Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review
Abstract
:1. Introduction
2. Types of Polyurethane
2.1. Thermoplastics PUs
2.2. Flexible PUs
2.3. Rigid PUs
2.4. PUs Ionomer
2.5. Water-Borne PUs
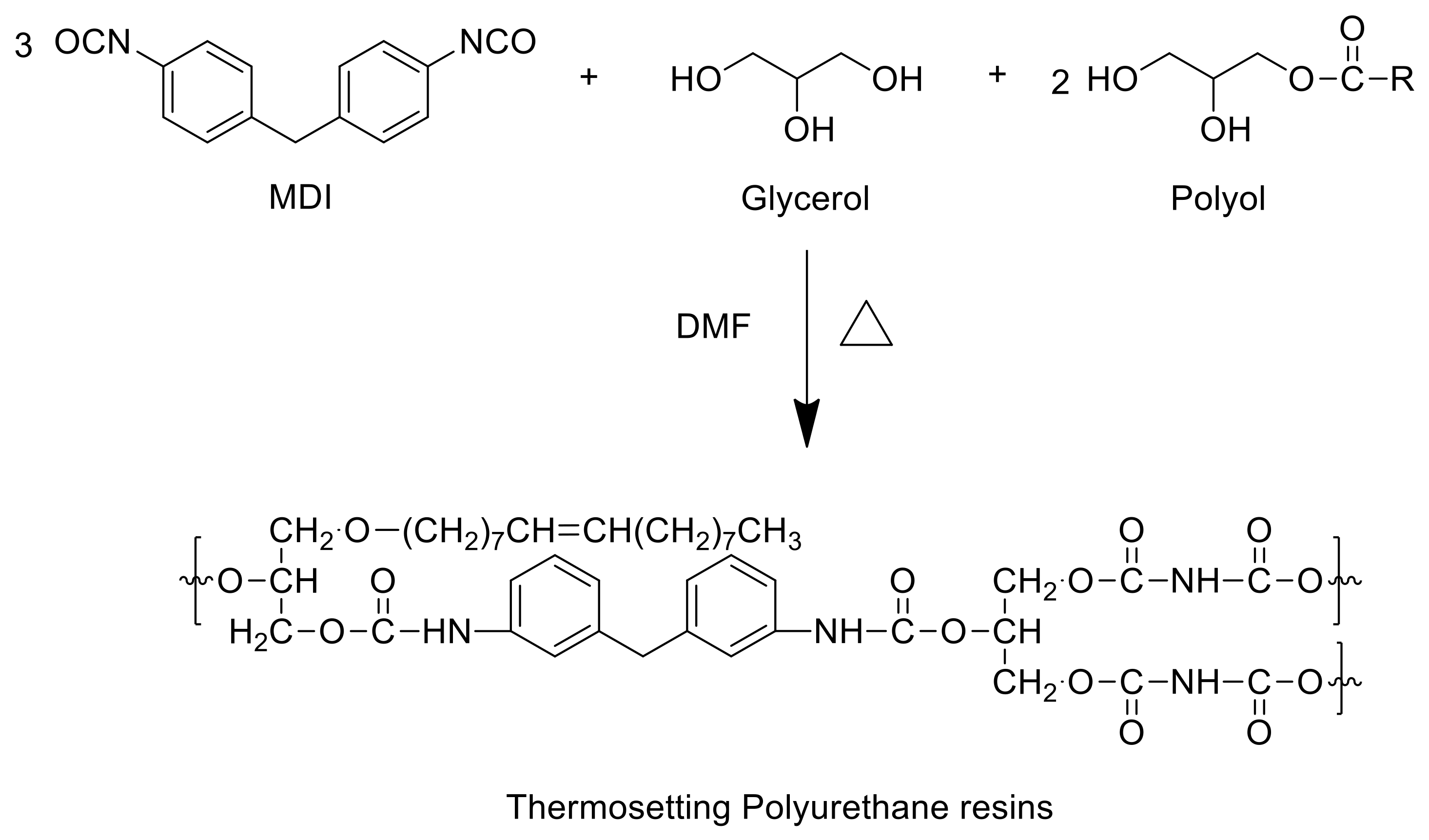
2.6. Thermosetting PUs
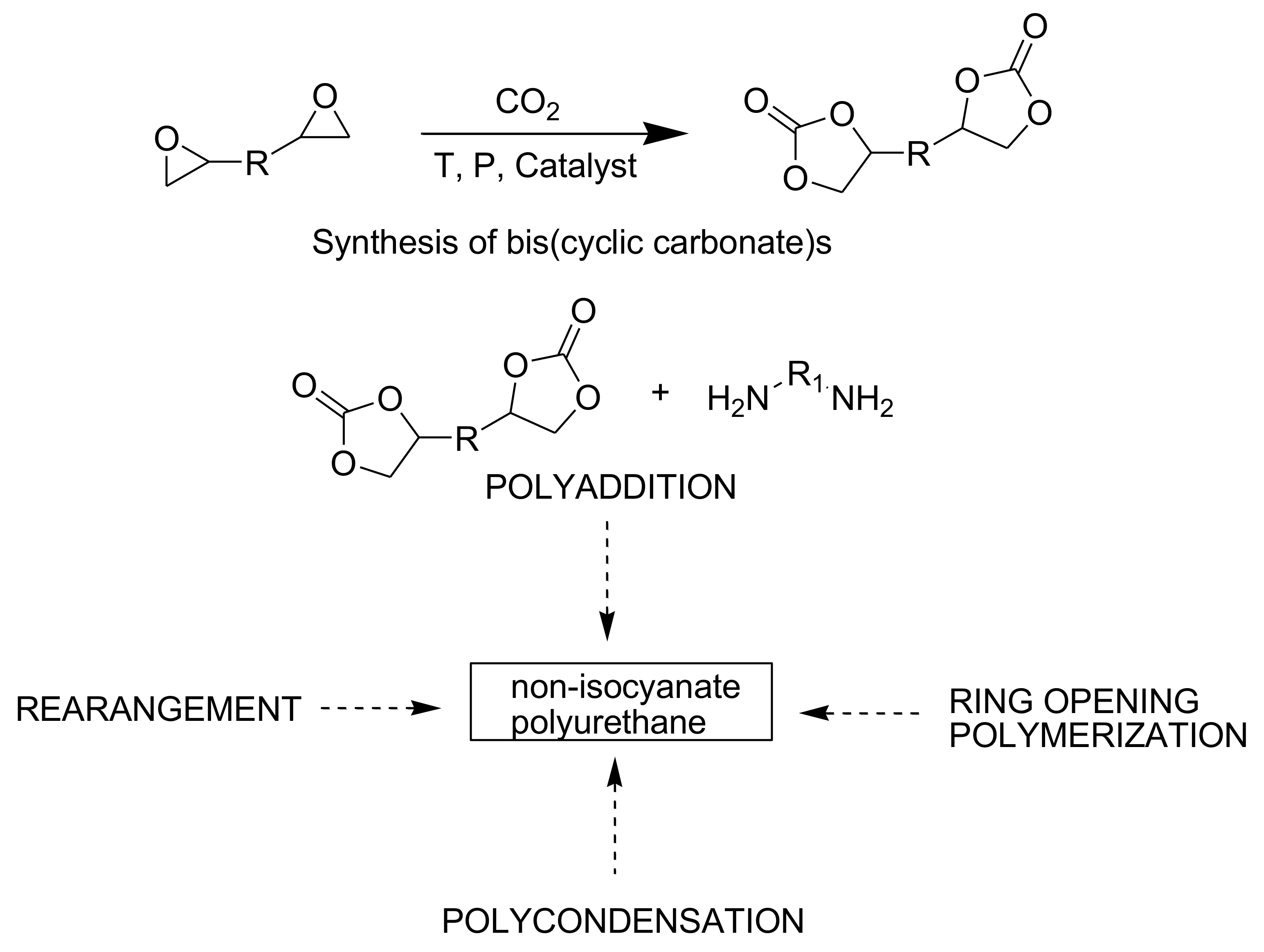
3. Route for Non-Isocyanate PUs (NIPUs)
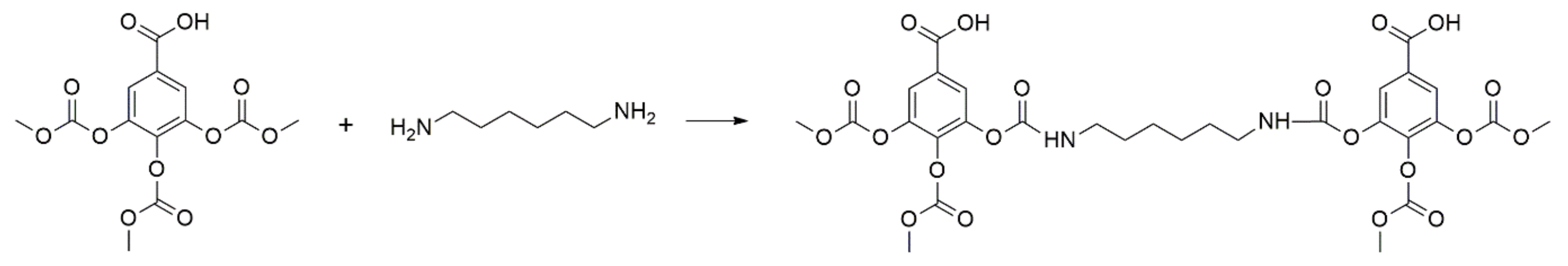
4. Lignin-Based NIPUs Adhesive
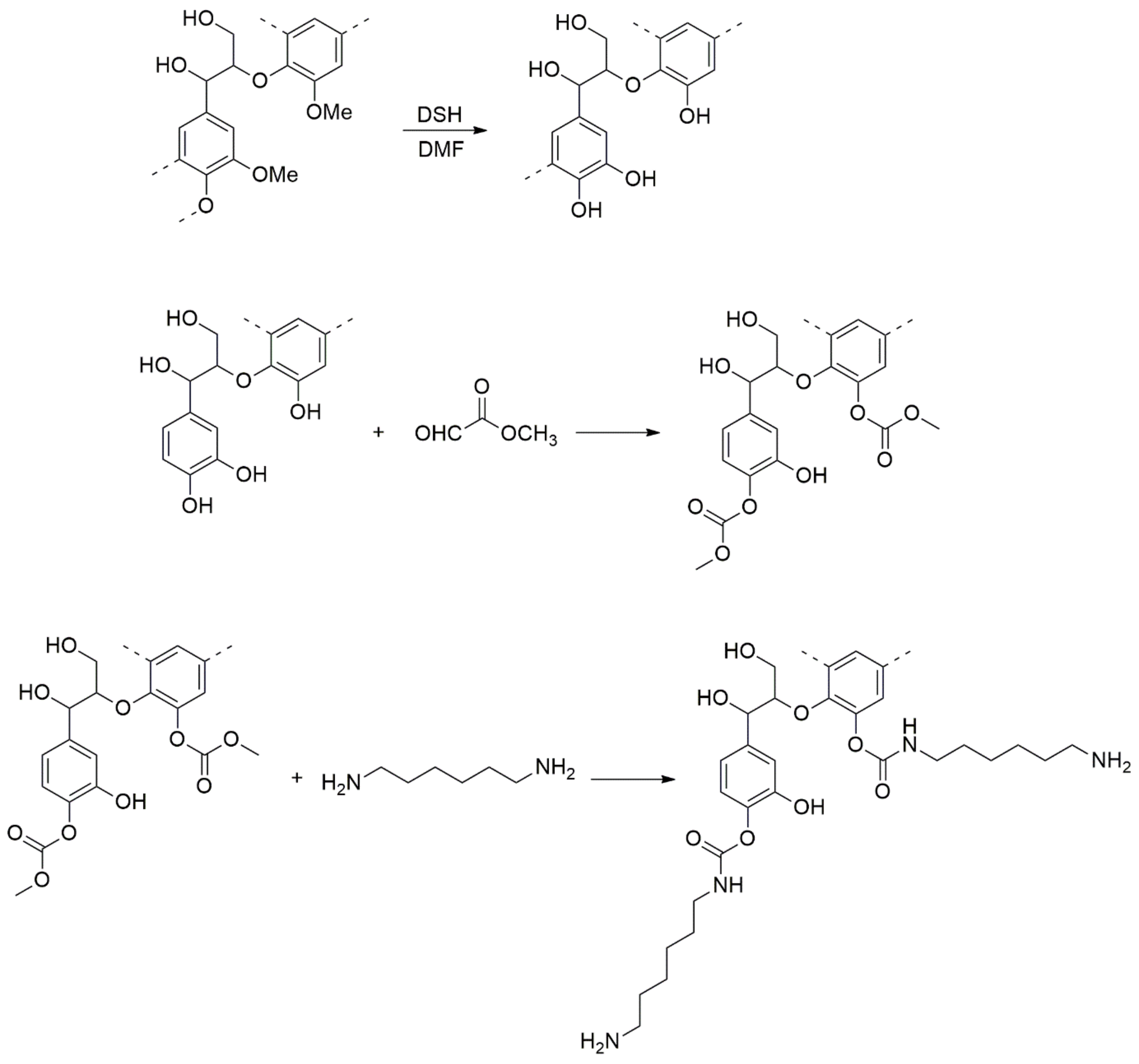
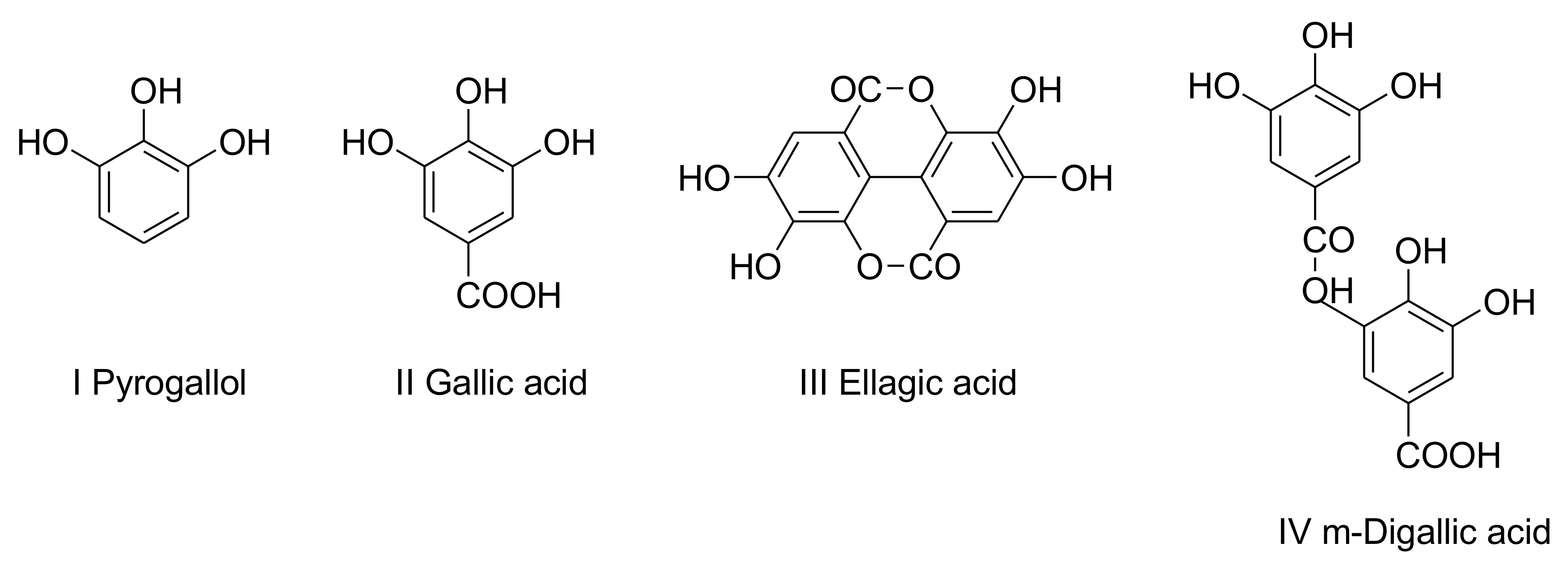
5. Tannin-Derived NIPUs Adhesive
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Zia, K.M.; Anjum, S.; Zuber, M.; Mujahid, M.; Jamil, T. Synthesis and molecular characterization of chitosan based polyurethane elastomers using aromatic diisocyanate. Int. J. Biol. Macromol. 2014, 66, 26–32. [Google Scholar] [CrossRef]
- Ionescu, M.; Radojčić, D.; Wan, X.; Shrestha, M.L.; Petrović, Z.S.; Upshaw, T.A. Highly functional polyols from castor oil for rigid polyurethanes. Eur. Polym. J. 2016, 84, 736–749. [Google Scholar] [CrossRef] [Green Version]
- Ghahri, S.; Chen, X.; Pizzi, A.; Hajihassani, R.; Papadopoulos, A.N. Natural Tannins as New Cross-Linking Materials for Soy-Based Adhesives. Polymers 2021, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood composites and their polymer binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef]
- Bekhta, P.; Sedliačik, J.; Kačík, F.; Noshchenko, G.; Kleinová, A. Lignocellulosic waste fibers and their application as a component of urea-formaldehyde adhesive composition in the manufacture of plywood. Eur. J. Wood Wood Prod. 2019, 77, 495–508. [Google Scholar] [CrossRef]
- Rafiee, Z.; Keshavarz, V. Synthesis and characterization of polyurethane/microcrystalline cellulose bionanocomposites. Prog. Org. Coat. 2015, 86, 190–193. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Liu, G.; Curtis, J.M. Characterization of canola oil based polyurethane wood adhesives. Int. J. Adhes. Adhes. 2011, 31, 559–564. [Google Scholar] [CrossRef]
- Frisch, K.C.; Klempner, D. Advances in Urethane: Science & Technology; CRC Press: London, UK, 1998; ISBN 9781566766753. [Google Scholar]
- Caraculacu, A.A.; Coseri, S. Isocyanates in polyaddition processes. Structure and reaction mechanisms. Prog. Polym. Sci. 2001, 26.5, 799–851. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of vegetable-oil-based polymers. J. Appl. Polym. Sci. 2014, 131, 9016–9028. [Google Scholar] [CrossRef] [Green Version]
- Datta, J.; Włoch, M. Progress in non-isocyanate polyurethanes synthesized from cyclic carbonate intermediates and di- or polyamines in the context of structure–properties relationship and from an environmental point of view. Polym. Bull. 2016, 73, 1459–1496. [Google Scholar] [CrossRef]
- Błażek, K.; Datta, J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 173–211. [Google Scholar] [CrossRef]
- Datta, J.; Kasprzyk, P.; Błażek, K.; Włoch, M. Synthesis, structure and properties of poly(ester-urethane)s obtained using bio-based and petrochemical 1,3-propanediol and 1,4-butanediol. J. Therm. Anal. Calorim. 2017, 130, 261–276. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Effect of reactive organoclay on physicochemical properties of vegetable oil-based waterborne polyurethane nanocomposites. RSC Adv. 2015, 5, 11524–11533. [Google Scholar] [CrossRef]
- Alagi, P.; Choi, Y.J.; Hong, S.C. Preparation of vegetable oil-based polyols with controlled hydroxyl functionalities for thermoplastic polyurethane. Eur. Polym. J. 2016, 78, 46–60. [Google Scholar] [CrossRef]
- Alagi, P.; Hong, S.C. Vegetable oil-based polyols for sustainable polyurethanes. Macromol. Res. 2015, 23, 1079–1086. [Google Scholar] [CrossRef]
- Faruk, O.; Sain, M. Continuous extrusion foaming of lignin enhanced thermoplastic polyurethane (TPU). J. Biobased Mater. Bioenergy 2013, 7, 309–314. [Google Scholar] [CrossRef]
- Alinejad, M.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for and Adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thébault, M.; Pizzi, A.; Essawy, H.A.; Barhoum, A.; Van Assche, G. Isocyanate free condensed tannin-based polyurethanes. Eur. Polym. J. 2015, 67, 513–526. [Google Scholar] [CrossRef]
- Santiago-Medina, F.J.; Basso, M.C.; Pizzi, A.; Delmotte, L. Polyurethanes from kraft lignin without using isocyanates. J. Renew. Mater. 2018, 6, 413–425. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Dumarçay, S.; Gerardin, P.; Fredon, E.; Delmotte, L. Polyurethanes from hydrolysable tannins obtained without using isocyanates. Ind. Crops Prod. 2014, 59, 329–336. [Google Scholar] [CrossRef]
- Norström, E.; Demircan, D.; Fogelström, L.; Khabbaz, F.; Malmström, E. Green Binders for Wood Adhesives. Appl. Adhes. Bond. Sci. Technol. 2018, 1, 13–70. [Google Scholar]
- Pizzi, A. Phenolic resin adhesives. In Handbook of Adhesive Technology, 3rd ed.; CRC Press: London, UK, 2017; pp. 223–262. ISBN 9781498736473. [Google Scholar]
- Bekhta, P.; Sedliačik, J.; Saldan, R.; Novák, I. Effect of different hardeners for urea-formaldehyde resin on properties of birch plywood. Acta Fac. Xylologiae 2016, 58, 65–72. [Google Scholar]
- Kumar, R.N.P. Urea Formaldehyde Resins; Pizzi, A., Ed.; Wiley-Scrivener: Hoboken, NJ, USA, 2019. [Google Scholar]
- Lubis, M.A.R.; Park, B.-D. Influence of Initial Molar Ratios on the Performance of Low Molar Ratio Urea-Formaldehyde Resin Adhesives. J. Korean Wood Sci. Technol. 2020, 482, 136–153. [Google Scholar]
- Mirski, R.; Bekhta, P.; Dziurka, D. Relationships between thermoplastic type and properties of polymer-triticale boards. Polymers 2019, 11, 1750. [Google Scholar] [CrossRef] [Green Version]
- Wibowo, E.S.; Lubis, M.A.R.; Park, B.D. In-situ modification of low molar ratio urea–formaldehyde resins with cellulose nanofibrils for plywood. J. Adhes. Sci. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- US Consumer Product Safety Commission. An update on formaldehyde. In Proceedings of the An Update on Formaldehyde; US Consumer Product Safety Commission: Bethesda, MD, USA, 2013. [Google Scholar]
