1. Introduction
Protease is added to detergents to decompose stains during laundry [
1,
2]. The protease breaks the polypeptide chain, the macromolecular protein is broken down into small molecule polypeptides or amino acids, and it is peeled from the fabric under the action of surfactant and external force [
3]. The earliest protease used for washing was trypsin, which only needs a small amount to achieve good washing results. However, trypsin is mainly extracted from animal materials [
4,
5], and the raw materials and processes have certain limitations. Thus, it has been gradually replaced with alkaline protease, which can be produced by large-scale fermentation. Current enzyme-added detergents only add alkaline protease and exhibit a single washing effect. With the large-scale production of multiple varieties of proteases, the production cost of proteases has been reduced. With the improvement of living conditions, people’s requirements for washing have increased, and new types of protein stain washing solutions are needed.
In 1963, Novozymes introduced protease, which led to a revolution in the industrial enzyme market and began a rapid expansion of detergent enzyme products. Currently, enzymes for detergents already account for 40% of all industrial enzymes. In the European and American markets, enzyme detergents already account for 80% of the detergent market, and almost all detergents are enzyme detergents in Japan. The current trend of enzymatic detergent research and development is extended from single protease to multiple enzymes, such as lipase, amylase, cellulase, mannanase, peroxidase, laccase, etc., and from a single type of stain to a comprehensive washing for multiple stains [
6]. Improving the storage stability of proteases in detergents and washing stability under high/low temperature and high alkaline/acidic conditions have also become new research hotspots. In order to meet the diverse functional requirements of the consumer market for detergents, Novozymes has developed a hybrid liquid detergent Medley solution. The enzymes used in the new Medley solution maintain stability and washing performance at high water content. Alkaline proteases have become key ingredients in detergent formulations [
7]. With the emergence of new strains, researchers have found that other proteases can be added to detergents, such as keratinase [
8]. The current problems of protease detergents are the compatibility of protease with washing auxiliaries and the stability of protease at different temperatures and pH [
9,
10].
The effect of trypsin [
11], keratinase [
12,
13], and alkaline protease [
14] on cleaning is obvious. The compound washing effect is better after the protease is proportioned. Detergents are added with different ingredients [
15], such as surfactants [
16], water-softening agents [
17], anti-redeposition agents [
18], softening agents [
19,
20], and stabilizers, to improve the washing efficiency. We selected 30 common detergent auxiliaries, matched them with protease, and compared the washing effects of different compound protease detergents, and the optimal formula was obtained. The washing power of the composite protease detergent was better than those of several common commercial detergents [
21].
2. Materials and Methods
2.1. Materials
Selected proteases included alkaline protease (5.0 × 105 U/g), weakly alkaline protease (4.8 × 105 U/g), neutral protease (1.5 × 105 U/g), acid protease (8.0 × 105 U/g), trypsin (4.0 × 103 U/g), aminopeptidase (6.0 × 104 U/g), flavourzyme (5.0 × 105 U/g), and keratinase (1.0 × 105 U/g) in food-grade solid powder form from Lonct Enzymes Co., Ltd. (Linyi, China). Protease activity is expressed as protease activity units. At 40 °C and a certain pH, the amount of enzyme required for protease to hydrolyze casein to produce 1 μg of tyrosine per minute is one unit of enzyme activity. The pH of the reaction conditions for measuring the enzymatic activity of alkaline proteases, weakly basic proteases, aminopeptidases, and keratinases was 10.5. The pH of the reaction conditions for measuring the enzymatic activity of neutral proteases, trypsin, and flavored proteases was 7.5. The pH of the reaction conditions for measuring the enzymatic activity of acidic proteases was 3.0.
