Influence of Thermomechanical Treatments on Corrosion of Carbon Steel in Synthetic Geopolymer Fly Ash Pore Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
4. Conclusions
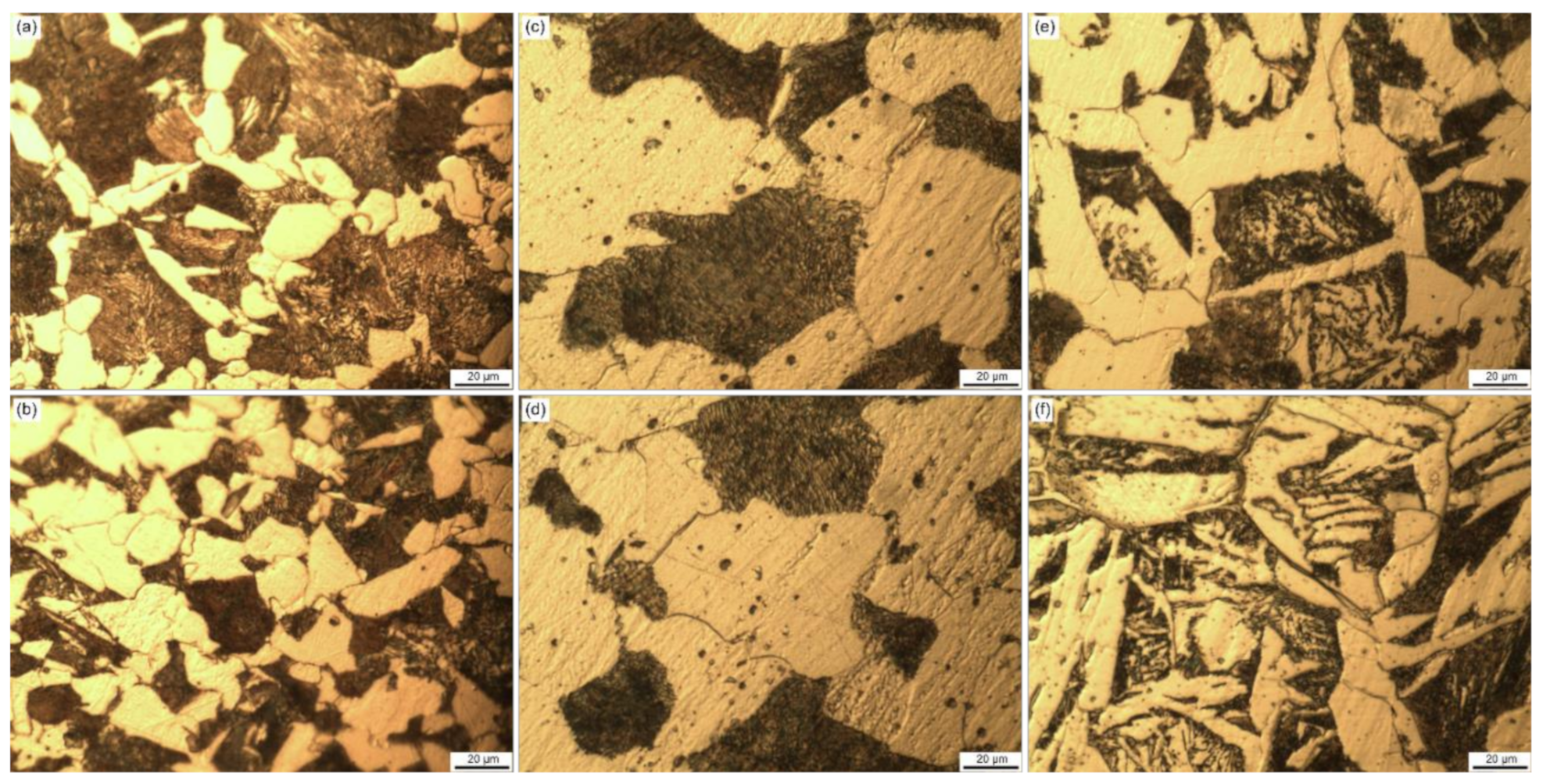
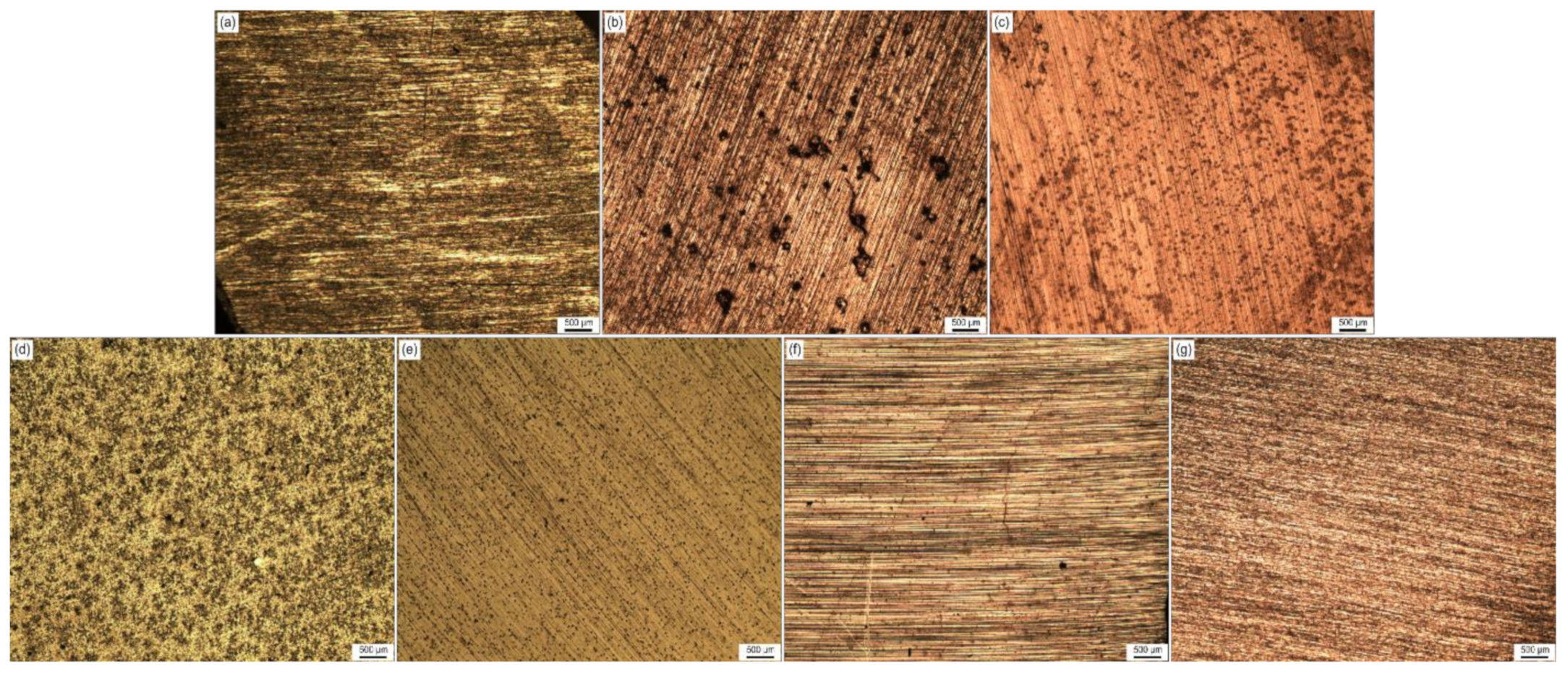
- Martensite was observed on the as-received sample near the edge of the rebar, whereas pearlite and cementite were found on the annealed sample with larger grain size. Smaller grains as well as Widmanstätten ferrite was found on the normalized sample near the edge. A full