The Effect of Cu (II) on Swelling and Shrinkage Characteristics of Sodium Bentonite in Landfills
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials and Samples
2.2. Free Swell Ratio (FSR) Experiment
2.3. Non-Load Swell Ratio Experiment
2.4. Shrinkage Test
2.5. Clay Mineralogy
3. Results
3.1. Free Swell Rate
3.2. Non-Load Swell Rate
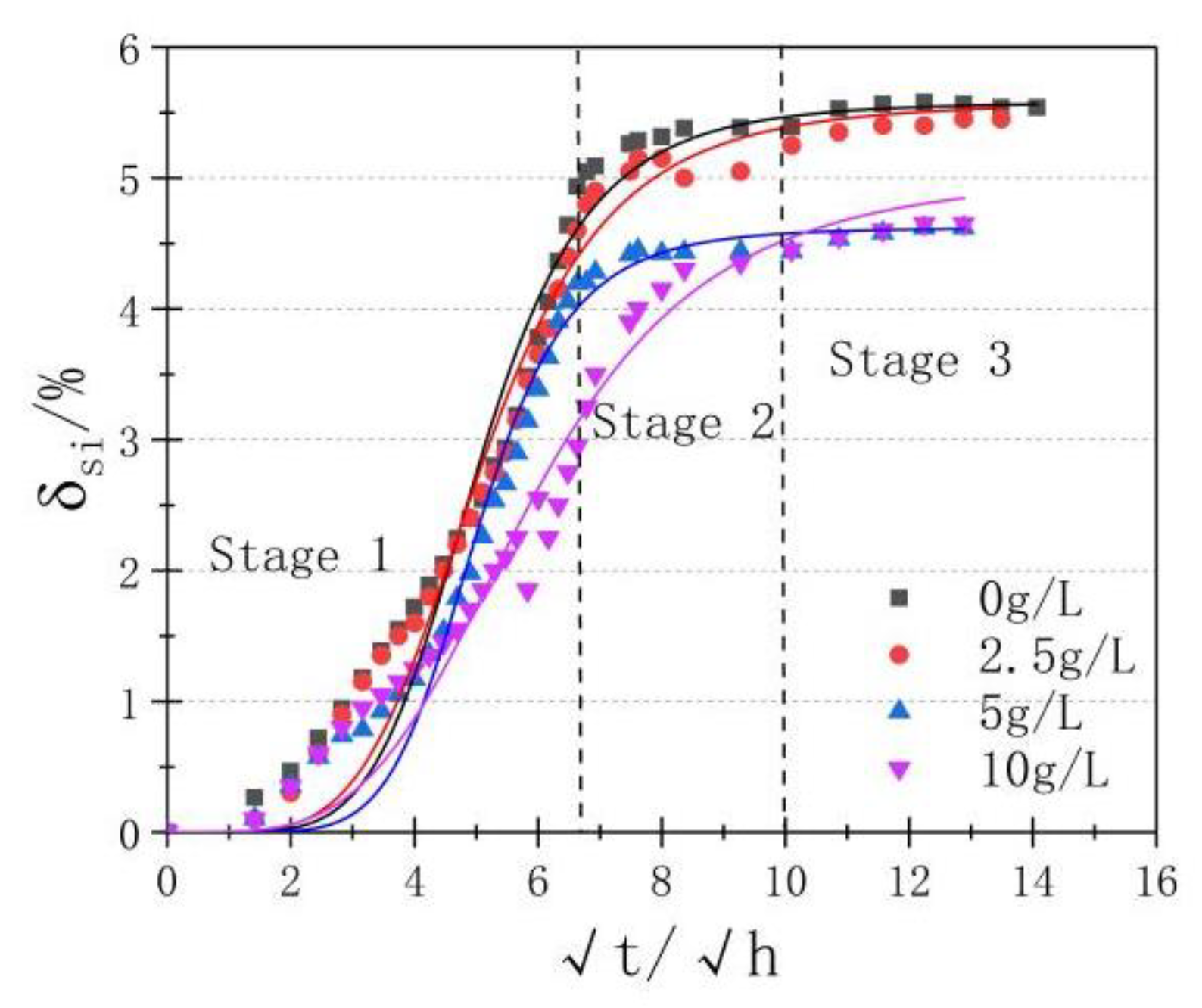
3.3. Shrinkage
3.4. Mineralogy Results
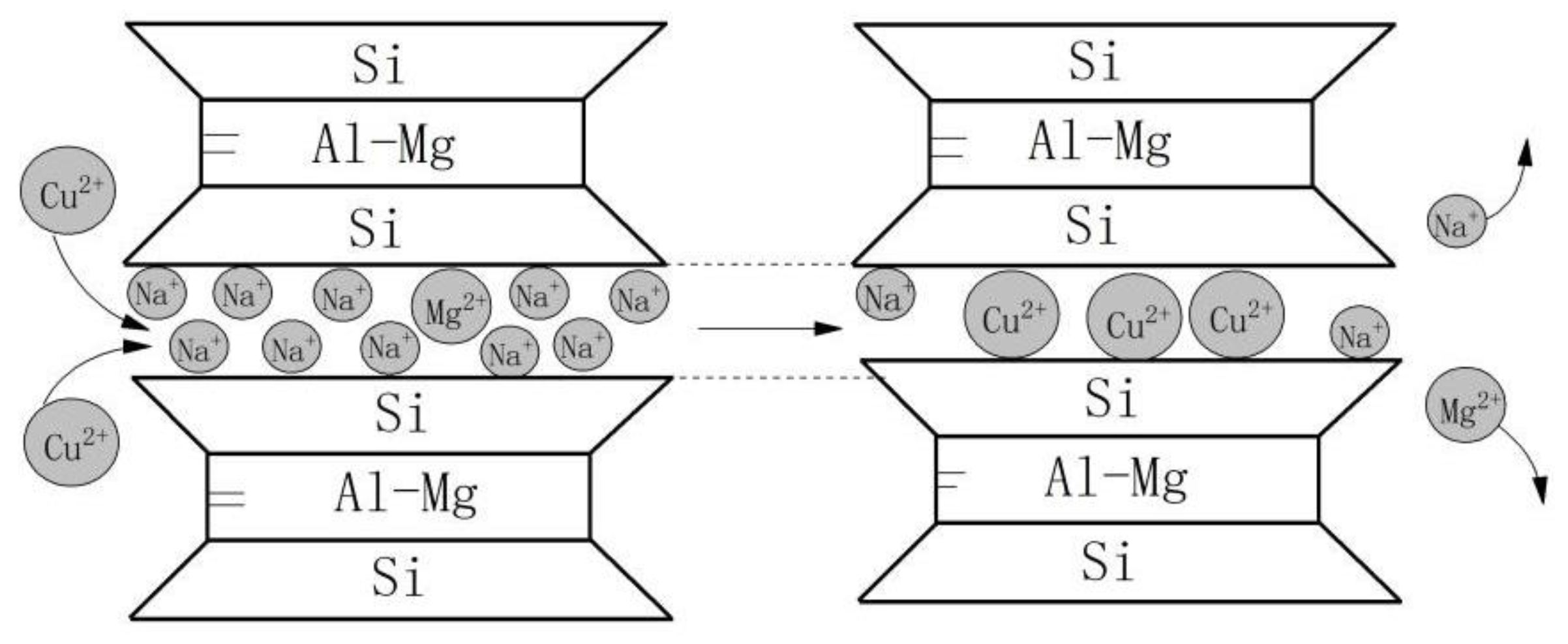
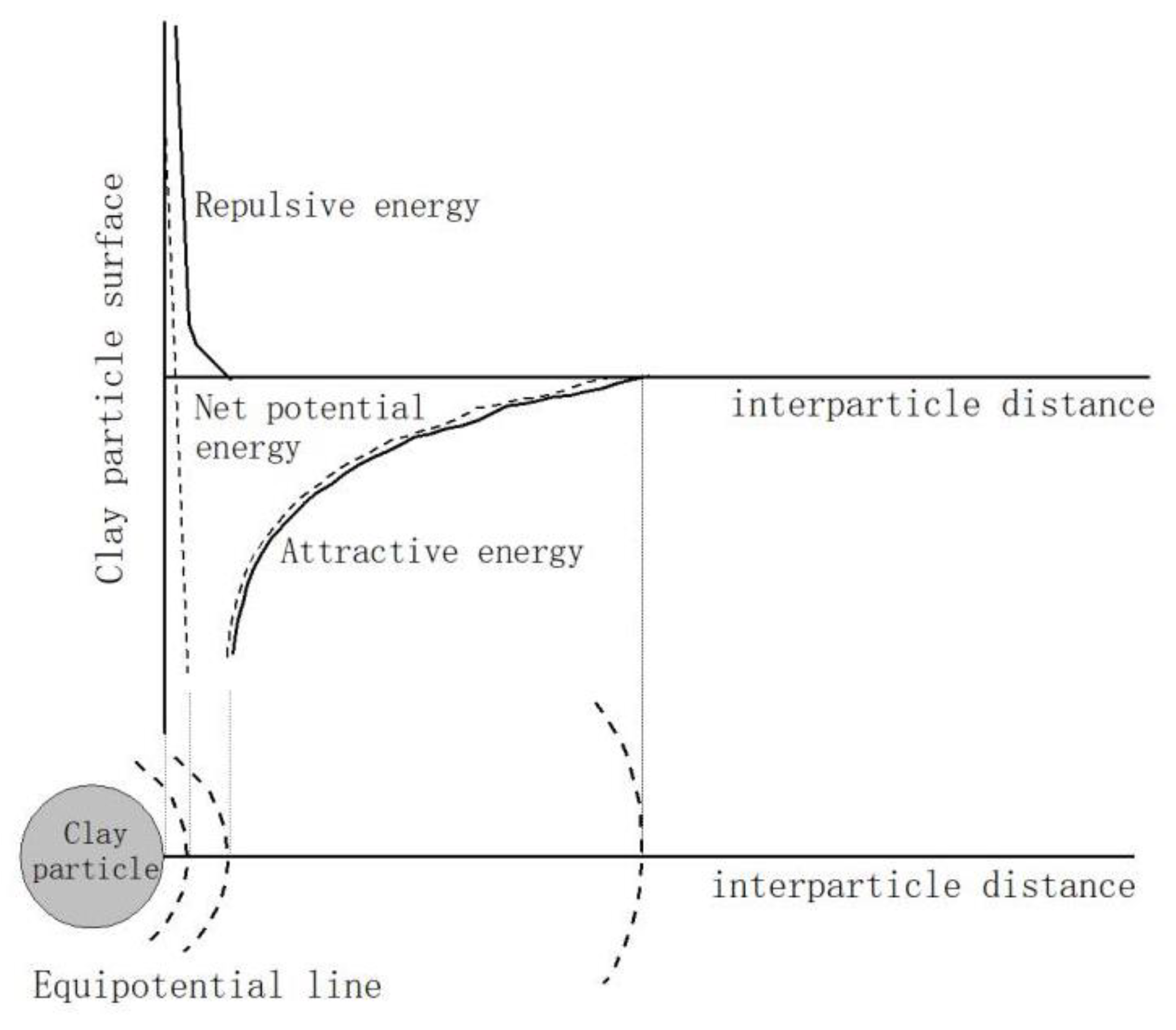
4. Mechanism of Bentonite Erosion Caused by Cu (II)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morselli, L.; Luzi, J.; De Robertis, C.; Vassura, I.; Carrillo, V.; Passarini, F. Assessment and comparison of the environmental performances of a regional incinerator network. Waste Manag. 2007, 27, S85–S91. [Google Scholar] [CrossRef]
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and long-term composition of MSW landfill leachate: A review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Chofqi, A.; Younsi, A.; Lhadi, E.K.; Mania, J.; Mudry, J.; Veron, A. Environmental impact of an urban landfill on a coastal aquifer (El Jadida, Morocco). J. Afr. Earth Sci. 2004, 39, 509–516. [Google Scholar] [CrossRef]
- Wang, Y. Leachate Management in the Aftercare Period of Municipal Waste Landfills. Ph.D. Thesis, Aalto University, Espoo, Finland, 2013. [Google Scholar]