- Bekhta, P.; Sedliačik, J.; Noshchenko, G.; Kačík, F.; Bekhta, N. Characteristics of beech bark and its effect on properties of UF adhesive and on bonding strength and formaldehyde emission of plywood panels. Eur. J. Wood Wood Prod. 2021, 79, 423–433. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. (June 2004) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 88 (2006): Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. Retrieved 10 June. 2011. Available online: http://monographs.iarc.fr/ENG/Monographs/vol88/index.php (accessed on 6 May 2021).
- International Agency for Research on Cancer. IARC Classifies Formaldehyde as Carcinogenic. Oncol. Times 2004, 26, 72. [Google Scholar] [CrossRef]
- Enivronmental Protection Agency. Proposed Designation of Di-Ethylhexyl Phthalate (DEHP) as a High-Priority Substance for Risk Evaluation. 2019. United States. Available online: https://www.epa.gov/sites/production/files/2019-08/documents/di-ethylhexyl_phthalate_117-81-7_proposeddesignation_082219.pdf (accessed on 6 May 2021).
- Antov, P.; Savov, V.; Neykov, N. Sustainable bio-based adhesives for eco-friendly wood composites a review. Wood Res. 2020, 65, 51–62. [Google Scholar] [CrossRef]
- Tudor, E.M.; Barbu, M.C.; Petutschnigg, A.; Réh, R.; Krišťák, Ľ. Analysis of larch-bark capacity for formaldehyde removal in wood adhesives. Int. J. Environ. Res. Public Health 2020, 17, 764. [Google Scholar] [CrossRef] [Green Version]
- Hüttermann, A.; Mai, C.; Kharazipour, A. Modification of lignin for the production of new compounded materials. Appl. Microbiol. Biotechnol. 2001, 55, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Youngquist, J.A. Wood-Based Composites and Panel Products. In Wood Handbook: Wood as an Engineering Material; General Technical Report FPL-113; USDA, Forest Service, Forest Products Laboratory: Madison, WI, USA, 1999; Chapter 10; pp. 1–31. [Google Scholar]
- Lubis, M.A.R.; Park, B.-D. Enhancing the performance of low molar ratio urea–formaldehyde resin adhesives via in-situ modification with intercalated nanoclay. J. Adhes. 2020, 1–20. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.; Wang, C.; Li, S.; Zhang, M.; Chu, F. Preparation and properties of lignin-phenol-formaldehyde resins based on different biorefinery residues of agricultural biomass. Ind. Crops Prod. 2013, 43, 326–333. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, X.; Zheng, Z. Preparation and characterization of phenol-formaldehyde adhesives modified with enzymatic hydrolysis lignin. Bioresour. Technol. 2010, 101, 2046–2048. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.; Xu, Y.; Wang, C.; Chu, F. Lignocellulosic ethanol residue-based lignin-phenol-formaldehyde resin adhesive. Int. J. Adhes. Adhes. 2013, 40, 11–18. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Yuan, T.Q.; Sun, R.C. Lignin-phenol-formaldehyde resin adhesives prepared with biorefinery technical lignins. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Papadopoulos, A.N. Advances in wood composites. Polymers 2020, 12, 48. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, A.N. Advances in wood composites ii. Polymers 2020, 12, 1552. [Google Scholar] [CrossRef]
- Papadopoulos, A.N. Advances in wood composites iii. Polymers 2021, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Lubis, M.A.R.; Hong, M.-K.; Park, B.-D.; Lee, S.-M. Effects of recycled fiber content on the properties of medium density fiberboard. Eur. J. Wood Wood Prod. 2018, 76, 1515–1526. [Google Scholar] [CrossRef]
- He, G.; Yan, N. Effect of moisture content on curing kinetics of pMDI resin and wood mixtures. Int. J. Adhes. Adhes. 2005, 25, 450–455. [Google Scholar] [CrossRef]
- Leitsch, E.K.; Heath, W.H.; Torkelson, J.M. Polyurethane/polyhydroxyurethane hybrid polymers and their applications as adhesive bonding agents. Int. J. Adhes. Adhes. 2016, 64, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhao, S.; Pang, H.; Zhang, W.; Zhang, S.; Li, J. Developing Eco-friendly High-Strength Soy Adhesives with Improved Ductility through Multiphase Core–Shell Hyperbranched Polysiloxane. ACS Sustain. Chem. Eng. 2019, 7, 7784–7794. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Taghiyari, H.R. Innovative wood surface treatments based on nanotechnology. Coatings 2019, 9, 866. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhang, F.; Wu, L.; Gao, Z.; Zhang, L. Assessment of soybean protein-based adhesive formulations, prepared by different liquefaction technologies for particleboard applications. Wood Sci. Technol. 2021, 55, 33–48. [Google Scholar] [CrossRef]
- Frihart, C.R.; Satori, H. Soy flour dispersibility and performance as wood adhesive. J. Adhes. Sci. Technol. 2013, 27, 2043–2052. [Google Scholar] [CrossRef] [Green Version]
- Ghahri, S.; Pizzi, A. Improving soy-based adhesives for wood particleboard by tannins addition. Wood Sci. Technol. 2018, 52, 261–279. [Google Scholar] [CrossRef]
- Zhao, X.F.; Peng, L.Q.; Wang, H.L.; Wang, Y.B.; Zhang, H. Environment-friendly urea-oxidized starch adhesive with zero formaldehyde-emission. Carbohydr. Polym. 2018, 181, 1112–1118. [Google Scholar] [CrossRef]
- Gu, Y.; Cheng, L.; Gu, Z.; Hong, Y.; Li, Z.; Li, C. Preparation, characterization and properties of starch-based adhesive for wood-based panels. Int. J. Biol. Macromol. 2019, 134, 247–254. [Google Scholar] [CrossRef]
- Trosa, A.; Pizzi, A. A no-aldehyde emission hardener for tannin-based wood adhesives for exterior panels. Holz Roh Werkst. 2001, 59, 266–271. [Google Scholar] [CrossRef]
- Ndiwe, B.; Pizzi, A.; Tibi, B.; Danwe, R.; Konai, N.; Amirou, S. African tree bark exudate extracts as biohardeners of fully biosourced thermoset tannin adhesives for wood panels. Ind. Crops Prod. 2019, 132, 253–268. [Google Scholar] [CrossRef]