Detergent builders included polyethoxylated fatty alcohols, sodium ethoxy alkyl sulfate, dodecylbenzenesulfonic acid, triethanolamine, anhydrous sodium citrate, SNS-80, each in industrial-grade (≥90%) from Research Institute of Daily Chemical Industry (Taiyuan, China). 2-morpholineethanesulfonic acid (MES) in molecular biology grade (>99%) from Coolaber. Fatty acid methyl ester ethoxylate (FMEE), alkyl glycoside (APG), layered sodium disilicate (SKS-6) from Yousuo Chemical Technology Co., Ltd. (Linyi, China), modified oil ethoxylate (SOE) from Junxin Chemical Technology Co., Ltd. (Guangzhou, China), tea saponin from Zhongye Biotechnology Co., Ltd. (Lishui, China), sodium alginate, hyaluronic acid from Boxbio Science & Technology Co., Ltd. (Beijing, China), all of the above are industrial grade (≥90%). Sodium carboxymethyl cellulose (CMC), polyvinyl alcohol type 1799 (PVA), polyethylene glycol 6000 (PEG), polyaspartic acid (PASP), silicon dioxide (SiO2), ethylenediaminetetraacetic acid tetrasodium salt (EDTA), disodium maleate were used in chemically pure forms (≥99.5%) from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Hydroxypropyl methylcellulose sodium (HPMC), hydroxyethyl cellulose sodium, and sodium polyacrylate were used in food-grade (>99%) from Best Food Additives Co., Ltd. (Zhengzhou, China). 4A zeolite was industrial-grade (≥90%) from Runfeng Synthetic Technology Co., Ltd. (Nantong, China). Sodium tartrate and sodium gluconate in food-grade (>99%) were acquired from Gukang Biological Engineering Co., Ltd. (Jinan, China). Sodium laurate in industrial-grade (≥90%) was from Longhui Chemical Co., Ltd. (Jinan, China). α-cyclodextrin, β-cyclodextrin, and γ-cyclodextrin were used in food-grade (>99%) from Youlezi Food Ingredients Co., Ltd. (Shanghai, China). Sulfobutyl-β-cyclodextrin (Captisol), methyl-β-cyclodextrin, and 2-hydroxypropyl-β-cyclodextrin were analytical reagents (≥99.9%) from Aladdin (Shanghai, China). In the experiment, the materials used to simulate protein stains were eggs and carbon powder. Blood used was porcine anticoagulated whole blood. Double distilled water was used in the experiments.
2.2. Preparation of Soiled Cloth
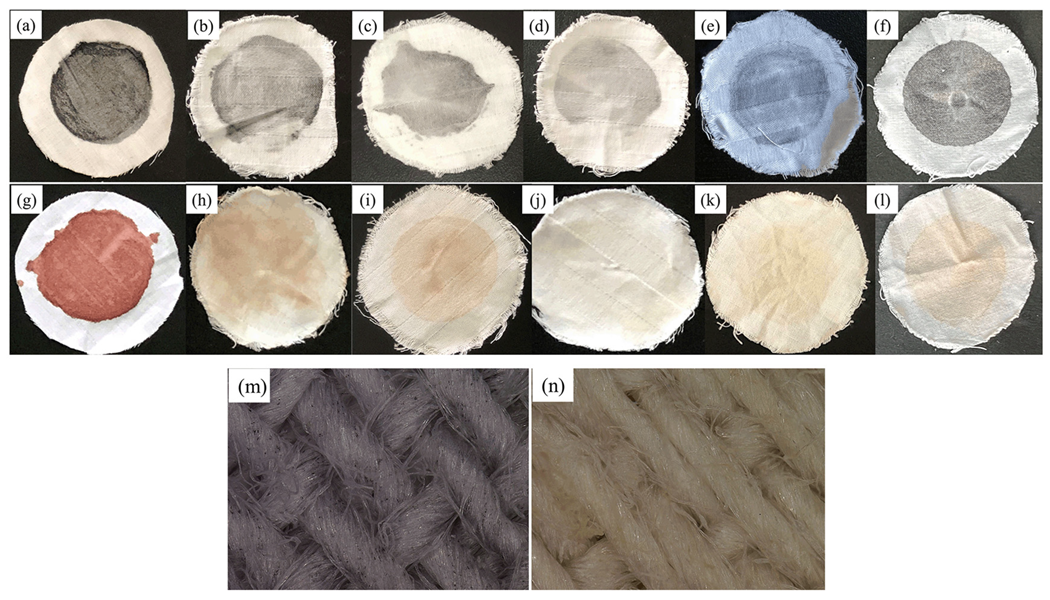
The protein stain was prepared as follows: Weigh 2.4 g of gum arabic powder and dissolve it with a little water, add 1.6 g of carbon black powder and grind for about 2 min. Transfer this carbon black stain to 120 mL of aqueous solution containing 13.8 g of whole milk powder, add another 120 mL of distilled water, homogenize with an emulsifier at 4000–5000 r/min for 30 min, then slowly add 120 mL of aqueous solution containing 25 g of egg liquid (egg white: yolk = 3:2) and continue to homogenize for 1 h.
White cotton cloth was cut into circular pieces of ~6 cm. When preparing the protein fouling cloth, the protein stain solution was heated to 40 °C and filtered. Then, 200 μL was added dropwise onto the white cotton cloth, soaked, pressed, and then dried. The same method was followed when preparing the dirty blood cloth.
2.3. Washing Procedure
The preparation method of the basal detergent is as follows. Add 4% polyethoxylated fatty alcohol, 2% ethoxylated alkyl sulfate, 8% dodecylbenzene sulfonic acid, 0.5% triethanolamine, and 0.5% anhydrous sodium citrate in a volume of water, stir to dissolve, and use sodium hydroxide solution to adjust the pH of the solution to 8.5–9.0.
