectral range: 7.5–13 μm detector size: 320 × 240 pixels |
| [51] | n = 24 (12/12) | WBC | Assessing the distribution and dynamics of temperature changes on the surface of selected body parts after systemic cryostimulation | Temperature/time −130 °C/3 min | pre-, post-WBC and in 1st, 5th, and 10th day of treatment | upper and lower extremities | Flir Therma CAM TM Sc500 nd |
| [52] | n = 60 (0/60) | WBC | Assessing the impact of cryotherapy on changes of surface body temperature and efficiency of thermoregulatory processes in relation to the intensity of cryogenic stimuli | Temperature/time 1. −110 °C/3 min 2. −120 °C/3 min 3. −140 °C/3 min | pre- WBC and 5 and 30 min after | regions of trunk, lower limbs and upper limbs | ThermoVision A20 M Researcher spectral range: 7.5–13 μm spatial resolution: 2.7 mrad automatic emissivity correction |
| [53] | n = 10 (2/8) | LC: cold pack | Evaluating the impact of the exposure duration of local cryotherapy on the skin temperature of the thigh and of the knee | Temperature/time Nd/5 and 10 min | every 30 s for 20 min after LC | skin of the thigh and of the knee | VarioCAM® hr head accuracy: ±2% spectral range: 7.5–14 μm detector size: 640 × 480 pixels |
| [54] | n = 36 (0/36) | LC: 1. cold water immersion 2. ice massage | Evaluating the effectiveness of LC in supporting recovery and preventing delayed-onset muscle soreness after maximum anaerobic physical effort | Temperature/time 1. 8 °C/3 min 2. nd/3 min | pre-, post-LC and 30 min after | front and back surface of lower limbs | Flir A325 thermal sensitivity: −20 °C to 350 °C accuracy: ±2% or ±2 °C sensitivity: <0.05 °C infrared spectral band: 7.5–13 μm refresh rate: 60 Hz detector size: 320 × 240 pixels |
| [55] | n = 15 (5/10) | WBC | Exploring the use of a new WBC technology based on forced convection (frontal unilateral wind) through the measurement of skin temperature | Temperature/time 1. −20 °C/30 s 2. −40 °C/3 min | pre-, post-WBC and within 20 min after | all body | VarioCAM® hr head; Thermal sensitivity: −40 °C to 1200 °C accuracy: ±2% detector size: 640 × 480 pixels spectral range: 7.5–14 μm |
| [56] | n = 20 (0/20) | WBC LC: liquid nitrogen vapors | Verifying whether local cryotherapy improves thermal diagnosis, similar to whole-body cryotherapy in spinal diseases | Temperature/time WBC: −110 °C/3 min LC: −160 °C/3 min | pre-, post-cryotherapy | spinal region (Th5/Th6-L5/S1) | Thermovision Camera E60 emissivity: 0.97–0.98 |
| [57] | n = 10 (5/5) | PBC WBC | Observing the differences between PBC and WBC treatments, based on the analysis of skin temperature distribution | Temperature/time −140 °C/3 min | pre-, post-cryotherapy | all body | Flir Thermal SC620 nd |
| [58] | n = 40 (0/40) | WBC | Evaluating changes in the distribution of body surface temperature under the influence of WBC, classical massage, and hot stone massage | Temperature/time −120 °C/3 min | pre-WBC and up to 10 min after | 12 areas in whole body | Thermo Vision A20M Sensitivity: 0.12 °C, temperature range: −20 °C to 900 °C detector size: 160 × 120 pixels frequency: 50/60 Hz |
| [59] | n = 22 (22/0) | LC: cold pack | Searching for associations between skin surface temperature and pressure pain tolerance thresholds of healthy individuals undergoing cryotherapy and thermotherapy | Temperature/time nd/20 min | pre-, post-LC and 20 min after | right knee | Flir E4; nd |
| [60] | n = 21 (0/21) | LC: wetted ice (500 g ice + 500 mL water) crushed ice CryoCuff® | Investigating differences in the cooling ability of different cryotherapy modalities in a rugby union population in an attempt to describe optimum cooling protocols | Temperature/time nd/20 min | post- and 5 times during LC and 20 min after | anterior thigh | Flir A40M; emissivity: 0.97–0.98 |