- Santos, J.; Antorrena, G.; Freire, M.S.; Pizzi, A.; González-Álvarez, J. Environmentally friendly wood adhesives based on chestnut (Castanea sativa) shell tannins. Eur. J. Wood Wood Prod. 2017, 75, 89–100. [Google Scholar] [CrossRef]
- Kunaver, M.; Medved, S.; Čuk, N.; Jasiukaityte, E.; Poljanšek, I.; Strnad, T. Application of liquefied wood as a new particle board adhesive system. Bioresour. Technol. 2010, 101, 1361–1368. [Google Scholar] [CrossRef]
- Klašnja, B.; Kopitović, S. Lignin-Phenol-Formaldehyde resins as adhesives in the production of plywood. Holz Roh Werkst. 1992, 50, 282–285. [Google Scholar] [CrossRef]
- Savov, V.; Valchev, I.; Yavorov, N.; Sabev, K. Influence of press factor and additional thermal treatment on technology for production of eco-friendly MDF based on lignosulfonate adhesives. Bulg. Chem. Commun. 2020, 52, 48–52. [Google Scholar]
- Antov, P.; Mantanis, G.I.; Savov, V. Development of Wood Composites from Recycled Fibres Bonded with Magnesium Lignosulfonate. Forests 2020, 11, 613. [Google Scholar] [CrossRef]
- Yotov, N.; Savov, V.; Petrin, S.; Valchev, I.; Karatotev, V. Study on possibility for the utilization of technical hydrolysis lignin in composition of medium density fiberboard. Innov. Wood. Ind. Eng. Des. 2015, 1, 74–80. [Google Scholar]
- El Mansouri, N.E.; Pizzi, A.; Salvadó, J. Lignin-based wood panel adhesives without formaldehyde. Holz Roh Werkst. 2007, 65, 65–70. [Google Scholar] [CrossRef]
- Antov, P.; Jivkov, V.; Savov, V.; Simeonova, R.; Yavorov, N. Structural application of eco-friendly composites from recycled wood fibres bonded with magnesium lignosulfonate. Appl. Sci. 2020, 10, 7526. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Krišt’ák, L.; Réh, R.; Mantanis, G.I. Eco-friendly, high-density fiberboards bonded with urea-formaldehyde and ammonium lignosulfonate. Polymers 2021, 13, 1–13. [Google Scholar]
- Chen, X.; Xi, X.; Pizzi, A.; Fredon, E.; Du, G.; Gerardin, C.; Amirou, S. Oxidized demethylated lignin as a bio-based adhesive for wood bonding. J. Adhes. 2020, 1–18. [Google Scholar] [CrossRef]
- Saražin, J.; Pizzi, A.; Amirou, S.; Schmiedl, D.; Šernek, M. Organosolv Lignin for Non-Isocyanate Based Polyurethanes (NIPU) as Wood Adhesive. J. Renew. Mater. 2021, 9, 881–907. [Google Scholar] [CrossRef]
- Antov, P.; Krišt’ák, L.; Réh, R.; Savov, V.; Papadopoulos, A.N. Eco-Friendly Fiberboard Panels from Recycled Fibers Bonded with Calcium Lignosulfonate. Polymers 2021, 13, 639. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Song, F.; Li, Q.; Xia, H.; Li, M.; Shu, X.; Zhou, Y. Recent development of cardanol based polymer materials-a review. J. Renew. Mater. 2019, 7, 601–619. [Google Scholar] [CrossRef] [Green Version]
- Tasooji, M.; Tabarsa, T.; Khazaeian, A.; Wool, R.P. Acrylated epoxidized soy oil as an alternative to urea-formaldehyde in making wheat straw particleboards. Wood Adhes. 2011, 24, 1717–1727. [Google Scholar]
- Zimele, Z.; Irbe, I.; Grinins, J.; Bikovens, O.; Verovkins, A.; Bajare, D. Novel mycelium-based biocomposites (Mbb) as building materials. J. Renew. Mater. 2020, 8, 1067–1076. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethane Volume 2; Smithers Rapra Technology Ltd.: Shrewsbury, UK, 2016; Volume 2, ISBN 978-1-84735-035-0. [Google Scholar]
- More, A.S.; Lebarbé, T.; Maisonneuve, L.; Gadenne, B.; Alfos, C.; Cramail, H. Novel fatty acid based di-isocyanates towards the synthesis of thermoplastic polyurethanes. Eur. Polym. J. 2013, 49, 823–833. [Google Scholar] [CrossRef]
- Palaskar, D.V.; Boyer, A.; Cloutet, E.; Alfos, C.; Cramail, H. Synthesis of biobased polyurethane from oleic and ricinoleic acids as the renewable resources via the AB-type self-condensation approach. Biomacromolecules 2010, 11, 1202–1211. [Google Scholar] [CrossRef]
- Claeys, B.; Vervaeck, A.; Hillewaere, X.K.; Possemiers, S.; Hansen, L.; De Beer, T.; Remon, J.P.; Vervaet, C. Thermoplastic Polyurethanes for the Manufacturing of Highly Dosed Oral Sustained Release Matrices via Hot Melt Extrusion and Injection Molding. Eur. J. Pharm. Biopharm. 2015, 90, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Unverferth, M.; Kreye, O.; Prohammer, A.; Meier, M.A.R. Renewable Non-Isocyanate Based Thermoplastic Polyurethanes via Polycondensation of Dimethyl Carbamate Monomers with Diols. Macromol. Rapid Commun. 2013, 34, 1569–1574. [Google Scholar] [CrossRef]
- Lingier, S.; Espeel, P.; Suarez, S.S.; Türünç, O.; De Wildeman, S.; Du Prez, F.E. Renewable thermoplastic polyurethanes containing rigid spiroacetal moieties. Eur. Polym. J. 2015, 70, 232–239. [Google Scholar] [CrossRef]
- Charlon, M.; Heinrich, B.; Matter, Y.; Couzigné, E.; Donnio, B.; Avérous, L. Synthesis, structure and properties of fully biobased thermoplastic polyurethanes, obtained from a diisocyanate based on modified dimer fatty acids, and different renewable diols. Eur. Polym. J. 2014, 61, 197–205. [Google Scholar] [CrossRef]
- Johnson, T.J.; Gupta, K.M.; Fabian, J.; Albright, T.H.; Kiser, P.F. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 2010, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Takács, E.; Krakovský, I.; Horváth, Z.E.; Rosta, L.; Almásy, L. Study on the microstructure of polyester polyurethane irradiated in air and water. Polymers 2015, 7, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.; Wei, R.; Oeser, T.; Dedavid e Silva, L.A.; Breite, D.; Schulze, A.; Zimmermann, W. Degradation of polyester polyurethane by bacterial polyester hydrolases. Polymers 2017, 9, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokharkar, V.; Sivaram, S. Poly(alkylene carbonate)s by the carbonate interchange reaction of aliphatic diols with dimethyl carbonate: Synthesis and characterization. Polymer 1995, 36, 4851–4854. [Google Scholar] [CrossRef]