martensitic microstructure was obtained after quenching, whereas a mix of bainite and martensite is revealed after a subcritical annealing process.
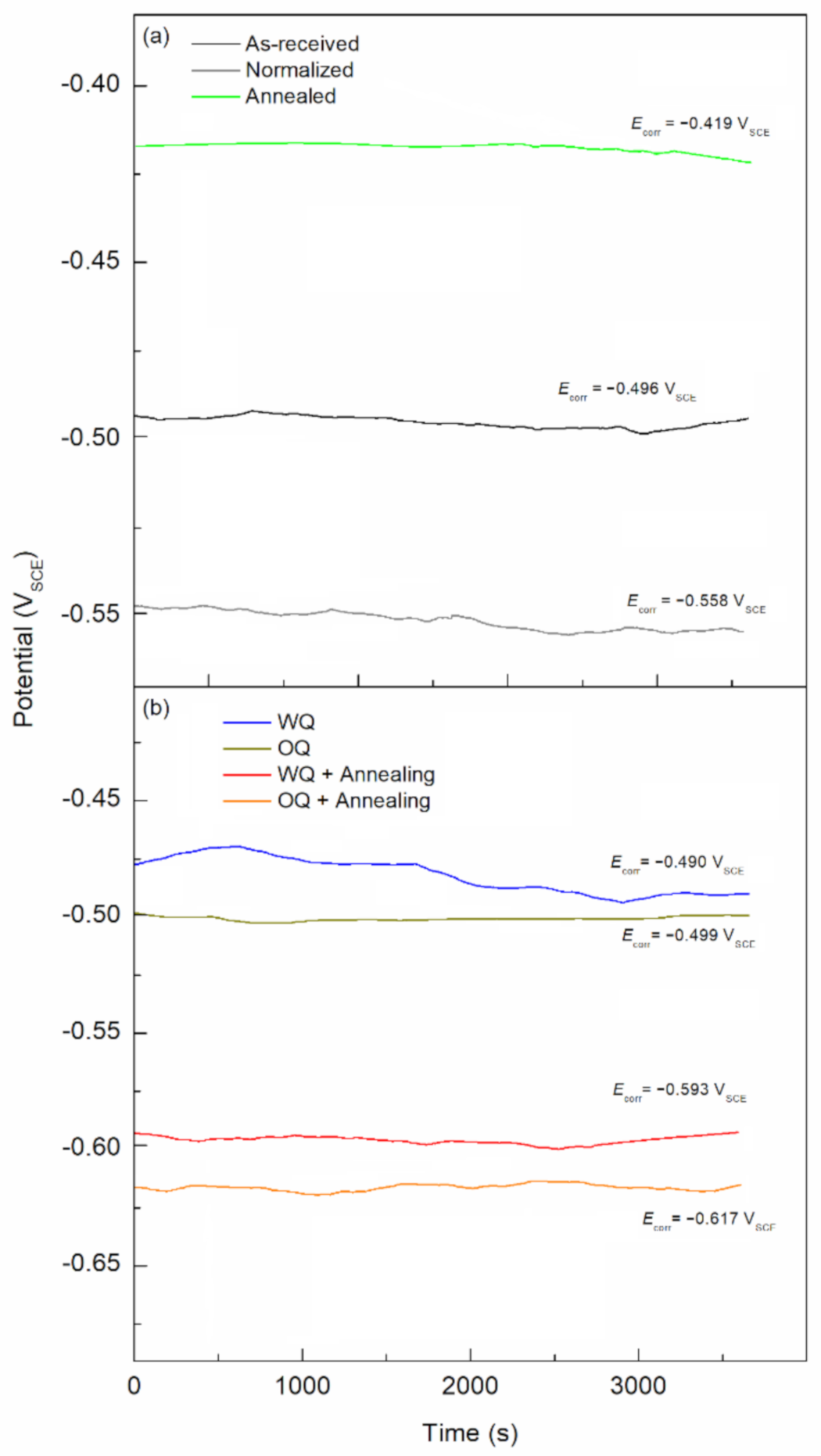
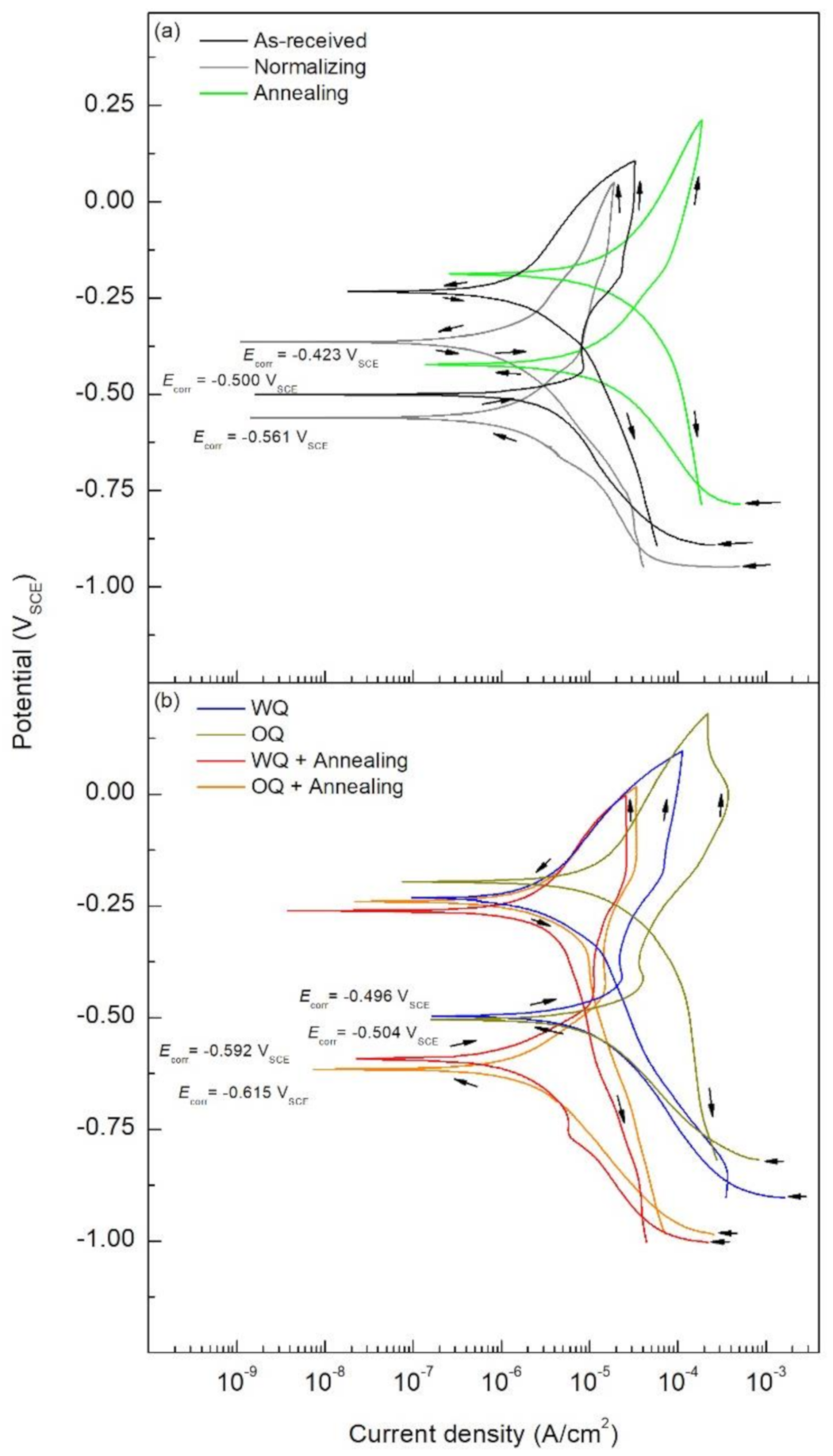
- Steel exposed to FA pore solution showed no active corrosion for the as-received samples due to the formation of a passive layer that protected the material even with chloride concentrations of 0.6 M NaCl. Such behavior worsens as the cooling rate of the rebars is increased due to the more active microstructure. The highest corrosion susceptibility was found to be in the martensite structure, followed by coarse and bigger grain size, bainitic and pearlitic steel.
- Higher icorr values were observed for the samples containing martensite, namely the quenched specimens. These icorr values were in the range of 3.18 × 10−5 and 2.20 × 10−5 A/cm2, whereas the as-received sample showed an icorr values of 2.13 × 10−6 A/cm2. Additionally, an increased icorr value of 2.09 × 10−5 A/cm2 was found on the annealed sample presenting coarse grain.
