Laboratory Measurements of Zeta Potential in Fractured Lewisian Gneiss: Implications for the Characterization of Flow in Fractured Crystalline Bedrock
Abstract
:1. Introduction
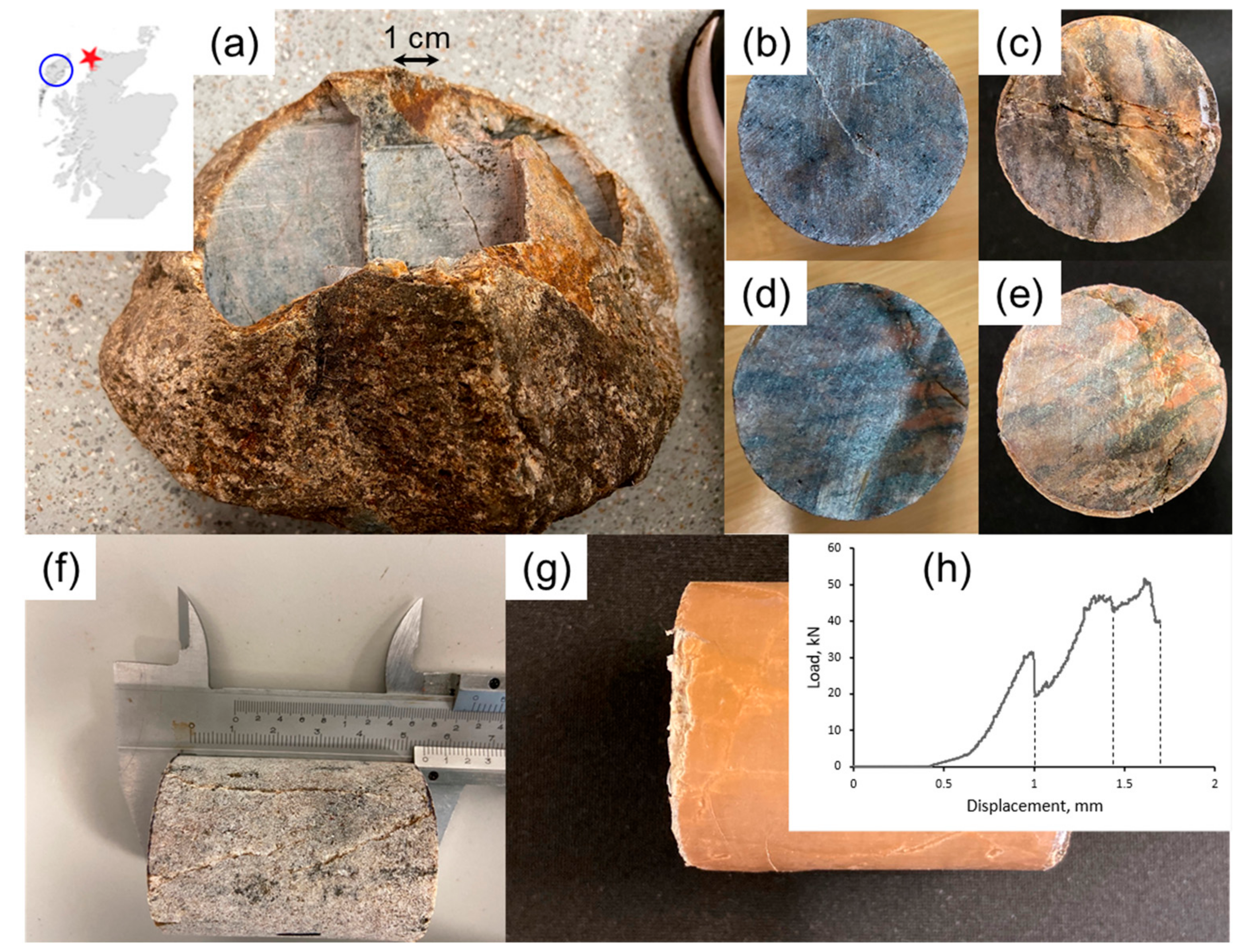
2. Materials and Methods
| Sample; Mineralogy | Solution | Zeta Potential, mV | Source | |
|---|---|---|---|---|
| LS | HS | |||
| Grey shaded area | ||||
| Fontainebleau SS; >99% quartz | NaCl | −52 | −20 | a, b |
| Lochaline SS; >99% quartz | NaCl | −77 | −25 | b |
| Stainton SS; 90% quartz, 5% clays and feldspar | NaCl | −26 | −16 | a |
| St Bess SS; 90% quartz, <5% clays | NaCl | −31 | −16 | a, c |
| St Bees SS; 90% quartz, <5% clays | SW | N/A | −13 | a |
| Doddington SS; 69% quartz, 5% clays | NaCl | −22 | −10 | e |
| SP; >99% quartz | TW | −100 | N/A | a |
| SP; >99% quartz | NaCl | −20 | −12 | d |
| Berea SS; 90% quartz, up to 4% clays | NaCl | −20 | −17 | f |