- Tanaka, H.; Kunimura, M. Mechanical Properties of Thermoplastic Polyurethanes. Polym. Eng. Sci. 2002, 42, 1333–1349. [Google Scholar] [CrossRef]
- Vogels, R.R.M.; Lambertz, A.; Schuster, P.; Jockenhoevel, S.; Bouvy, N.D.; Disselhorst-Klug, C.; Neumann, U.P.; Klinge, U.; Klink, C.D. Biocompatibility and biomechanical analysis of elastic TPU threads as new suture material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Rogulska, M.; Kultys, A.; Pikus, S. The effect of chain extender structure on the properties of new thermoplastic poly(carbonate–urethane)s derived from MDI. J. Therm. Anal. Calorim. 2017, 127, 2325–2339. [Google Scholar] [CrossRef] [Green Version]
- Kull, K.L.; Bass, R.W.; Craft, G.; Julien, T.; Marangon, E.; Marrouat, C.; Harmon, J.P. Synthesis and characterization of an ultra-soft poly(carbonate urethane). Eur. Polym. J. 2015, 71, 510–522. [Google Scholar] [CrossRef]
- Foy, E.; Farrell, J.B.; Higginbotham, C. Synthesis of Linear Aliphatic Polycarbonate Macroglycols Using Dimethylcarbonate. J. Appl. Polym. Sci. 2009, 111, 217–227. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, Y.; Kang, M.; Wang, J.; Wang, X.; Feng, Y.; Yin, N.; Li, Q. The effects of the molecular weight and structure of polycarbonatediols on the properties of waterborne polyurethanes. Prog. Org. Coat. 2015, 82, 46–56. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Meijs, G.F.; Mccarthy, S.J.; Adhikari, R.; Sherriff, N. Synthesis and characterization of a series of poly(alkylene carbonate) macrodiols and the effect of their structure on the properties of polyurethanes. J. Appl. Polym. Sci. 1998, 69, 1621–1633. [Google Scholar] [CrossRef]
- Kojio, K.; Nonaka, Y.; Masubuchi, T.; Furukawa, M. Effect of the composition ratio of copolymerized poly(carbonate) glycol on the microphase-separated structures and mechanical properties of polyurethane elastomers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 4448–4458. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, M.; Ti, Y.; Wang, B. Study on structure and performance of polycarbonate urethane synthesized via different copolymerization methods. J. Mater. Sci. 2007, 42, 5508–5515. [Google Scholar] [CrossRef]
- Kojio, K.; Furukawa, M.; Motokucho, S.; Shimada, M.; Sakai, M. Structure-mechanical property relationships for polycarbonate urethane elastomers with novel soft segments. Macromolecules 2009, 42, 8322–8327. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M.; Pikus, S.; Skrzypiec, K. The synthesis and characterization of new thermoplastic poly(carbonate-urethane) elastomers derived from HDI and aliphatic-aromatic chain extenders. Eur. Polym. J. 2009, 45, 2629–2643. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, Y.; Zhang, Z.; Ma, D.; Wang, X. Synthesis of polycarbonate urethane elastomers and effects of the chemical structures on their thermal, mechanical and biocompatibility properties. Heliyon 2016, 2, e00125. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Jing, M.; Liu, Z.; Dong, P.; Liu, T.; Fu, Q. Microfibrillated cellulose reinforced bio-based poly(propylene carbonate) with dual-responsive shape memory properties. RSC Adv. 2016, 6, 7560–7567. [Google Scholar] [CrossRef]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Singhal, P.; Small, W.; Cosgriff-Hernandez, E.; Maitland, D.J.; Wilson, T.S. Low density biodegradable shape memory polyurethane foams for embolic biomedical applications. Acta Biomater. 2014, 10, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Hodlur, R.M.; Rabinal, M.K. Self assembled graphene layers on polyurethane foam as a highly pressure sensitive conducting composite. Compos. Sci. Technol. 2014, 90, 160–165. [Google Scholar] [CrossRef]
- Kang, S.M.; Kwon, S.H.; Park, J.H.; Kim, B.K. Carbon nanotube reinforced shape memory polyurethane foam. Polym. Bull. 2013, 70, 885–893. [Google Scholar] [CrossRef]
- Liu, H.D.; Liu, Z.Y.; Yang, M.B.; He, Q. Surperhydrophobic polyurethane foam modified by graphene oxide. J. Appl. Polym. Sci. 2013, 130, 3530–3536. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, J.; Zhu, K.; Li, J. Electro-mechanical performance of polyurethane dielectric elastomer flexible micro-actuator composite modified with titanium dioxide-graphene hybrid fillers. Mater. Des. 2016, 90, 1069–1076. [Google Scholar] [CrossRef]
- Li, T.T.; Dai, W.; Huang, S.Y.; Wang, H.; Lin, Q.; Lou, C.W.; Lin, J.H. Preparation and characterization of SEBS-g-MAH-filled flexible polyurethane foam composites with gradient-changing structure. Mater. Des. 2019, 183, 108150. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, S.; Editors, K. Energy Sustainability through Green Energy in Green Energy and Technology; Springer India: Rae Bareli, India, 2015; ISBN 8132223373. [Google Scholar]
- Heinen, M.; Gerbase, A.E.; Petzhold, C.L. Vegetable oil-based rigid polyurethanes and phosphorylated flame-retardants derived from epoxydized soybean oil. Polym. Degrad. Stab. 2014, 108, 76–86. [Google Scholar] [CrossRef]
- Arniza, M.Z.; Hoong, S.S.; Idris, Z.; Yeong, S.K.; Hassan, H.A.; Din, A.K.; Choo, Y.M. Synthesis of transesterified palm olein-based Polyol and rigid polyurethanes from this polyol. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 243–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, V.B.; Menger, R.K.; Forte, M.M.D.C.; Petzhold, C.L. Rigid Polyurethane Foam Based on Modified Vegetable Oil. J. Appl. Polym. Sci. 2011, 120, 530–537. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Z.; Zhang, J.; Chen, S.; Zhou, Y. Effects of a novel phosphorus-nitrogen flame retardant on rosin-based rigid polyurethane foams. Polym. Degrad. Stab. 2015, 120, 427–434. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Chen, S.; Zhou, Y. Synthesis and fire properties of rigid polyurethane foams made from a polyol derived from melamine and cardanol. Polym. Degrad. Stab. 2014, 110, 27–34. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols. Ind. Crops Prod. 2014, 52, 380–388. [Google Scholar] [CrossRef]