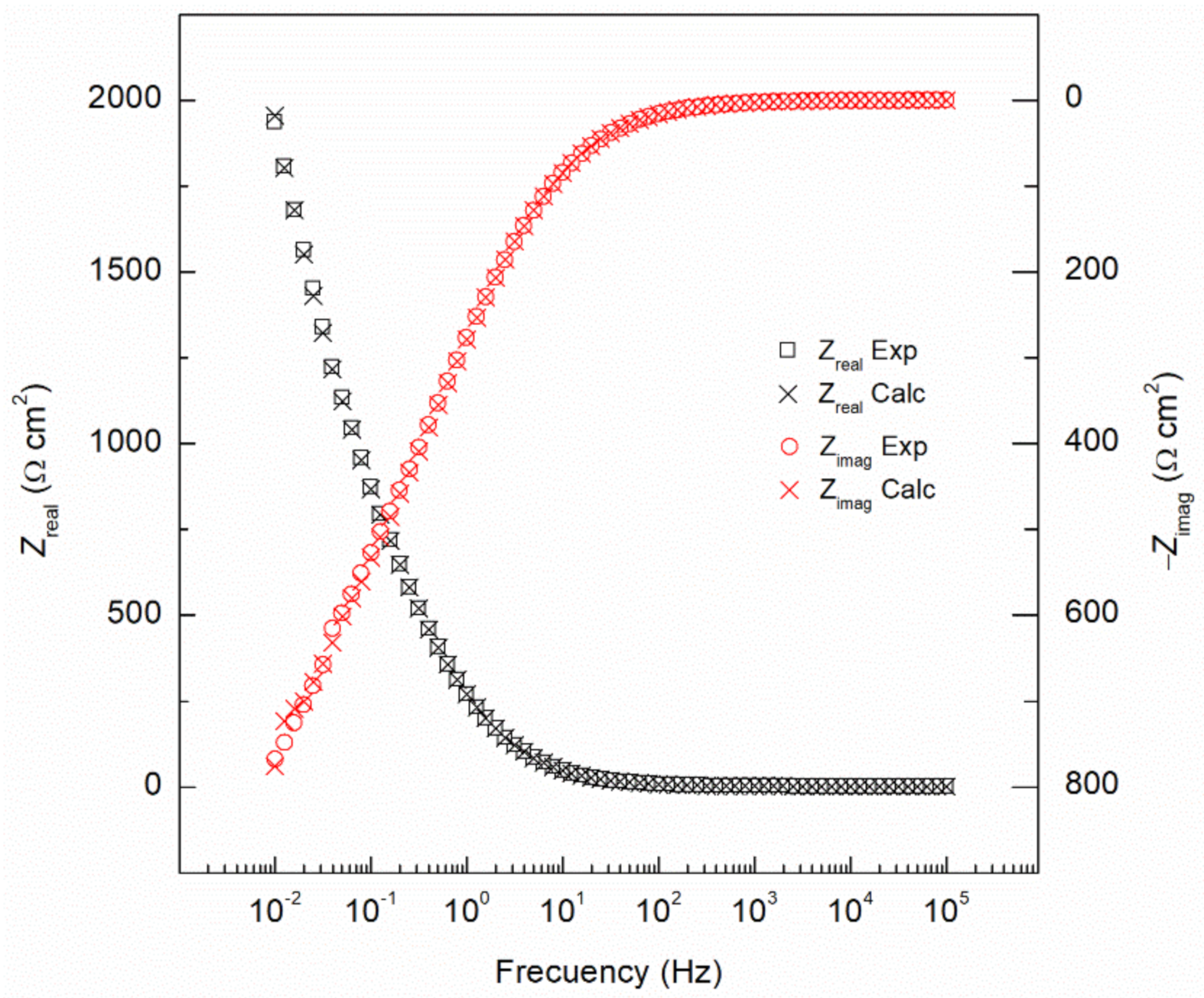
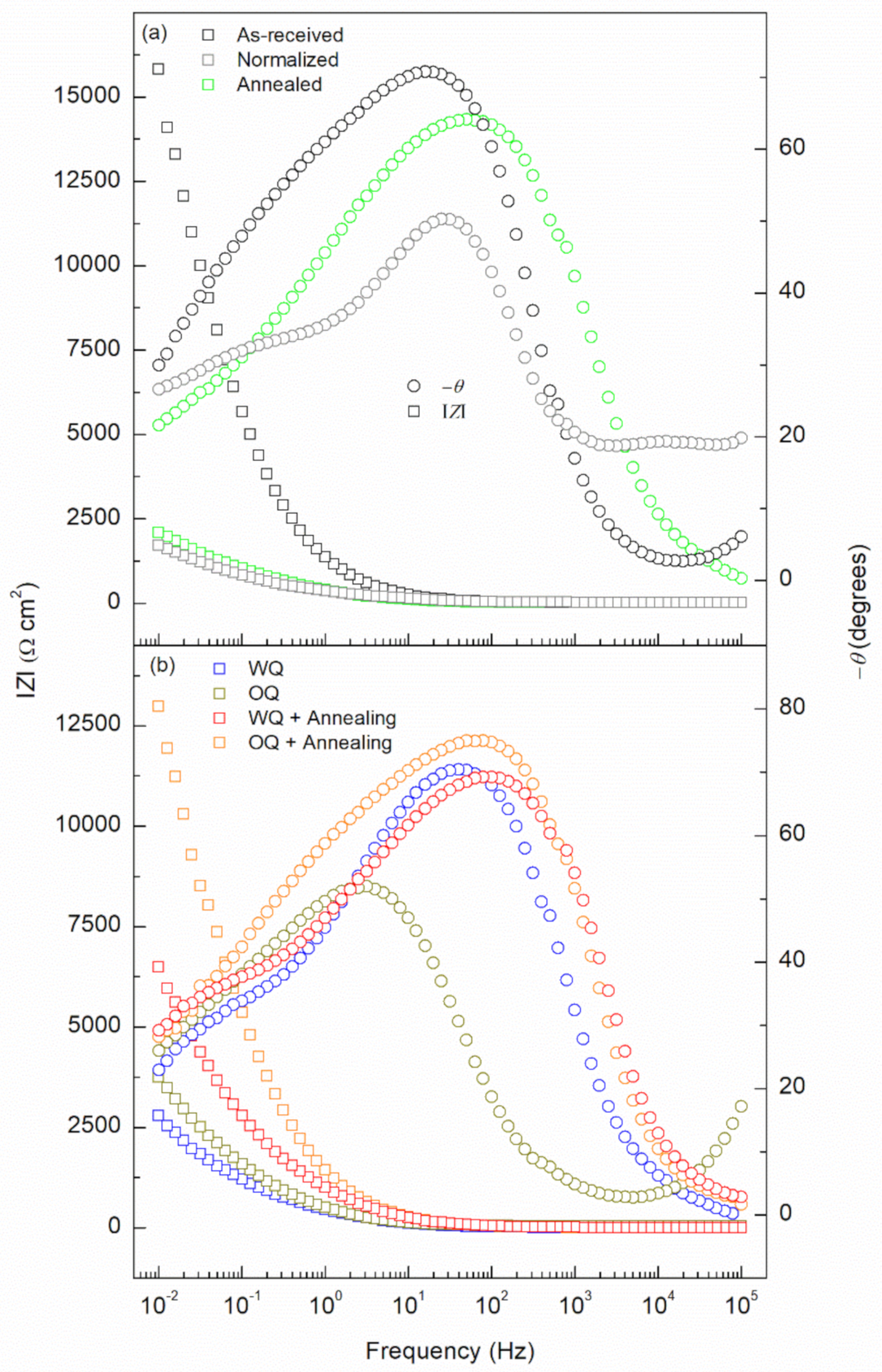
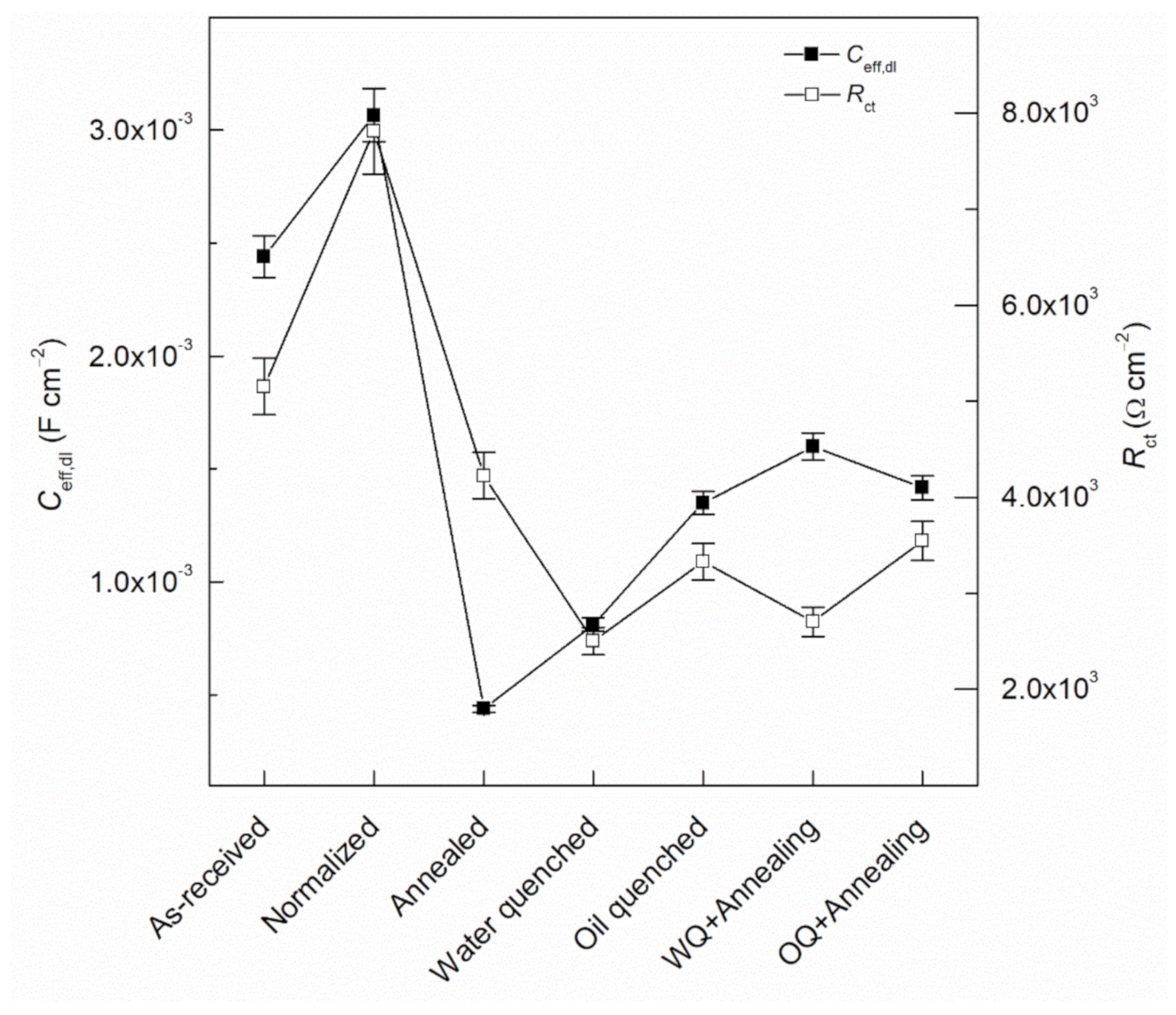
- Ceff,dl shows the lower stability of the passive film as the cooling rate increased, from 1.11 × 10−3 to 8.12 × 10−4 µF/cm2 for as-received and water quenched samples, respectively.