| Boise SS; 47% quartz, 26% plagioclase, 3% clays | NaCl | −20 | −20 | b |
| Inada granite; N/A | KCl | −35 | N/A | g |
| Westerly granite, 38% plagioclase 29% quartz | NaCl | −20 | N/A | h |
| Crushed Westerly granite; N/A | KCl/NaCl | −65 | N/A | i |
| Inada granite; N/A | KNO3 | −70 | N/A | j |
| White shaded area | ||||
| Ketton LS; 97% calcite, 3% dolomite | NaCl | N/A | −6 | k |
| Estaillades LS; 95% calcite, 4% dolomite, 1% anhydrite | NaCl | N/A | −6 | k |
| Portland LS; 96.6% calcite, 3.4% quartz | NaCl | N/A | −9 | k |
| Estaillades LS; 95% calcite, 4% dolomite, 1% anhydrite | SW | N/A | −1.25 | l |
| Reservoir BA LS; N/A | ALSW | −4 | N/A | m |
| Reservoir BD LS; N/A | ALSW | −10 | N/A | m |
| Outcrop TE LS; >99% calcite | Artificial | −15 | −2 | m |
| Crushed Iceland spar; 100% calcite | NaCl | −5 [n] | +4 [o] | n, o |

3. Results and Discussion
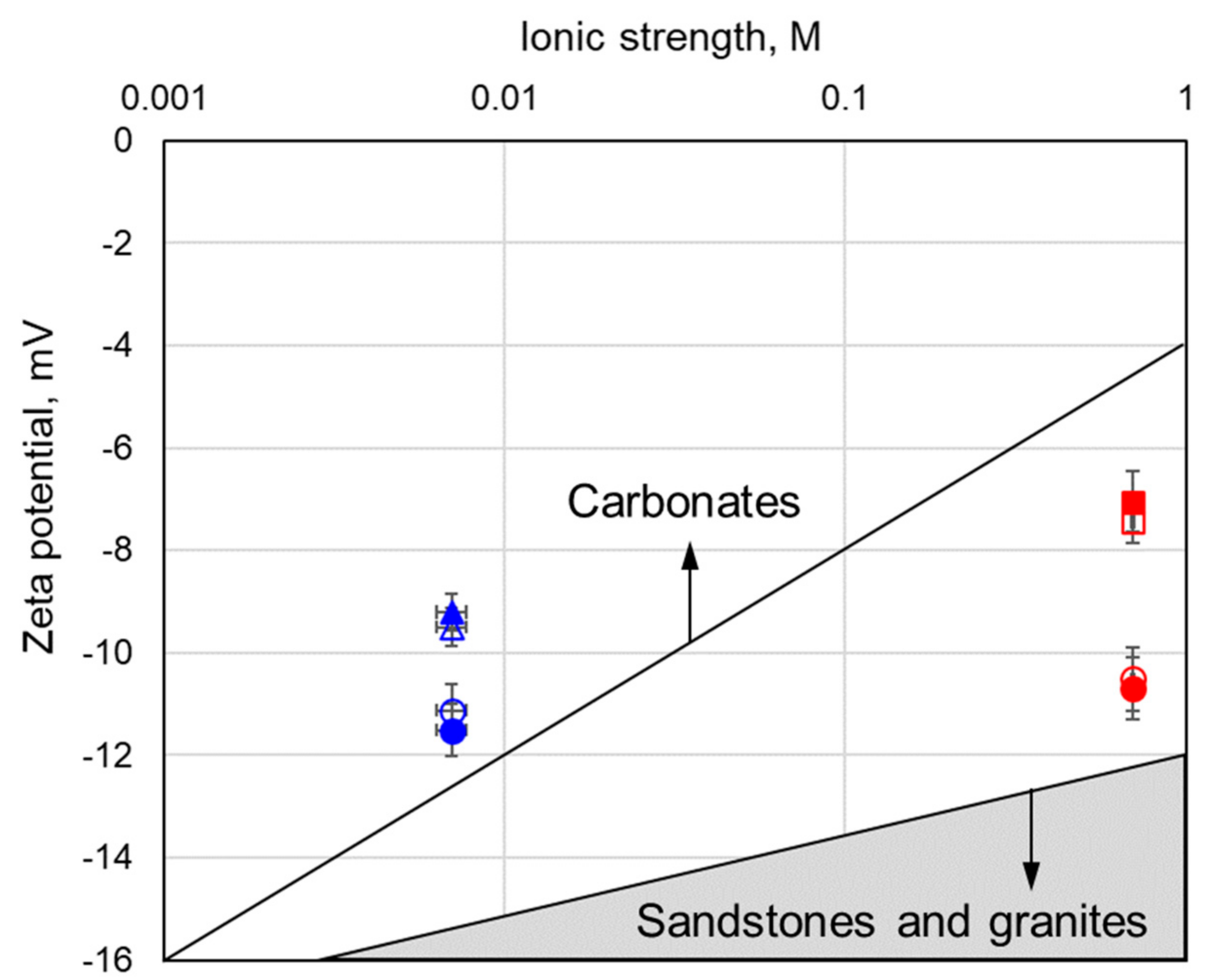
3.1. Effect of Rock Type and Mineralogy
3.2. Effect of the Ionic Strength and Chemical Composition
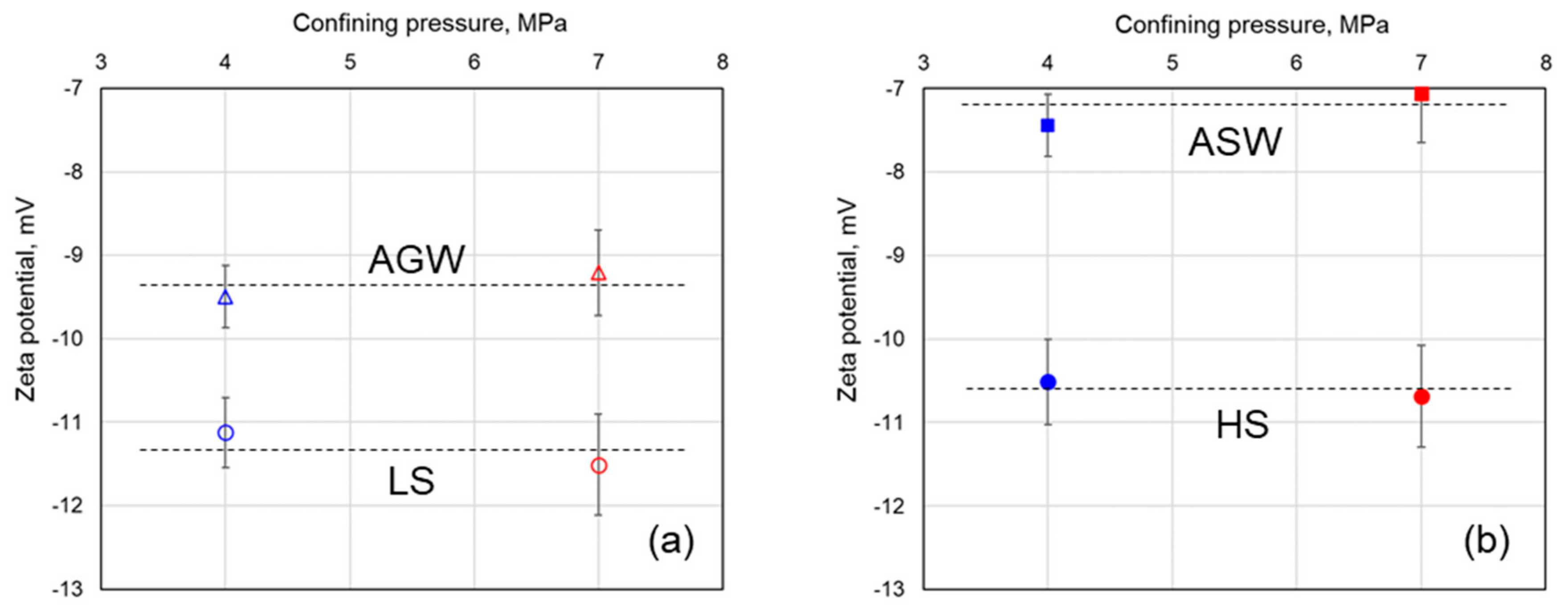
3.3. Effect of Confining Pressure
4. Conclusions
- Zeta potentials of gneiss are unique and dissimilar to sandstones, carbonates and even to individual gneiss constitutive minerals, i.e., mica and feldspar.
- The negative zeta potential decreases with increasing salinity when the sample is saturated with AGW and ASW, but the rate of the decrease is smaller compared to any other mineral.
- The negative zeta potential is independent of salinity when using NaCl; this feature is similar to what was observed with clayey sandstone [45] but the zeta potential of gneiss was found to be more positive compared with clayey sandstones saturated with NaCl.