- Jaudouin, O.; Robin, J.J.; Lopez-Cuesta, J.M.; Perrin, D.; Imbert, C. Ionomer-based polyurethanes: A comparative study of properties and applications. Polym. Int. 2012, 61, 495–510. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Dou, S.; Colby, R.H.; Runt, J. Molecular mobility, ion mobility and mobile ion concentration in polyethylene oxide-based polyurethane ionomers. Macromolecules 2008, 41, 5723–5728. [Google Scholar] [CrossRef]
- Gu, S.; Yan, B.; Liu, L.; Ren, J. Carbon nanotube-polyurethane shape memory nanocomposites with low trigger temperature. Eur. Polym. J. 2013, 49, 3867–3877. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Sonseca, A.; Gimenez, E.; Marcos-Fernandez, A.; Kenny, J.M. Synthesis and characterization of PCL-PLLA polyurethane with shape memory behavior. Eur. Polym. J. 2013, 49, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Hayashi, N.; Hayashi, S. Structure and properties of shape-memory polyurethane block copolymers. J. Appl. Polym. Sci. 1996, 60, 1061–1069. [Google Scholar] [CrossRef]
- Takahara, A.; Hergenrother, R.W.; Coury, A.J.; Coopert, S.L. Effect of soft segment chemistry on the biostability degradation and lipid sorption. Biomed. Mater. Res. 1992, 26, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Brostowitz, N.R.; Cavicchi, K.A.; Weiss, R.A. Perspective: Ionomer research and applications. Macromol. React. Eng. 2014, 8, 81–99. [Google Scholar] [CrossRef]
- Airinei, A.; Buruiana, E.C. Polyurethane Cationomers Containing an- Thryl and Nitroaromatic. Eur. Polym. J. 1997, 33, 877–880. [Google Scholar]
- Charnetskaya, A.G.; Polizos, G.; Shtompel, V.I.; Privalko, E.G.; Kercha, Y.Y.; Pissis, P. Phase morphology and molecular dynamics of a polyurethane ionomer reinforced with a liquid crystalline filler. Eur. Polym. J. 2003, 39, 2167–2174. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Choi, K.F.; Meng, Q.; Chen, S.; Yeung, K.W. Shape memory effect and reversible phase crystallization process in SMPU ionomer. Polym. Adv. Technol. 2008, 19, 328–333. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Lu, J.; Yeung, L.Y.; Yeung, K.W. Shape memory fiber spun with segmented polyurethane ionomer. Polym. Adv. Technol. 2008, 19, 1745–1753. [Google Scholar] [CrossRef]
- Raasch, J.; Ivey, M.; Aldrich, D.; Nobes, D.S.; Ayranci, C. Characterization of polyurethane shape memory polymer processed by material extrusion additive manufacturing. Addit. Manuf. 2015, 8, 132–141. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhao, Y.; Purnawali, H.; Huang, W.M.; Sun, L. Chemically induced morphing in polyurethane shape memory polymer micro fibers/springs. React. Funct. Polym. 2012, 72, 757–764. [Google Scholar] [CrossRef]
- Casado, U.M.; Quintanilla, R.M.; Aranguren, M.I.; Marcovich, N.E. Composite films based on shape memory polyurethanes and nanostructured polyaniline or cellulose-polyaniline particles. Synth. Met. 2012, 162, 1654–1664. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.X.; Huang, W.B.; Yang, J.H.; Wang, Y.; Zhou, Z.W.; Zhang, J.H. Carbon nanotube network structure induced strain sensitivity and shape memory behavior changes of thermoplastic polyurethane. Mater. Des. 2015, 69, 105–113. [Google Scholar] [CrossRef]
- Li, Y.; Nakamura, N.; Wang, Y. Synthesis and hemocompatibilities of new segmented polyurethanes and poly (urethane urea) s with poly (butadiene) and phosphatidylcholine analogues in the main. Chem. Mater. 1997, 4756, 1570–1577. [Google Scholar] [CrossRef]
- Chen, G.N.; Chen, K.N. Self-curing behaviors of single pack aqueous-based polyurethane system. J. Appl. Polym. Sci. 1997, 63, 1609–1623. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.Y.; Dai, J.B.; Li, W.H. Synthesis and characterization of yellow water-borne polyurethane using a diol colorant as extender. Chin. Chem. Lett. 2010, 21, 143–145. [Google Scholar] [CrossRef]
- Huber, J.; Mecking, S. Aqueous poly(arylacetylene) dispersions. Macromolecules 2010, 43, 8718–8723. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Zhou, X.; Yu, Q.; Liu, S.; Guo, D.; Yu, R.; Hu, J. Synthesis and characterization of low crystalline waterborne polyurethane for potential application in water-based ink binder. Prog. Org. Coat. 2014, 77, 61–71. [Google Scholar] [CrossRef]
- Peng, S.J.; Jin, Y.; Cheng, X.F.; Sun, T.B.; Qi, R.; Fan, B.Z. A new method to synthesize high solid content waterborne polyurethanes by strict control of bimodal particle size distribution. Prog. Org. Coat. 2015, 86, 1–10. [Google Scholar] [CrossRef]
- Chu, F.; Guyot, A. High solids content latexes with low viscosity. Colloid Polym. Sci. 2001, 279, 361–367. [Google Scholar] [CrossRef]
- Ai, Z.; Deng, R.; Zhou, Q.; Liao, S.; Zhang, H. High solid content latex: Preparation methods and application. Adv. Colloid Interface Sci. 2010, 159, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Liu, Z.; Li, W.; Dai, J. Studies on waterborne polyurethanes based on new medium length fluorinated diols. J. Fluor. Chem. 2015, 175, 12–17. [Google Scholar] [CrossRef]
- Fan, W.; Du, W.; Li, Z.; Dan, N.; Huang, J. Abrasion resistance of waterborne polyurethane films incorporated with PU/silica hybrids. Prog. Org. Coat. 2015, 86, 125–133. [Google Scholar] [CrossRef]
- Fu, C.; Zheng, Z.; Yang, Z.; Chen, Y.; Shen, L. A fully bio-based waterborne polyurethane dispersion from vegetable oils: From synthesis of precursors by thiol-ene reaction to study of final material. Prog. Org. Coat. 2014, 77, 53–60. [Google Scholar] [CrossRef]
- Baysal, G.; Kasapbası, E. Polyurethanes and Usage Areas. Global J. Sc. Front. Res. B Chem. 2017, 17, 1–9. [Google Scholar]