The water used during washing was hard water (250 mg/kg), and the molar ratio of Ca2+ to Mg2+ was 6:4. The configuration method is as follows: weigh 1.67 g CaCl2 and 2.04 g MgCl2·6H2O, add water to make 10.0 L, which is 250 mg/kg hard water.
Then, 400 U/g of protease was added to the basal detergent and mixed well to prepare a 0.2% solution. A 100 mL aliquot of the solution was added to an Erlenmeyer flask, and a cloth piece was added. Various proteases have their maximum enzyme activity at 50–60 °C. Thus, 50 °C was selected as the reaction temperature [
22,
23]. The rotating speed was maintained at 150 r/min at 50 °C and washed for 50 min. Each piece of cloth was rinsed, dehydrated, and then dried.
2.4. Characterization of the Cleaning Effect
A whiteness tester was used to detect the whiteness reflectance value of the cloth surface before and after washing at the wavelength of 457 nm. We took two points on the front and back sides of the cloth piece before washing or after washing and measured the whiteness value. The average value of the four measurements is the whiteness value of the cloth piece. The stain residues on the surface and inside of the fiber before and after washing were observed with a super depth-of-field microscope (Leica DVM6A, Chongqing, China) at magnifications of 200× and 500× [
24]. The state of the fibers before and after washing the cloth and the stain residues between the fibers were observed via scanning electron microscopy (SEM, Phenom pure plus, Shanghai, China) at a voltage of 10 kV [
13,
25]. The magnifications were 410×, 430×, and 440×. Fourier transform infrared spectroscopy (ATR-FTIR, Nicolet10, Waltham, MA, USA) was performed before and after washing of the fabric sheet [
26,
27]. The wavenumber range of residual functional groups was 500–4000 cm
−1. Energy dispersion analysis was performed using X-ray photoelectron spectrometry (XPS, ESCALABXi+, Waltham, MA, USA) [
27].
2.5. Detection of Stain Protein Molecular Weight
Eighty units of enzyme activities of alkaline protease, keratinase, and 4 U enzyme activities of trypsin were added to the protein-contaminated liquid to verify the decomposing effect of the proteases on protein stains. The reaction was carried out at 50 °C for 50 min, and the reaction solution was analyzed by SDS–PAGE [
14,
28]. Eighty units of alkaline protease, keratinase, and trypsin were added to the blood to verify the decomposing effect of the proteases on bloodstains. The reaction was carried out at 50 °C for 10–50 min, and the reaction solution was analyzed by SDS–PAGE. The electrophoresis gel was prepared using the Meilun protein gel kit. The sample was added with 80 μL Tricine-SDS-PAGE loading buffer (5×), boiled for 5 min, centrifuged to obtain the supernatant, and then loaded with 10–15 μL for each sample. Electrophoresis was performed after adding the electrophoresis buffer to the electrophoresis system.
2.6. Evaluation of the Effect of Blood Stains Redeposition
5 mL of 4% blood dilution solution was prepared; added with 80 U of alkaline protease, keratinase, and trypsin; and then shaken in a water bath at 50 °C for 10, 20, 30, 40, and 50 min. The reaction solution was boiled for 3 min, and then protein gel electrophoresis was performed. Double-distilled water (5 mL) was added to the reaction solution, in which the white cotton cloth was immersed and allowed to stand for 12 h at 30 °C. The cloth was rinsed with water and dried, and then the extent of surface deposition and the relationship between the extent of protein deposition on the surface of the cloth and the molecular weight of the protein were observed.
2.7. Optimization of Protease Washing Performance
Alkaline protease and keratinase were mixed in the detergent at different ratios, and the total enzyme activity of each experiment was set to 80 U. In the experiment, the enzyme activity ratios of alkaline protease and keratinase were 20 U:60 U, 40 U:40 U, 30 U:50 U, 60 U:20 U, and 10 U:70 U. On the basis of adding 80 U protease activity per 0.2 g detergent, the enzyme activity of trypsin was added as 5 U, 10 U, 15 U, 20 U successively, and the remaining enzyme activity was supplemented to 80 U by alkaline protease and keratinase. The selected detergent auxiliaries were added to the basal detergent, and the addition amount was 1% of the mass of basal detergent. One or two additives, enzymes, and basal detergents with the best cleaning effect were selected from the surfactants, anti-deposition agents, water softeners, and cyclodextrins for subsequent experiments. The optimized formula was compared with four common commercial detergents in terms of washing effect.