- Higher pit depth was observed for the quenched samples, with an average pit depth of 5 μm. Similarly, the annealed sample showed four times the average pith depth compared to the as-received sample, 4 and 1 μm, respectively. These findings are in accordance with the EIS and CPP results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CE | Counter electrode |
| Ceff,dl | Effective capacitance of the double layer |
| CPE | Constant phase element |
| CPP | Cyclic potentiodynamic polarization |
| CS | Carbon steel |
| Ecorr | Corrosion potential |
| EEC | Electrical equivalent circuit |
| Ef | Flade potential |
| EIS | Electrochemical impedance spectroscopy |
| FA | Fly ash |
| i0,ORR | Exchange current density of ORR |
| icorr | Corrosion current density |
| IFM | Infinite focus microscope |
| OM | Optical microscope |
| OPC | Ordinary Portland cement |
| ORR | Oxygen reduction reaction |
| OQ | Oil quenched |
| RCS | Reinforced concrete structures |
| Rct | Charge transfer resistance |
| RE | Reference electrode |
| Rp | Polarization resistance |
| WE | Working electrode |
| WQ | Water quenched |
| Y | Pseudo-capacitance |
| α | Charge transfer coefficient |
| β | Tafel slope |
| χ2 | Chi-square goodness of fit |
References
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.X.; Huang, L.; Zhao, Y.B.; Chen, P.; Zeng, L.; Zheng, W. Modeling of carbon dioxide measurement on cement plants. Adv. Mater. Res. 2012, 610–613, 2120–2128. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Energy Technology Perspectives 2010: Scenarios and Strategies to 2050; OECD Publishing: Paris, France, 2009. [Google Scholar] [CrossRef]
- Mahasenan, N.; Smith, S.; Humphreys, K. The cement industry and global climate change current and potential future cement industry CO2 emissions. In Proceedings of the Greenhouse Gas Control Technologies—6th International Conference, Kyoto, Japan, 1–4 October 2002; Pergamon: Oxford, UK, 2003; pp. 995–1000. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; Van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- John, S.K.; Nadir, Y.; Girija, K. Effect of source materials, additives on the mechanical properties and durability of fly ash and fly ash-slag geopolymer mortar: A review. Constr. Build. Mater. 2021, 280, 122443. [Google Scholar] [CrossRef]
- Fu, X.; Li, Q.; Zhai, J.; Sheng, G.; Li, F. The physical–chemical characterization of mechanically-treated CFBC fly ash. Cem. Concr. Compos. 2008, 30, 220–226. [Google Scholar] [CrossRef]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Research Report; Curtin University of Technology: Perth, Australia, 2005. [Google Scholar]
- Parande, A.K.; Babu, B.R.; Karthik, M.A.; Deepak, K.K.; Palaniswamy, N. Study on strength and corrosion performance for steel embedded in metakaolin blended concrete/mortar. Constr. Build. Mater. 2008, 22, 127–134. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Mehrali, M.; Alengaram, U.J.; Jumaat, M.Z. Graphene nanoplatelet-fly ash based geopolymer composites. Cem. Concr. Res. 2015, 76, 222–231. [Google Scholar] [CrossRef]
- Ariza-Figueroa, H.A.; Bosch, J.; Baltazar-Zamora, M.A.; Croche, R.; Santiago-Hurtado, G.; Landa-Ruiz, L.; Mendoza-Rangel, J.M.; Bastidas, J.M.; Almeraya-Calderón, F.; Bastidas, D.M. Corrosion behavior of AISI 304 stainless steel reinforcements in SCBA-SF ternary ecological concrete exposed to MgSO4. Materials 2020, 13, 2412. [Google Scholar] [CrossRef]
- Masi, G. Steel corrosion behavior in light weight fly-ash based alkali activated mortars. Appl. Sci. 2021, 11, 1908. [Google Scholar] [CrossRef]
- Georget, F.; Soja, W.; Scrivener, K.L. Characteristic lengths of the carbonation front in naturally carbonated cement pastes: Implications for reactive transport models. Cem. Concr. Res. 2020, 134, 106080. [Google Scholar] [CrossRef]
- Ma, F.-Y. Corrosive effects of chlorides on metals. In Pitting Corrosion; InTech Europe: Rijeka, Croatia, 2012; Volume 294, pp. 139–178. [Google Scholar]
- Zuo, Y.; Nedeljković, M.; Ye, G. Pore solution composition of alkali-activated slag/fly ash pastes. Cem. Concr. Res. 2019, 115, 230–250. [Google Scholar] [CrossRef]
- Babaee, M.; Castel, A. Chloride diffusivity, chloride threshold, and corrosion initiation in reinforced alkali-activated mortars: Role of calcium, alkali, and silicate content. Cem. Concr. Res. 2018, 111, 56–71. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Gao, X.; Huang, Y.; Yu, R.; Xiao, X. Modification on the chloride binding capacity of cementitious materials by aluminum compound addition. Constr. Build. Mater. 2019, 222, 15–25. [Google Scholar] [CrossRef]
- Gunasekara, C.; Law, D.; Bhuiyan, S.; Setunge, S.; Ward, L. Chloride induced corrosion in different fly ash based geopolymer concretes. Constr. Build. Mater. 2019, 200, 502–513. [Google Scholar] [CrossRef]
- Nedeljković, M.; Ghiassi, B.; van der Laan, S.; Li, Z.; Ye, G. Effect of curing conditions on the pore solution and carbonation resistance of alkali-activated fly ash and slag pastes. Cem. Concr. Res. 2019, 116, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Hu, J.; Ma, Y.; Guo, W.; Huang, H.; Wei, J.; Yin, S.; Yu, Q. Characterization of the passive film formed on the reinforcement surface in alkali activated fly ash: Surface analysis and electrochemical evaluation. Corros. Sci. 2020, 165, 108393. [Google Scholar] [CrossRef]