- Gupta, A.; Zhao, R.; Novak, J.T.; Goldsmith, C.D. Variation in organic matter characteristics of landfill leachates in different stabilisation stages. Waste Manag. Res. 2014, 32, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.B.; Clifford, E.; Devroedt, C.; Morrison, L.; Healy, M.G. Treatment of landfill leachate in municipal wastewater treatment plants and impacts on effluent ammonium concentrations. J. Environ. Manag. 2017, 188, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Shaheen, S.M.; Jiang, Y.; Li, R.; Slaný, M.; Abdelrahman, H.; Kwon, E.; Bolan, N.; Rinklebe, J.; Zhang, Z. Fe/Mn- and P-modified drinking water treatment residuals reduced Cu and Pb phytoavailability and uptake in a mining soil. J. Hazard. Mater. 2021, 403, 123628. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Ren, A.H. Adsorption of zinc from aqueous solutions to bentonite. J. Hazard. Mater. 2005, 125, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Cooper, Z.; Bringolf, R.; Cooper, R.; Loftis, K.; Bryan, A.L.; Martin, J.A. Heavy metal bioaccumulation in two passerines with differing migration strategies. Sci. Total Environ. 2017, 592, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Gao, Z.; Liu, Y.; Ran, C.; Mao, X.; Kang, Q.; Dai, J. Microwave-assisted pyrolysis of textile dyeing sludge, and migration and distribution of heavy metals. J. Hazard. Mater. 2018, 355, 128–135. [Google Scholar] [CrossRef]