| [61] | n = 16 (16/0) | LC: ice bag watered ice (500 g of ice in 500 mL of water) wetted ice (500 g ice and 500 mL water) | Comparing the effects of different cryotherapeutic preparations | Temperature/time nd/20 min | pre-, post-LC and 5, 10, 15, and 20 min after | anterior thigh | Flir A325sc accuracy: 0.07 °C emissivity: 0.98 |
| [62] | n = 10 (3/7) | LC: GameReady GRPro 2.1 | Evaluating changes in temperature in response to a cold stress test after a strength-fatiguing exercise protocol | Temperature/time 0–3 °C/3 min | pre-, post-LC | anterior thigh | Flir E60bx detector size: 320 × 240 pixels accuracy: ±2%, NETD: <0.05 °C |
| [63] | n = 14 (4/10) | LC: Game Ready GRPro 2.1 | Determining the reproducibility of lower limbs skin temperature after cold stress test using the Game Ready system | Temperature/time 0–3°C/3 min | pre-, post-LC and 30, 60, 120, and 180 s after | lower limbs skin | Flir E-60bx; detector size: 320 × 240 pixels NETD: <0.05 °C resolution: 1.36 mrad accuracy: ±2% |
| [64] | n = 23 (11/12) | LC: 1. liquid nitrogen vapors 2. ice bag 3. cold air | Evaluating the dynamics of temperature changes in the knee joint area in response to different kinds of local cryotherapy | Temperature/time 1.: −160 °C/3 min 2.: −30 °C/3 min 3.: 0 °C/3 min | pre-, post-LC and 1–5 min after (with frequency 1 min) and 5–90 min (with frequency 5 min) | knee joint | Flir A655s NETD: <0.05 °C detector size: 640 × 480 pixels accuracy: ±2% frequency: 50/200 Hz temperature range: −40 °C to 150 °C, emissivity: 0.98 |
| Laser therapy | |||||||
| [65] | n = 41 (17/24) | LLLT | Evaluating the usefulness of thermography as a prognostic tool in the treatment of stage III and IV pressure ulcers | λ = 808 nm; power: 400 mW; frequency: 5000 Hz time: 5–20 min | pre-treatment and 2 and 4 weeks after | the pressure ulcer | VIGOcam v50 camera; resolution: 1 mrad detector size: 384 × 288 pixels NETD: 0.07 °C emissivity: 0.950 frequency: 60 Hz |
| [66] | n = 14 (0/14) | LLLT | Verifying the influence of PMB on muscle fatigue and the temperature of the biceps brachii | λ = 808 nm; power: 100 mW/point; energy of 3 Joules/point Time:nd | pre-, post-treatment and 5, 10, and 15 min after | biceps brachii muscle | FLIR S65 emissivity: 0.98 |
| Electrotherapy | |||||||
| [67] | n = 45 (25/20) | NMES | Verifying whether unilateral application of NMES can result in local and cross-education thermal effects | 400 μs, 8 Hz time: 12 min | pre-, post-treatment and 10 and 20 min after | anterior region of both thigh | Flir E60 detector size: 320 × 240 poixels NETD: < 0.05 °C frequency: 60 Hz |
| Diathermy | |||||||
| [68] | n = 60 (26/34) | ESW | Detecting thermal conditions in the region of tested muscles to assess the effects of the radial ESW stimulation | pressure of 1.5 bar; frequency of 5 pulses per s [Hz] 1500 shots | pre-, post-treatment | carpal flexor muscles | MobIR M8 m detector size: 160 × 120 pixels spectral range: 8–14 μm thermal sensivity: ≤100 mk temperature range: −20 °C to 250 °C accuracy: ± 2% or ± 2 °C |
| [69] | n = 40 (40/0) | short- and microwave diathermy | Evaluating the behavior of temperature and arterial blood flow after the application | 1. 27.12 MHz 240 W 2. 2.45 GHz 200 W time: 20 min | pre-treatment and 10, 20, 30, and 40 min after | lower limb | Flir T300; accuracy: 0.05 °C emissivity: 0.98 |