- Bîrca, A.; Gherasim, O.; Grumezescu, V.; Grumezescu, A.M. Introduction in thermoplastic and thermosetting polymers in Materials for Biomedical Engineering. Thermoset Thermoplast. Polym. 2019, 1–28. [Google Scholar] [CrossRef]
- Mohammed, I.A.; Al-Mulla, E.A.J.; Kadar, N.K.A.; Ibrahim, M. Structure-property studies of thermoplastic and thermosetting polyurethanes using palm and soya oils-based polyols. J. Oleo Sci. 2013, 62, 1059–1072. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, H. Chemistry and Technology of Isocyanate. J. Appl. Toxicol. 1997, 17, 195. [Google Scholar]
- Sharmin, E.; Zafar, F. Polyurethane: An Introduction. In Polyurethane; IntechOpen: London, UK, 2012; pp. 3–16. ISBN 978-953-51-6229-2. [Google Scholar]
- Rokicki, G.; Piotrowska, A. A new route to polyurethanes from ethylene carbonate, diamines and diols. Polymer 2002, 43, 2927–2935. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Optimization study of lignin oxypropylation in view of the preparation of polyurethane rigid foams. Ind. Eng. Chem. Res. 2009, 48, 2583–2589. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Zhou, K.; Yuen, R.K.K.; Gui, Z.; Hu, Y.; Jiang, S. Novel CuCo2O4/graphitic carbon nitride nanohybrids: Highly effective catalysts for reducing CO generation and fire hazards of thermoplastic polyurethane nanocomposites. J. Hazard. Mater. 2015, 293, 87–96. [Google Scholar] [CrossRef]
- Parcheta, P.; Datta, J. Environmental impact and industrial development of biorenewable resources for polyurethanes. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1986–2016. [Google Scholar] [CrossRef]
- Blattmann, H.; Fleischer, M.; Bähr, M.; Mülhaupt, R. Isocyanate- and phosgene-free routes to polyfunctional cyclic carbonates and green polyurethanes by fixation of carbon dioxide. Macromol. Rapid Commun. 2014, 35, 1238–1254. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B. A Perspective Approach to Sustainable Routes for Non-Isocyanate Polyurethanes. Eur. Polym. J. 2016, 87, 535–552. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.; Boutevin, B.; Lyon, F.G. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Figovsky, O.; Shapovalov, L.; Leykin, A.; Birukova, O.; Potashnikova, R. Recent advances in the development of non-isocyanate polyurethanes based on. PU Mag. 2013, 10, 1–9. [Google Scholar]
- Figovsky, O.; Shapovalov, L.; Leykin, A.; Birukova, O.; Potashnikova, R. Progress in Elaboration of Nonisocyanate Polyurethanes Based on Cyclic Carbonates. Int. Lett. Chem. Phys. Astron. 2013, 3, 52–66. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lamarzelle, O.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-Free Routes to Polyurethanes and Poly(hydroxy Urethane)s. Chem. Rev. 2015, 115, 12407–12439. [Google Scholar] [CrossRef] [Green Version]
- Dyer, E.; Scott, H. The Preparation of Polymeric and Cyclic Urethans and Ureas from Ethylene Carbonate and Amines. J. Am. Chem. Soc. 1957, 79, 672–675. [Google Scholar] [CrossRef]
- Ochiai, B.; Utsuno, T. Non-isocyanate synthesis and application of telechelic polyurethanes via polycondensation of diurethanes obtained from ethylene carbonate and diamines. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 525–533. [Google Scholar] [CrossRef]
- Drechsel, E.K.; Groszos, S.J. Method of Preparing A Polyurethane. U.S. Patent No. 2,802,022, 6 August 1957. [Google Scholar]
- Javni, I.; Hong, D.P.; Petrović, Z.S. Polyurethanes from soybean oil, aromatic, and cycloaliphatic diamines by nonisocyanate route. J. Appl. Polym. Sci. 2013, 128, 566–571. [Google Scholar] [CrossRef]
- Hahn, C.; Keul, H.; Möller, M. Hydroxyl-functional polyurethanes and polyesters: Synthesis, properties and potential biomedical application. Polym. Int. 2012, 61, 1048–1060. [Google Scholar] [CrossRef]
- Fleischer, M.; Blattmann, H.; Mülhaupt, R. Glycerol-, pentaerythritol- and trimethylolpropane-based polyurethanes and their cellulose carbonate composites prepared via the non-isocyanate route with catalytic carbon dioxide fixation. Green Chem. 2013, 15, 934–942. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environment impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.L.; Guerrero-Ramírez, G.L.; Vilas-Vilela, J.L. Lignin-based hydrogels: Synthesis and applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef] [Green Version]
- Falah, F.; Lubis, M.A.R.; Triastuti, T.; Fatriasari, W.; Sari, F.P. Utilization of Lignin from the Waste of Bioethanol Production as a Mortar Additive. J. Sylva Lestari 2020, 8, 326. [Google Scholar] [CrossRef]
- Aristri, M.A.; Lubis, M.A.R.; Laksana, R.P.B.; Fatriasari, W.; Ismayati, M.; Wulandari, A.P.; Ridho, M.R. Bio-Polyurethane Resins Derived from Liquid Fractions of Lignin for the Modification of Ramie Fibers. J. Sylva Lestari 2021, 1–16, (Article in press). Available online: http://jurnal.fp.unila.ac.id/index.php/JHT/article/view/4832/3472 (accessed on 6 May 2021).
- Solihat, N.N.; Sari, F.P.; Falah, F.; Ismayati, M.; Lubis, M.A.R.; Fatriasari, W.; Santoso, E.B.; Syafii, W. Lignin as an Active Biomaterial: A Review. J. Sylva Lestari 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Frihart, C.R. Wood Adhesion and Adhesives. In Handbook of Wood Chemistry and Wood Composites; CRC Press: London, UK, 2013; Chapter 9; ISBN 978-1-4398-5380-1. [Google Scholar]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Bio-Renewable Sources for Synthesis of Eco-Friendly Polyurethane Adhesives—Review. Open J. Polym. Chem. 2017, 7, 57–75. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, A.W.; Vick, C.B.; Okkonen, E.A. Enhanced Durability of One-Part Polyurethane Bonds to Wood Due to the Use of HMR Primer. In Proceedings of the Forest Produtcs Society Annual Meeting, South Lake Tahoe, NV, USA, 2001; pp. 489–494. Available online: https://www.fpl.fs.fed.us/documnts/pdf2000/chris00d.pdf (accessed on 6 May 2021).