- De Stefanis, A.; Tomlinson, A.A.G.; Steriotis, T.A.; Charalambopoulou, G.C.; Keiderling, U. Study of structural irregularities of smectite clay systems by small-angle neutron scattering and adsorption. Appl. Surf. Sci. 2007, 253, 5633–5639. [Google Scholar] [CrossRef]
- Birmili, W.; Charron, A.; Harrison, R. Treatment of Textile Wastewater using Bentonite Clay as a Natural Coagulant. Pediatr. Transplant. 2014, 11, 895–900. [Google Scholar] [CrossRef]
- Xiang, G.; Ye, W.; Yu, F.; Wang, Y.; Fang, Y. Surface fractal dimension of bentonite affected by long-term corrosion in alkaline solution. Appl. Clay Sci. 2019, 175, 94–101. [Google Scholar] [CrossRef]
- Suzuki, S.; Prayongphan, S.; Ichikawa, Y.; Chae, B.G. In situ observations of the swelling of bentonite aggregates in NaCl solution. Appl. Clay Sci. 2005, 29, 89–98. [Google Scholar] [CrossRef]
- Segad, M.; Hanski, S.; Olsson, U.; Ruokolainen, J.; Åkesson, T.; Jönsson, B. Microstructural and swelling properties of Ca and Na montmorillonite: (in situ) observations with Cryo-TEM and SAXS. J. Phys. Chem. C 2012, 116, 7596–7601. [Google Scholar] [CrossRef]
- Xu, Y.; Xiang, G.; Jiang, H.; Chen, T.; Chu, F. Role of osmotic suction in volume change of clays in salt solution. Appl. Clay Sci. 2014, 101, 354–361. [Google Scholar] [CrossRef]