- Mangat, P.S.; Ojedokun, O.O.; Lambert, P. Chloride-initiated corrosion in alkali activated reinforced concrete. Cem. Concr. Compos. 2021, 115, 103823. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; Van Deventer, J.S.J. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Mundra, S.; Criado, M.; Bernal, S.A.; Provis, J.L. Chloride-induced corrosion of steel rebars in simulated pore solutions of alkali-activated concretes. Cem. Concr. Res. 2017, 100, 385–397. [Google Scholar] [CrossRef]
- Lu, J.; Liu, H.; Chen, Z.; Liao, X. Experimental investigation into the post-fire mechanical properties of hot-rolled and cold-formed steels. J. Constr. Steel Res. 2016, 121, 291–310. [Google Scholar] [CrossRef]
- Gunalan, S.; Mahendran, M. Experimental investigation of post-fire mechanical properties of cold-formed steels. Thin-Walled Struct. 2014, 84, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lo, K.H.; Kwok, C.T.; Sun, Y.F.; Lai, K.K. Post-fire mechanical and corrosion properties of duplex stainless steel: Comparison with ordinary reinforcing-bar steel. Constr. Build. Mater. 2018, 174, 150–158. [Google Scholar] [CrossRef]
- Panda, B.; Balasubramaniam, R.; Dwivedi, G. On the corrosion behaviour of novel high carbon rail steels in simulated cyclic wet-dry salt fog conditions. Corros. Sci. 2008, 50, 1684–1692. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Misra, S.; Mondal, K. Effect of different cooling rates on the corrosion behavior of high-carbon pearlitic steel. J. Mater. Eng. Perform. 2018, 27, 1753–1762. [Google Scholar] [CrossRef]
- Atienza, J.M.; Elices, M. Behavior of prestressing steels after a simulated fire: Fire-induced damages. Constr. Build. Mater. 2009, 23, 2932–2940. [Google Scholar] [CrossRef]
- Torbati-Sarraf, H.; Poursaee, A. The influence of phase distribution and microstructure of the carbon steel on its chloride threshold value in a simulated concrete pore solution. Constr. Build. Mater. 2020, 259, 119784. [Google Scholar] [CrossRef]
- Bautista, A.; Pomares, J.C.; González, M.N.; Velasco, F. Influence of the microstructure of TMT reinforcing bars on their corrosion behavior in concrete with chlorides. Constr. Build. Mater. 2019, 229, 116899. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.; Mukerjee, D.; Sen, S.K.; Bhattacharya, A.; Dhua, S.K.; Prasad, M.S.; Banerjee, N.; Popli, A.M.; Sahu, A.K. Microstructure and properties of thermomechanically strengthened reinforcement bars: A comparative assessment of plain-carbon and low-alloy steel grades. J. Mater. Eng. Perform. 1997, 6, 335–343. [Google Scholar] [CrossRef]
- Hao, X.; Dong, J.; Etim, I.I.N.; Wei, J.; Ke, W. Sustained effect of remaining cementite on the corrosion behavior of ferrite-pearlite steel under the simulated bottom plate environment of cargo oil tank. Corros. Sci. 2016, 110, 296–304. [Google Scholar] [CrossRef]
- Hao, X.; Zhao, X.; Chen, H.; Huang, B.; Ma, J.; Wang, C.; Yang, Y. Comparative study on corrosion behaviors of ferrite-pearlite steel with dual-phase steel in the simulated bottom plate environment of cargo oil tanks. J. Mater. Res. Technol. 2021, 12, 399–411. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Mohammed, J.A.; Luqman, M.; Ragab, S.A.; Almotairy, S.M. Influence of chloride ions on electrochemical corrosion behavior of dual-phase steel over conventional rebar in pore solution. Appl. Sci. 2020, 10, 4568. [Google Scholar] [CrossRef]
- Trejo, D.; Monteiro, P.; Thomas, G.; Wang, X. Mechanical properties and corrosion susceptibility of dual-phase steel in concrete. Cem. Concr. Res. 1994, 24, 1245–1254. [Google Scholar] [CrossRef]
- Moser, R.D.; Singh, P.M.; Kahn, L.F.; Kurtis, K.E. Chloride-induced corrosion resistance of high-strength stainless steels in simulated alkaline and carbonated concrete pore solutions. Corros. Sci. 2012, 57, 241–253. [Google Scholar] [CrossRef]
- ASTM. G3-14 Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Grewal, R.; Aranas, C.; Chadha, K.; Shahriari, D.; Jahazi, M.; Jonas, J.J. Formation of Widmanstätten ferrite at very high temperatures in the austenite phase field. Acta Mater. 2016, 109, 23–31. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, J.; Shen, X.; Wang, Y.; Zhao, W. Surface hardening of 30CrMnSiA steel using continuous electron beam. Nucl. Instrum. Methods Phys. Res. B 2017, 410, 207–214. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of cyclic potentiodynamic polarization test results for study of corrosion behavior of metals: A review. Prot. Met. Phys. Chem. Surfaces 2018, 54, 976–989. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.S.; Montemor, M.F. The electrochemical behaviour of stainless steel AISI 304 in alkaline solutions with different pH in the presence of chlorides. Electrochim. Acta 2011, 56, 5280–5289. [Google Scholar] [CrossRef]
- Zhang, W.; François, R.; Cai, Y.; Charron, J.P.; Yu, L. Influence of artificial cracks and interfacial defects on the corrosion behavior of steel in concrete during corrosion initiation under a chloride environment. Constr. Build. Mater. 2020, 253, 119165. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 75005–75013. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Misra, S.; Mondal, K. Comparative corrosion behavior of five microstructures (pearlite, bainite, spheroidized, martensite, and tempered martensite) made from a high carbon steel. Metall. Mater. Trans. A 2019, 50, 1489–1501. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, S.; Shi, Q.; Tao, H.; Song, Y.; Zheng, J.; Xu, P.; Zhang, L. Improvement of corrosion resistance of SS316L manufactured by selective laser melting through subcritical annealing. Corros. Sci. 2020, 164, 108353. [Google Scholar] [CrossRef]