- Rowell, R.M. Moisture Properties in Handbook of Wood Chemistry and Wood Composites; CRC Press: London, UK, 2012; ISBN 9781439853818. [Google Scholar]
- Lima García, J.; Pans, G.; Phanopoulos, C. Use of lignin in polyurethane-based structural wood adhesives. J. Adhes. 2018, 94, 814–828. [Google Scholar] [CrossRef]
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels-A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Chahar, S.; Dastidar, M.G.; Choudhary, V.; Sharma, D.K. Synthesis and characterisation of polyurethanes derived from waste black liquor lignin. J. Adhes. Sci. Technol. 2004, 18, 169–179. [Google Scholar] [CrossRef]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-based polyurethane prepared from Kraft lignin and modified castor oil. Express Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Saraf, V.P.; Wolfgang, G.G.; Garth, L.W. Engineering plastics from lignin. VII. Structure property relationships of poly (butadiene glycol)-containing polyurethane networks. J. Appl. Polym. Sci. 1985, 30, 3809–3823. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Lang, J.M.; Shrestha, U.M.; Dadmun, M. The effect of Plant source on the Properties of lignin-Based Polyurethanes. Front. Energy Res. 2018, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Fast Curing Bio-Based Phenolic Resins via Lignin Demethylated under Mild Reaction Condition. Polym. Int. 2017, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Kuai, J.; Pan, H.; Wang, N.; Zhu, X. Study on the demethylation of enzymatic hydrolysis lignin and the properties of lignin—epoxy resin blends. Wood Sci. Technol. 2018, 52, 1343–1357. [Google Scholar] [CrossRef]
- Zhou, H.; Qiu, X.; Yang, D.; Xie, S. Laccase and Xylanase Incubation Enhanced the Sulfomethylation Reactivity of Alkali Lignin. ACS Sustain. Chem. Eng. 2016, 4, 1248–1254. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Yan, N.; Zhang, R.; Li, J. Demethylation of wheat straw alkali lignin for application in phenol formaldehyde adhesives. Polymers 2016, 8, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upton, B.M.; Kasko, A.M. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2018, 116, 2275–2306. [Google Scholar] [CrossRef]
- Mohammad, Z.; Muhammad, Z.; Aqdas, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar]
- Podschun, J.; Stu, A.; Buchholz, R.I.; Heitmann, M.; Schreiber, A.; Saake, B.; Lehnen, R. Phenolated Lignins as Reactive Precursors in Wood Veneer and Particleboard Adhesion. Ind. Eng. Chem. Res. 2016, 55, 5231–5237. [Google Scholar] [CrossRef]
- Zhao, M.; Jing, J.; Zhu, Y.; Yang, X.; Wang, X.; Wang, Z. Preparation and performance of lignin-phenol-formaldehyde adhesives. Int. J. Adhes. Adhes. 2016, 64, 163–167. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2007, 27, 202–207. [Google Scholar] [CrossRef]
- Gonc, A.R. Hydroxymethylation and oxidation of Organosolv lignins and utilization of the products. Bioresour. Technol. 2001, 79, 103–111. [Google Scholar]
- Akim, L.G.; Shevchenko, S.M.; Zarubin, M.Y.; Petersburg, S. 13 C NMR studies on lignins depolymerized with dry hydrogen iodide. Wood Sci. Technol. 1993, 248, 241–248. [Google Scholar]
- Chung, H.; Washburn, N.R. Improved Lignin Polyurethane Properties with Lewis Acid Treatment. Appl. Mater. Interfaces 2012, 4, 2840–2846. [Google Scholar] [CrossRef] [Green Version]
- Linyou, A.; Brian, Z.; Leonard, M.R.; Christopher, L.; Dekker, R.F.H.; Malek, L. Fungal demethylation of Kraft lignin. Enzyme Microb. Technol. 2015, 73, 44–50. [Google Scholar]
- Riley, R.; Salamov, A.A.; Brown, D.W.; Nagy, L.G.; Floudas, D.; Held, B.W.; Grigoriev, I. V Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. USA 2014, 111, 9923–9928. [Google Scholar] [CrossRef] [Green Version]
- Pizzi, A. Tannin-based polyurethane adhesives. J. Appl. Polym. Sci. 1979, 23, 1889–1891. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Park, B.D.; Hong, M.K. Tuning of adhesion and disintegration of oxidized starch adhesives for the recycling of medium density fiberboard. BioResources 2020, 15, 5156–5178. [Google Scholar]
- Frihart, C.R.; Lorenz, L.F. Speci fi c Oxidants Improve the Wood Bonding Strength of Soy and Other Plant Flours. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1017–1023. [Google Scholar] [CrossRef]
- Frihart, C.R.; Pizzi, A.; Xi, X.; Lorenz, L.F. Reactions of Soy Flour and Soy Protein by Specific Oxidation. Polymers 2019, 11, 1478. [Google Scholar] [CrossRef] [Green Version]
- Kim, U.; Kuga, S.; Wada, M.; Okano, T.; Kondo, T. Periodate Oxidation of Crystalline Cellulose. Biomacromolecules 2000, 1, 488–492. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Kasbe, P.S.; Mahanwar, P.A.; Gadekar, P.T. Synthesis and characterization of lignin-polyurethane based wood adhesive. Int. J. Adhes. Adhes. 2019, 95, 102427. [Google Scholar] [CrossRef]
- Heinrich, L.A.; Heinrich, L.A. Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef] [Green Version]
- Xi, X.; Wu, Z.; Pizzi, A.; Gerardin, C.; Lei, H.; Zhang, B.; Du, G. Non-isocyanate polyurethane adhesive from sucrose used for particleboard. Wood Sci. Technol. 2019, 53, 393–405. [Google Scholar] [CrossRef]
- Tudor, E.M.; Dettendorfer, A.; Kain, G.; Barbu, M.C.; Réh, R.; Krišt’ák, L. Sound-absorption coefficient of bark-based insulation panels. Polymers 2020, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Tudor, E.M.; Scheriau, C.; Barbu, M.C.; Réh, R.; Krišt’ák, L.; Schnabel, T. Enhanced resistance to fire of the bark-based panels bonded with clay. Appl. Sci. 2020, 10, 5594. [Google Scholar] [CrossRef]
- Réh, R.; Igaz, R.; Krišt’ák, L.; Ružiak, I.; Gajtanska, M.; Božíková, M.; Kučerka, M. Functionality of beech bark in adhesive mixtures used in plywood and its effect on the stability associated with material systems. Materials 2019, 12, 1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merle, J.; Birot, M.; Deleuze, H.; Mitterer, C.; Carré, H.; Bouhtoury, F.C. El New biobased foams from wood byproducts. Mater. Des. 2016, 91, 186–192. [Google Scholar] [CrossRef]
- Esmaeili, N.; Zohuriaan-Mehr, M.J.; Salimi, A.; Vafayan, M.; Meyer, W. Tannic acid derived non-isocyanate polyurethane networks: Synthesis, curing kinetics, antioxidizing activity and cell viability. Thermochim. Acta 2018, 664, 64–72. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-Based Wood Adhesives in Wood Adhesives Chemistry and Technology; Dekker, M., Ed.; CRC Press: New York, NY, USA, 1983; Volume 1. [Google Scholar]
- Valenzuela, J.; Von Leyser, E.; Pizzi, A.; Westermeyer, C.; Gorrini, B. Industrial production of pine tannin-bonded particleboard and MDF. Eur. J. Wood Wood Prod. 2012, 70, 735–740. [Google Scholar] [CrossRef]
- Jahanshahi, S.; Pizzi, A.; Abdulkhani, A.; Doosthoseini, K.; Shakeri, A.; Lagel, M.C.; Delmotte, L. MALDI-TOF, 13C NMR and FT-MIR analysis and strength characterization of glycidyl ether tannin epoxy resins. Ind. Crops Prod. 2016, 83, 177–185. [Google Scholar] [CrossRef]
- Benyahya, S.; Aouf, C.; Caillol, S.; Boutevin, B.; Pascault, J.P.; Fulcrand, H. Functionalized green tea tannins as phenolic prepolymers for bio-based epoxy resins. Ind. Crops Prod. 2014, 53, 296–307. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-Based Adhesives. J. Macromol. Sci. Part C 1980, 18, 247–315. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-based biofoams-A review. J. Renew. Mater. 2019, 7, 477–492. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, H.R.; Navarrete, P.; Pizzi, A.; Tapin-Lingua, S.; Benjelloun-Mlayah, B.; Pasch, H.; Rigolet, S. Synthetic-resin-free wood panel adhesives from mixed low molecular mass lignin and tannin. Eur. J. Wood Wood Prod. 2011, 69, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Ghahri, S.; Pizzi, A.; Mohebby, B.; Mirshokraie, A.; Mansouri, H.R. Soy-based, tannin-modified plywood adhesives. J. Adhes. 2018, 94, 218–237. [Google Scholar] [CrossRef]
- Abdullah, U.H.B.; Pizzi, A. Tannin-furfuryl alcohol wood panel adhesives without formaldehyde. Eur. J. Wood Wood Prod. 2013, 71, 131–132. [Google Scholar] [CrossRef]
- Pizzi, A.; Meikleham, N.; Stephanou, A. Induced accelerated autocondensation of polyflavonoid tannins for phenolic polycondensates. II. Cellulose effect and application. J. Appl. Polym. Sci. 1995, 55, 929–933. [Google Scholar] [CrossRef]