- Chen, Y.G.; Zhu, C.M.; Ye, W.M.; Cui, Y.J.; Chen, B. Effects of solution concentration and vertical stress on the swelling behavior of compacted GMZ01 bentonite. Appl. Clay Sci. 2016, 124–125, 11–20. [Google Scholar] [CrossRef]
- Widomski, M.K.; Stępniewski, W.; Musz-Pomorska, A. Clays of Different Plasticity as Materials for Landfill Liners in Rural Systems of Sustainable Waste Management. Sustainability 2018, 10, 2489. [Google Scholar] [CrossRef] [Green Version]
- Slaný, M.; Jankovič, L.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Manikandan, R.; Ramamurthy, K. Swelling characteristic of bentonite on pelletization and properties of fly ash aggregates. J. Mater. Civil. Eng. 2009, 21, 578–586. [Google Scholar] [CrossRef]
- Camillis, D.M.; Emidio, D.G.; Bezuijen, A.; Verástegui-Flores, R.D. Hydraulic conductivity and swelling ability of a polymer modified bentonite subjected to wet–dry cycles in seawater. Geotext. Geomembr. 2016, 44, 739–747. [Google Scholar] [CrossRef]
- Zhou, S.W.; Xu, M.G.; Ma, Y.B.; Chen, S.B.; Wei, D.P. Aging mechanism of copper added to bentonite. Geoderma 2008, 147, 86–92. [Google Scholar] [CrossRef]
- Gomes, P.C.; Fontes, M.P.F.; Silva, D.A.G.; Mendonça, D.S.E.; Netto, A.R. Selectivity sequence and competitive adsorption of heavy metals by Brazilian soils. Soil Sci. Soc. Am. J. 2001, 65, 1115–1121. [Google Scholar] [CrossRef]