| [70] | n = 10 (4/6) | TT | Determining if TT, administered in two modes, affects the intramuscular blood flow, perfusion of skin microcirculation, and skin temperature | resistive and capacitive TT time: 8 min | pre-, post-treatment | volar forearm | Voltcraft Infrared IR 500-8S; nd |
| [71] | n = 32 (0/32) | TT | Analyzing the acute effect of TT on latent myofascial trigger points on skin temperature, ankle range of motion, and pain in professional basketball players | 0.5 MHz radiofrequency signals at a variable power with a maximum of 300 W time: 25 min | pre-treatment and 15 and 30 min after | medial gastrocnemius | Flir E6 nd |
| [72] | n = 26 (26/0) | SWD | Evaluating local and general temperature changes after treatment with SWD | 1 MHz; 300 m wavelength; 11 m short-wave diathermy time: 8 min | pre-, post-treatment | area of the right knee joint | Flir ThermaCAM P640 detector size: 640 × 480 pixels temperature range: −200 to 9000 °C |
| [73] | n = 18 (0/18) | three capacitive technique of SWD | Analyzing which capacitive technique arrangement of SWD is the most effective in skin temperature change | 27.12 MHz; contra-, coplanar and longitudinal arrangement time: 20 min | pre-treatment and over 25 min after | anterior aspect of the thigh | Flir A325sc ccuracy: ±2% detector size: 320 × 240 pixels |
| Massage | |||||||
| [74] | n = 12 (10/2) | RM | Determining whether RM triggers measurable changes of body surface temperature and of heart rate variability | 10 RM sessions once or twice week time: 30 min | pre-treatment and 5 and 10 min after | dorsal region | Goratec Technology AVIO TVS 700 nd |
| [75] | n = 35 (35/0) | classical massage | Determining the relationship between classical sports massage of the hand and the forearm and the surface temperature of upper limb muscles | nd | pre-, post-treatment | upper limbs | Flir A 325 nd |
| [58] | n = 40 (0/40) | classical massage; hot stone massage | Evaluating changes in the distribution of body surface temperature under the influence of WBC, classical massage, and hot stone massage | temperature of hot stone: 60 °C; 19 stones in different size time: 30 min | pre-, post-treatment | 12 areas in the whole body | Thermo Vision A20M; temperaturę sensitivity: 0.12 °C temperature range: –20 °C to 900 °C, detector size: 160 × 120 pixels frequency: 50/60 Hz |
| [76] | n = 40 (40/0) | isometric and classic massage | Determining the relationship between isometric and classic massage and the chosen parameters of the quadriceps femoris muscles | nd time: 12 min | pre-, post-treatment | anterior part of the thigh | Flir A 325; nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubkowska, A.; Pluta, W. Infrared Thermography as a Non-Invasive Tool in Musculoskeletal Disease Rehabilitation—The Control Variables in Applicability—A Systematic Review. Appl. Sci. 2022, 12, 4302. https://doi.org/10.3390/app12094302
Lubkowska A, Pluta W. Infrared Thermography as a Non-Invasive Tool in Musculoskeletal Disease Rehabilitation—The Control Variables in Applicability—A Systematic Review. Applied Sciences. 2022; 12(9):4302. https://doi.org/10.3390/app12094302
Chicago/Turabian StyleLubkowska, Anna, and Waldemar Pluta. 2022. "Infrared Thermography as a Non-Invasive Tool in Musculoskeletal Disease Rehabilitation—The Control Variables in Applicability—A Systematic Review" Applied Sciences 12, no. 9: 4302. https://doi.org/10.3390/app12094302
APA StyleLubkowska, A., & Pluta, W. (2022). Infrared Thermography as a Non-Invasive Tool in Musculoskeletal Disease Rehabilitation—The Control Variables in Applicability—A Systematic Review. Applied Sciences, 12(9), 4302. https://doi.org/10.3390/app12094302