- Garcia, R.; Pizzi, A.; Merlin, A. Ionic polycondensation effects on the radical autocondensation of polyflavonoid tannins: An ESR study. J. Appl. Polym. Sci. 1997, 65, 2623–2633. [Google Scholar] [CrossRef]
- Garcia, R.; Pizzi, A. Polycondensation and autocondensation networks in polyflavonoid tannins. II. Polycondensation versus autocondensation. J. Appl. Polym. Sci. 1998, 70, 1093–1110. [Google Scholar] [CrossRef]
- Garcia, R.; Pizzi, A. Polycondensation and autocondensation networks in polyflavonoid tannins. I. Final networks. J. Appl. Polym. Sci. 1998, 70, 1083–1091. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and actual industrial applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojabri, L.; Kong, X.; Narine, S.S. Fatty Acid-Derived Diisocyanate and Biobased Polyurethane Produced from Vegetable Oil: Synthesis, Polymerization, and Characterization. Biomacromolecules 2009, 10, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Cornille, A.; Dworakowska, S.; Bogdal, D.; Boutevin, B.; Caillol, S. A new way of creating cellular polyurethane materials: NIPU foams. Eur. Polym. J. 2015, 66, 129–138. [Google Scholar] [CrossRef]
- Camara, F.; Benyahya, S.; Besse, V.; Boutevin, G.; Boutevin, B.; Caillol, S. Reactivity of secondary amines for the synthesis of Non Isocyanate Polyurethanes. Eur. Polym. J. 2014, 55, 17–26. [Google Scholar] [CrossRef]
- Cornille, A.; Guillet, C.; Benyahia, S.; Negrell, C. Room temperature flexible isocyanate-free polyurethane foams. Eur. Polym. J. 2016, 84, 873–888. [Google Scholar] [CrossRef]
- Cornille, A.; Michaud, G.; Simon, F.; Fouquay, S.; Auvergne, R.; Boutevin, B.; Caillol, S. Promising mechanical and adhesive properties of isocyanate-free poly (hydroxyurethane). Eur. Polym. J. 2016, 84, 404–420. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Zhou, Q. Waterborne isocyanate-free polyurethane epoxy hybrid coatings synthesized from sustainable fatty acid diamine. Green Chem. 2020, 22, 1329–1337. [Google Scholar] [CrossRef]
- Parcheta, P.; Datta, J. Structure analysis and thermal degradation characteristics of bio-based poly (propylene succinate) s obtained by using different catalyst amounts. J. Therm. Anal. Calorim. 2017, 130, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Schrock, A.K.; Hamilton, H.S.C.; Johnson, N.D.; Thompson, B.D.; Ulrich, K.; Coggio, W.D. Thermal characterization and crystallization kinetics of polyester polyols derived from adipic acid and bio-based succinic acid with 1, 4- butanediol and 1, 6-hexanediol. Polymer 2016, 101, 233–240. [Google Scholar] [CrossRef]
- Jacquel, N.; Saint-loup, R.; Pascault, J.; Rousseau, A.; Fenouillot, F. Bio-based alternatives in the synthesis of aliphatic-aromatic polyesters dedicated to biodegradable film applications. Polymer 2015, 59, 234–242. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.; Gruter, G.J.M.; Coelho, J.F.; Silvestre, A.J. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Papageorgiou, M.; Bikiaris, D.N. Evaluation of polyesters from renewable resources as alternatives to the current fossil-based polymers Phase transitions of poly (butylene). Polymer 2014, 55, 3846–3858. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2, 5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Mou, Z.; Chen, E.Y. Polyesters and Poly (ester-urethane) s from Bio-based Difuranic Polyols. ACS Sustain. Chem. Eng. 2016, 4, 7118–7129. [Google Scholar] [CrossRef]
- Muo, Z.; Feng, S.K.; Chen, E.Y.X. Bio-based difuranic polyol monomers and their derived linear and cross-linked polyurethanes. Polym. Chem. 2016, 7, 1593–1602. [Google Scholar] [CrossRef]
- Çaylı, G.; Küsefoğlu, S. A Simple One-Step Synthesis and Polymerization of Plant Oil Triglyceride Iodo Isocyanates. J. Appl. Polym. Sci. 2010, 116, 2433–2440. [Google Scholar] [CrossRef]



| Type of Polyurethane (PU) | |||||
|---|---|---|---|---|---|
| Thermoplastic PU | Flexible PU | Rigid PU | PU Ionomer | Water-Borne PU | Thermosetting PUs |
| Keyboard protector, external cases of mobile equipment, car instrument panels, casters, power equipment, sporting goods, medical equipment, drive belts, boots, inflatable rafts, and a wide range of extruded film, sheets of paper, and profile applications. | Cushion materials, carpet underlays, furniture bedding, automotive interior parts, packaging, biomedicine, and nanocomposites. | Thermal and sound insulators. | Artificial hearts, connector tubing for heart pacemakers, and haemodialysis tubes. | Coatings, adhesives, sealant, binders. | Beds, quilts, packaging materials, isolation materials, footbed, fender, door panel, vehicle exterior tire, seal, car bumper or synthetic leather. |
| Parameter | Lignin- and Tannin-Based NIPUs Adhesives | Other Wood Adhesives | References |
|---|---|---|---|
| Bonding properties | High delamination resistance, adhesion, and cohesion strength. Tensile strength and deformation are comparable to traditional isocyanate PUs | UF, MF, MUF give over penetration in wood, lower tear resistance, and lower adhesion compared to NIPUs | [150,152,154,171] |
| Physical properties | Comparable water resistance and dimensional stability to isocyanate PUs | Lower water resistance of formaldehyde-based adhesives compared to NIPUs | [147,148,149,150,151,152,153] |
| Mechanical properties | High value of hard to soft ratio resulting in satisfactory mechanical properties | Mechanical properties are lower than NIPUs adhesives | [14,15,16] |
| Chemical properties | Having greater chemical resistance for about 30–50% than other adhesives | Lower chemical resistance and permeability than NIPUs adhesives, except for isocyanates | [150,152,154,171] |
| Thermal properties | Thermal stability enhancement owing to the presence of aromatic lignin and tannin | Lower thermal stability than NIPUs, except for isocyanates | [14,15,16,149,150,151,152,153,154,155] |
| Renewability | Derived from renewable biomass of lignin and tannin | Derived from petroleum that is not renewable | [150,152,154,171] |
| Toxicity | Less toxicity due to being isocyanate free | Release formaldehyde and contain isocyanate that are carcinogenic | [150,152,154,171] |
| Price | Cheaper than isocyanate PUs, but still more expensive that those of formaldehyde-based resins | Isocyanates are expensive, but formaldehyde-based resins are cheaper | [14,15,16,150,152,154,171] |
| Cross-Linker | Type of Wood Composite | Bonding Strength (MPa) | References |
|---|---|---|---|
| Na2SO3 | Plywood | 1.10 | [199] |
| KH560 (Silane coupling agent) | Particleboard | 1.06 | [200] |
| NaIO4 | Plywood | 1.72 | [70] |
| Hexamine | Particleboard | 0.77 | [71] |
| Cross-Linker | Products | Bonding Strength (MPa) |
|---|---|---|
| 1% Cellulose Nanofibers | Particleboard | 0.96 |
| 2% Cellulose Nanofibers | Particleboard | 0.98 |
| 3% Cellulose Nanofibers | Particleboard | 0.86 |
| Hexamine | Medium Density Fibreboard | 0.15 |
| Hexamine pH 9 | Medium Density Fibreboard | 0.45 |
| Hexamine pH 10 | Medium Density Fibreboard | 0.65 |
| Furfuryl Alcohol | Particleboard | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aristri, M.A.; Lubis, M.A.R.; Yadav, S.M.; Antov, P.; Papadopoulos, A.N.; Pizzi, A.; Fatriasari, W.; Ismayati, M.; Iswanto, A.H. Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review. Appl. Sci. 2021, 11, 4242. https://doi.org/10.3390/app11094242
Aristri MA, Lubis MAR, Yadav SM, Antov P, Papadopoulos AN, Pizzi A, Fatriasari W, Ismayati M, Iswanto AH. Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review. Applied Sciences. 2021; 11(9):4242. https://doi.org/10.3390/app11094242
Chicago/Turabian StyleAristri, Manggar Arum, Muhammad Adly Rahandi Lubis, Sumit Manohar Yadav, Petar Antov, Antonios N. Papadopoulos, Antonio Pizzi, Widya Fatriasari, Maya Ismayati, and Apri Heri Iswanto. 2021. "Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review" Applied Sciences 11, no. 9: 4242. https://doi.org/10.3390/app11094242
APA StyleAristri, M. A., Lubis, M. A. R., Yadav, S. M., Antov, P., Papadopoulos, A. N., Pizzi, A., Fatriasari, W., Ismayati, M., & Iswanto, A. H. (2021). Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review. Applied Sciences, 11(9), 4242. https://doi.org/10.3390/app11094242