- Hu, W.; Lu, S.; Song, W.; Chen, T.; Hayat, T.; Alsaedi, N.S.; Liu, H. Competitive adsorption of U (VI) and Co (II) on montmorillonite: A batch and spectroscopic approach. Appl. Clay Sci. 2018, 157, 121–129. [Google Scholar] [CrossRef]
- Batchelor, B. Leach models for contaminants immobilized by pH-dependent mechanisms. Environ. Sci. Technol. 1998, 32, 1721–1726. [Google Scholar] [CrossRef]
- Tahervand, S.; Jalali, M. Sorption and desorption of potentially toxic metals (Cd, Cu, Ni and Zn) by soil amended with bentonite, calcite and zeolite as a function of pH. J. Geochem. Explor. 2017, 181, 148–159. [Google Scholar] [CrossRef]
- Zhang, H.; Tong, Y.; Jia, Q. Corrosion of GMS bentonite by diffusion of strong alkaline solution. Chin. J. Rock Mech. Eng. 2020, 39, 166–176. [Google Scholar] [CrossRef]
- Ito, H. Compaction properties of granular bentonites. Appl. Clay Sci. 2006, 31, 47–55. [Google Scholar] [CrossRef]
- Hoffmann, C.; Alonso, E.E.; Romero, E. Hydro-mechanical behaviour of bentonite pellet mixtures. Phys. Chem. Earth 2007, 32, 832–849. [Google Scholar] [CrossRef] [Green Version]
- Gleason, M.H.; Daniel, D.E.; Eykholt, G.R. Calcium and sodium bentonite for hydraulic containment applications. J. Geotech. Geoenviron. Eng. 1997, 123, 438–445. [Google Scholar] [CrossRef]