- Guidelli, R.; Compton, R.G.; Feliu, J.M.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Defining the transfer coefficient in electrochemistry: An assessment. Pure Appl. Chem. 2014, 86, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Bastidas, D.M. Interpretation of impedance data for porous electrodes and diffusion processes. Corrosion 2007, 63, 515–521. [Google Scholar] [CrossRef]
- Martin, U.; Ress, J.; Bosch, J.; Bastidas, D.M. Stress corrosion cracking mechanism of AISI 316LN stainless steel rebars in chloride contaminated concrete pore solution using the slow strain rate technique. Electrochim. Acta 2020, 335, 135565. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Abrantes, J.C. Application of electrochemical impedance spectroscopy (EIS) to monitor the corrosion of reinforced concrete: A new approach. Constr. Build. Mater. 2016, 111, 98–104. [Google Scholar] [CrossRef]
- Huang, J. Diffusion impedance of electroactive materials, electrolytic solutions and porous electrodes: Warburg impedance and beyond. Electrochim. Acta 2018, 281, 170–188. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Fajardo, S.; Llorente, I.; Jiménez, J.A.; Bastidas, J.M.; Bastidas, D.M. Effect of Mn additions on the corrosion behaviour of TWIP Fe-Mn-Al-Si austenitic steel in chloride solution. Corros. Sci. 2019, 154, 246–253. [Google Scholar] [CrossRef]
- Huang, V.M.-W.; Vivier, V.; Orazem, M.E.; Pébère, N.; Tribollet, B. The apparent constant-phase-element behavior of a disk electrode with faradaic reactions. J. Electrochem. Soc. 2007, 154, C99. [Google Scholar] [CrossRef] [Green Version]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Martin, U.; Ress, J.; Bosch, J.; Bastidas, D.M. Long-term stability and electronic properties of passive film of lean-duplex stainless steel reinforcements in chloride containing mortar. Constr. Build. Mater. 2021, 123319. [Google Scholar] [CrossRef]
- Handoko, W.; Pahlevani, F.; Sahajwalla, V. Enhancing corrosion resistance and hardness properties of carbon steel through modification of microstructure. Materials 2018, 11, 2404. [Google Scholar] [CrossRef] [Green Version]




| C | Mn | S | Cu | P | Ni | Cr | Si | Mo | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.28 | 1.08 | 0.043 | 0.37 | 0.019 | 0.16 | 0.16 | 0.20 | 0.050 | 0.0379 | Bal. |
| Specimen | icorr (A/cm2) | Ecorr (VSCE) | βa (V/dec) | βc (V/dec) | B (mV) | αa | αc | i0,ORR (A/cm2) |
|---|---|---|---|---|---|---|---|---|
| As-received | 2.13 × 10−6 | −0.500 | 0.091 | 0.181 | 26.33 | 0.282 | 0.142 | 5.91 × 10−8 |
| Normalized | 1.84 × 10−6 | −0.423 | 0.265 | 0.208 | 50.67 | 0.097 | 0.124 | 1.77 × 10−8 |
| Annealed | 2.09 × 10−5 | −0.561 | 0.252 | 0.269 | 56.57 | 0.102 | 0.096 | 1.18 × 10−9 |
| Water quenched | 3.18 × 10−5 | −0.496 | 0.292 | 0.295 | 63.80 | 0.088 | 0.087 | 4.86 × 10−9 |
| Oil quenched | 2.20 × 10−5 | −0.504 | 0.284 | 0.271 | 60.29 | 0.091 | 0.095 | 4.12 × 10−9 |
| WQ + Sub. Annealing | 2.18 × 10−6 | −0.592 | 0.182 | 0.231 | 44.26 | 0.141 | 0.111 | 3.48 × 10−9 |
| OQ + Sub. Annealing | 2.77 × 10−6 | −0.615 | 0.232 | 0.245 | 51.81 | 0.111 | 0.105 | 1.79 × 10−9 |
| Specimen | Rs Ω cm2 | Rfilm Ω cm2 | Yfilm µS/cm2 sn,film | nfilm | Rct Ω cm2 | Ydl S/cm2 sn,dl | ndl | Ceff,dl µF/cm2 | χ2 (*) |
|---|---|---|---|---|---|---|---|---|---|
| As-received | 13.3 | 457.3 | 82.2 | 0.91 | 5154 | 2.44 × 10−3 | 0.81 | 1.11 × 10−3 | 5.51 × 10−4 |
| Normalized | 16.2 | 131.9 | 162.5 | 0.87 | 7814 | 5.23 × 10−3 | 0.82 | 3.06 × 10−3 | 2.18 × 10−4 |
| Annealed | 16.6 | 174.1 | 170.0 | 0.84 | 4225 | 1.37 × 10−3 | 0.77 | 4.38 × 10−4 | 4.19 × 10−4 |
| Water quenched | 12.9 | 241.5 | 207.9 | 0.88 | 2500 | 1.86 × 10−3 | 0.82 | 8.12 × 10−4 | 2.83 × 10−4 |
| Oil quenched | 13.2 | 123.9 | 109.7 | 0.83 | 3326 | 3.10 × 10−3 | 0.79 | 1.35 × 10−3 | 1.54 × 10−4 |
| WQ+Sub. Annealing | 14.9 | 151.7 | 79.6 | 0.89 | 2702 | 2.41 × 10−3 | 0.89 | 1.61 × 10−3 | 1.11 × 10−4 |
| OQ+Sub. Annealing | 13.1 | 131.3 | 71.1 | 0.90 | 3545 | 1.99 × 10−3 | 0.92 | 1.42 × 10−3 | 2.44 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosch, J.; Martin, U.; Ress, J.; Klimek, K.; Bastidas, D.M. Influence of Thermomechanical Treatments on Corrosion of Carbon Steel in Synthetic Geopolymer Fly Ash Pore Solution. Appl. Sci. 2021, 11, 4054. https://doi.org/10.3390/app11094054
Bosch J, Martin U, Ress J, Klimek K, Bastidas DM. Influence of Thermomechanical Treatments on Corrosion of Carbon Steel in Synthetic Geopolymer Fly Ash Pore Solution. Applied Sciences. 2021; 11(9):4054. https://doi.org/10.3390/app11094054
Chicago/Turabian StyleBosch, Juan, Ulises Martin, Jacob Ress, Keston Klimek, and David M. Bastidas. 2021. "Influence of Thermomechanical Treatments on Corrosion of Carbon Steel in Synthetic Geopolymer Fly Ash Pore Solution" Applied Sciences 11, no. 9: 4054. https://doi.org/10.3390/app11094054
APA StyleBosch, J., Martin, U., Ress, J., Klimek, K., & Bastidas, D. M. (2021). Influence of Thermomechanical Treatments on Corrosion of Carbon Steel in Synthetic Geopolymer Fly Ash Pore Solution. Applied Sciences, 11(9), 4054. https://doi.org/10.3390/app11094054