- Yang, S.; Ren, X.; Zhao, G.; Shi, W.; Montavone, G.; Grambowe, B.; Wang, X. Competitive sorption and selective sequence of Cu (II) and Ni (II) on montmorillonite: Batch, modeling, EPR and XAS studies. Geochim. Cosmochim. Acta 2015, 166, 129–145. [Google Scholar] [CrossRef]
- Yan, C.; Li, G.; Xue, P.; Wei, Q.; Li, Q. Competitive effect of Cu (II) and Zn (II) on the biosorption of lead (II) by Myriophyllum spicatum. J. Hazard. Mater. 2010, 179, 721–728. [Google Scholar] [CrossRef]
- Turer, D. Effect of heavy metal and alkali contamination on the swelling properties of kaolinite. Environ. Geol. 2007, 52, 421–425. [Google Scholar] [CrossRef]
- Liu, Z.R.; Uddin, M.A.; Sun, Z.X. FT-IR and XRD analysis of natural Na-bentonite and Cu (II)-loaded Na-bentonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1013–1016. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Adebowale, K.O.; Olu-Owolabi, B.I.; Yang, L.Z.; Kong, L. Adsorption of Pb (II) and Cd (II) from aqueous solutions onto sodium tetraborate-modified Kaolinite clay: Equilibrium and thermodynamic studies. Hydrometallurgy 2008, 93, 1–9. [Google Scholar] [CrossRef]
- Mercier, L.; Detellier, C. Preparation, characterization, and applications as heavy metals sorbents of covalently grafted thiol functionalities on the interlamellar surface of montmorillonite. Environ. Sci. Technol. 1995, 29, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Kraepiel, A.M.L.; Keller, K.; Morel, F.M.M. A model for metal adsorption on montmorillonite. J. Colloid Interface Sci. 1999, 210, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agassi, M.; Shainberg, I.; Morin, J. Effect of electrolyte concentration and soil sodicity on infiltration rate and crust formation. Soil Sci. Soc. Am. J. 1981, 45, 848–851. [Google Scholar] [CrossRef]
- Kazman, Z.; Shainberg, I.; Gal, M. Effect of low levels of exchangeable sodium and applied phosphogypsum on the infiltration rate of various soils. Soil Sci. 1983, 135, 184–192. [Google Scholar] [CrossRef]

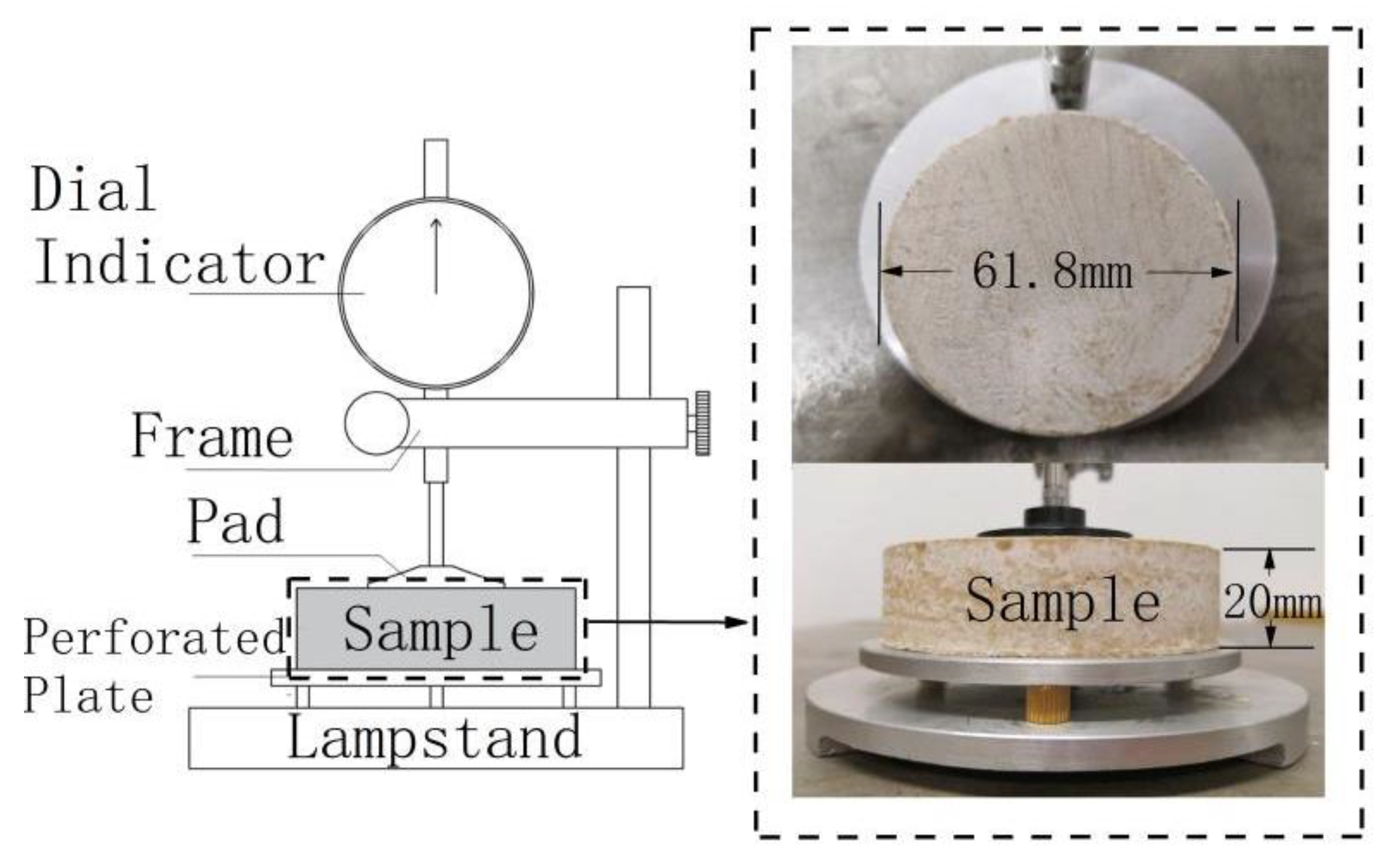
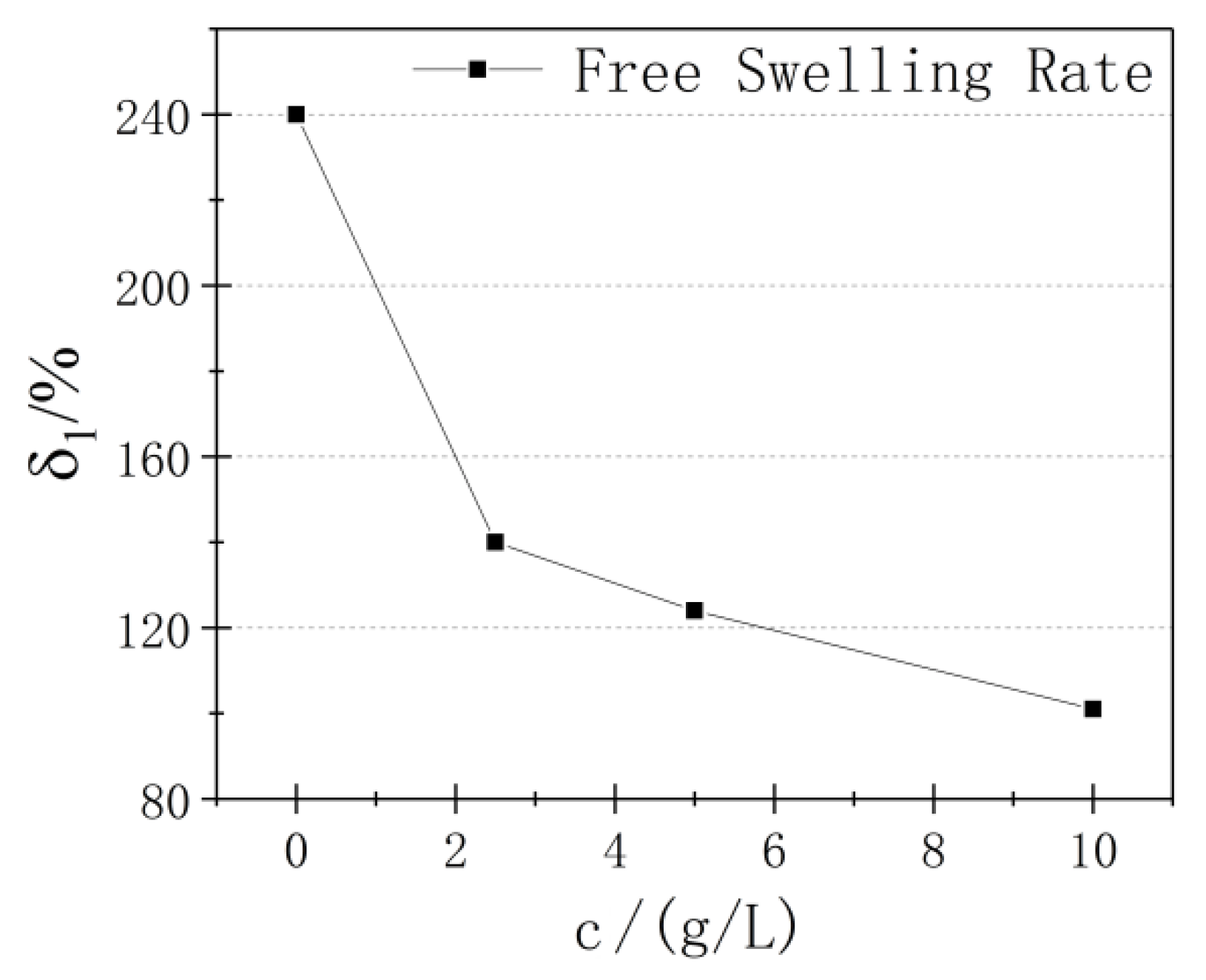
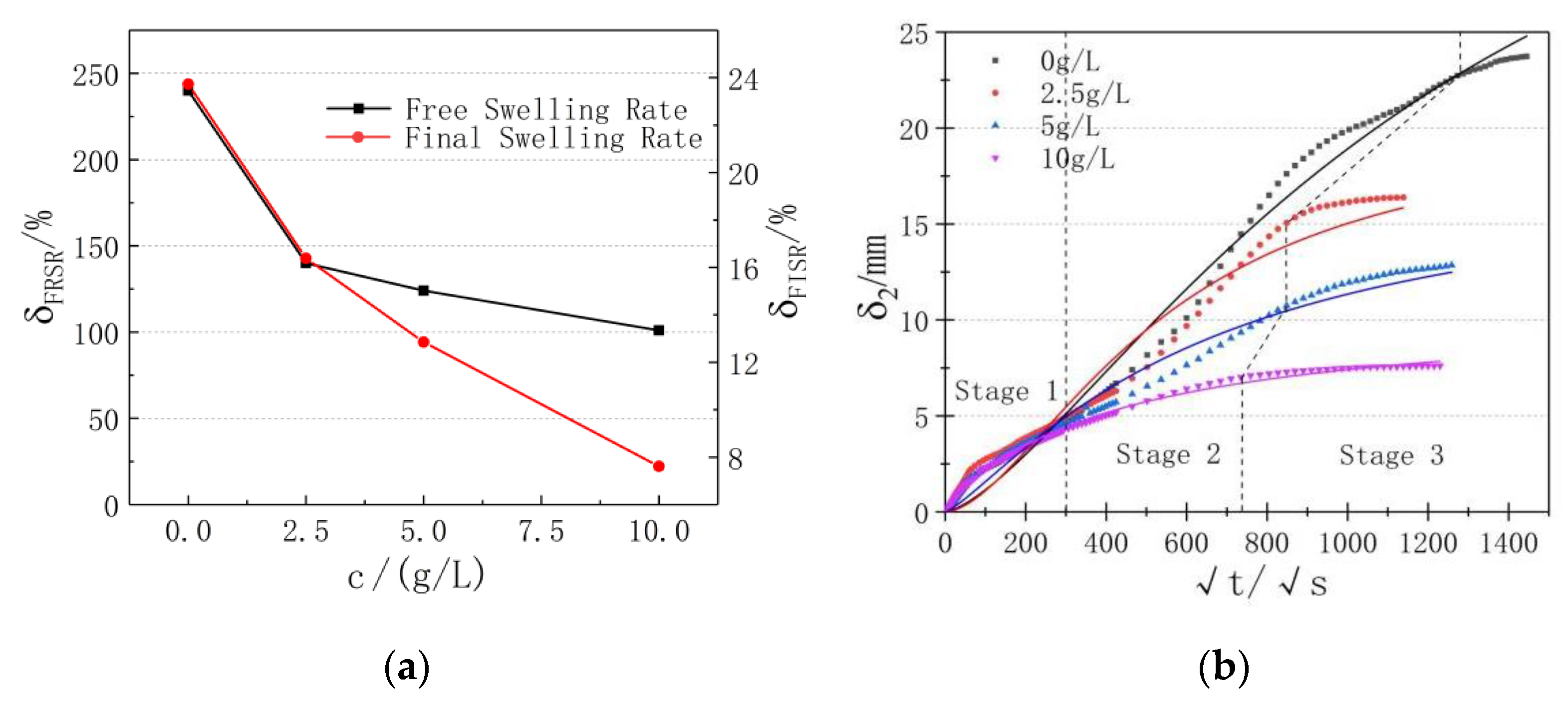
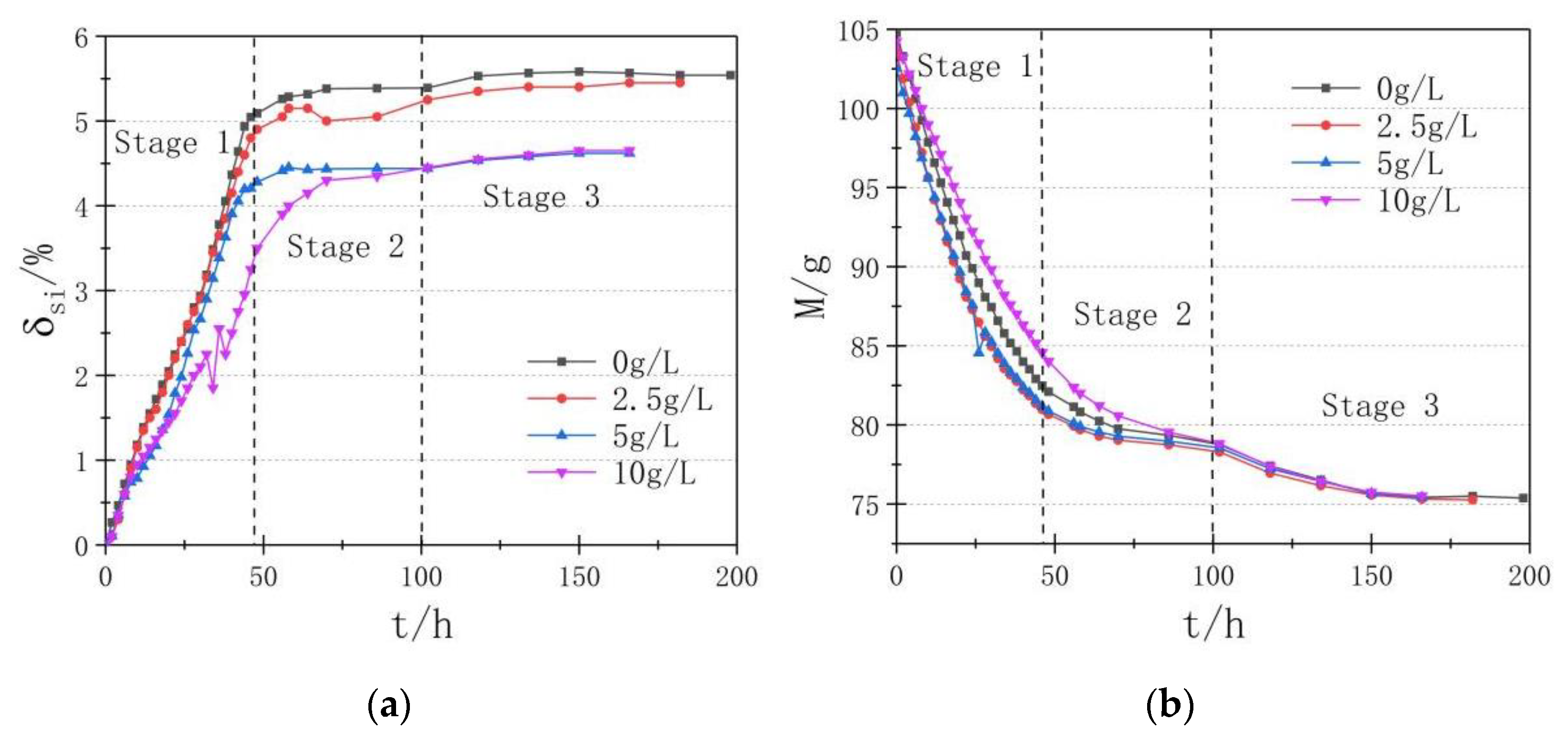


| Physical Properties | Value |
|---|---|
| Specific gravity | 2.73 |
| Liquid limit | 126.1% |
| Plastic limit | 44.1% |
| Plasticity index | 82 |
| Cation exchange capacity | 0.407 mEq/g |
| Content of montmorillonite | 65.2% |
| Solution | c (g/L) | |||
|---|---|---|---|---|
| 0 | 2.5 | 5 | 10 | |
| pH | 6.85 | 4.50 | 4.47 | 4.42 |
| Coefficient | c(g/L) | |||
|---|---|---|---|---|
| 0 | 2.5 | 5 | 10 | |
| A1 | 0.955 | 0.731 | 0.034 | −0.012 |
| A2 | 34.410 | 70.740 | 254.133 | 11.700 |
| t0 | 907.511 | 3008.984 | 55,932.723 | 530.176 |
| P | 1.845 | 1.161 | 0.758 | 0.893 |
| R2 | 0.986 | 0.987 | 0.996 | 0.997 |
| Coefficient | c(g/L) | |||
|---|---|---|---|---|
| 0 | 2.5 | 5 | 10 | |
| A1 | 0.610 | 0.411 | 0.449 | 0.410 |
| A2 | 5.698 | 5.580 | 4.659 | 5.045 |
| t0 | 5.350 | 5.267 | 5.236 | 6.199 |
| p | 5.640 | 4.907 | 6.744 | 4.082 |
| R2 | 0.980 | 0.983 | 0.988 | 0.973 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, G.; Xu, G.; Wei, T.; Zeng, J.; Liu, W.; Zhang, L. The Effect of Cu (II) on Swelling and Shrinkage Characteristics of Sodium Bentonite in Landfills. Appl. Sci. 2021, 11, 3881. https://doi.org/10.3390/app11093881
Xiao G, Xu G, Wei T, Zeng J, Liu W, Zhang L. The Effect of Cu (II) on Swelling and Shrinkage Characteristics of Sodium Bentonite in Landfills. Applied Sciences. 2021; 11(9):3881. https://doi.org/10.3390/app11093881
Chicago/Turabian StyleXiao, Guiyuan, Guangli Xu, Tongzhong Wei, Jian Zeng, Wenjun Liu, and Lu Zhang. 2021. "The Effect of Cu (II) on Swelling and Shrinkage Characteristics of Sodium Bentonite in Landfills" Applied Sciences 11, no. 9: 3881. https://doi.org/10.3390/app11093881
APA StyleXiao, G., Xu, G., Wei, T., Zeng, J., Liu, W., & Zhang, L. (2021). The Effect of Cu (II) on Swelling and Shrinkage Characteristics of Sodium Bentonite in Landfills. Applied Sciences, 11(9), 3881. https://doi.org/10.3390/app11093881