- Significant amounts of feldspar and mica present in our gneiss sample were found to be responsible for the high sensitivity of the sample to the presence of divalent ions (Ca2+, Mg2+ and SO42−) compared with quartz. Therefore, these ions were identified as PDIs for gneiss, making the sample respond to compositional and concentration variations in a fashion similar to that of carbonate samples.
- The reported values of gneiss CSP remained independent of the confining pressure, thus, suggesting that the surface electrical conductivity in gneiss aquifers could be neglected. A simple fracture model was developed to evaluate depths, and resulting in situ confining pressures, until which fracture aperture would remain large enough to neglect the surface electrical conductivity; this depth was found to be 380 m, which is much deeper than most water resources applications.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gustafson, G.; Krásný, J. Crystalline Rock Aquifers: Their Occurrence, Use And Importance. Hydrogeol. J. 1994, 2, 64–75. [Google Scholar] [CrossRef]
- Ofterdinger, U.; MacDonald, A.M.; Comte, J.C.; Young, M.E. Groundwater in fractured bedrock environments: Managing catchment and subsurface resources–an introduction. Geol. Soc. Lond. Spec. Publ. 2019, 479, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lachassagne, P.; Dewandel, B.; Wyns, R. Review: Hydrogeology of weathered crystalline/hard-rock aquifers—Guidelines for the operational survey and management of their groundwater resources. Hydrogeol. J. 2021, 29, 2561–2594. [Google Scholar] [CrossRef]
- Bianchi, M.; MacDonald, A.M.; Macdonald, D.M.J.; Asare, E.B. Investigating the productivity and sustainability of weathered basement aquifers in tropical Africa using numerical simulation and global sensitivity analysis. Water Resour. Res. 2020, 56, e2020WR027746. [Google Scholar] [CrossRef]
- Comte, J.-C.; Cassidy, R.; Nitsche, J.; Ofterdinger, U.; Pilatova, K.; Flynn, R. The typology of Irish hard-rock aquifers based on an integrated hydrogeological and geophysical approach. Hydrogeol. J. 2012, 20, 1569–1588. [Google Scholar] [CrossRef]
- Roques, C.; Bour, O.; Aquilina, L.; Dewandel, B. High-yielding aquifers in crystalline basement: Insights about the role of fault zones, exemplified by Armorican Massif, France. Hydrogeol. J. 2016, 24, 2157–2170. [Google Scholar] [CrossRef]
- Neuman, S.P. Trends, prospects and challenges in quantifying flow and transport through fractured rocks. Hydrogeol. J. 2005, 13, 124–147. [Google Scholar] [CrossRef]
- Le Borgne, T.; Bour, O.; Paillet, F.; Caudal, J.-P. Assessment of preferential flow path connectivity and hydraulic properties at single-borehole and cross-borehole scales in a fractured aquifer. J. Hydrol. 2006, 328, 347–359. [Google Scholar] [CrossRef]
- Krásný, J.; Sharp, J.M. Hydrogeology of fractured rocks from particular fractures to regional approaches: State-of-the-art and future challenges. In Groundwater of Fractured Rocks; Taylor and Francis: London, UK, 2007; pp. 1–32. [Google Scholar]
- Becker, M.W.; Shapiro, A. Interpreting tracer breakthrough tailing from different forced-gradient tracer experiment configurations in fractured bedrock. Water Resour. Res. 2003, 39. [Google Scholar] [CrossRef] [Green Version]
- Illman, W.A.; Liu, X.; Takeuchi, S.; Yeh, T.-C.J.; Ando, K.; Saegusa, H. Hydraulic tomography in fractured granite: Mizunami Underground Research site, Japan. Water Resour. Res. 2009, 45. [Google Scholar] [CrossRef] [Green Version]
- Comte, J.-C.; Ofterdinger, U.; Legchenko, A.; Caulfield, J.; Cassidy, R.; González, J.A.M. Catchment-scale heterogeneity of flow and storage properties in a weathered/fractured hard rock aquifer from resistivity and magnetic resonance surveys: Implications for groundwater flow paths and the distribution of residence times. Geol. Soc. Lond. Spec. Publ. 2019, 479, 35–58. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, P. Scale Effects in Fluid Flow through Fractured Geologic Media. In Scale Dependence and Scale Invariance in Hydrology; Sposito, G., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 335–353. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Ladderud, J.A.; Yager, R.M. Interpretation of hydraulic conductivity in a fractured-rock aquifer over increasingly larger length dimensions. Hydrogeol. J. 2015, 23, 1319–1339. [Google Scholar] [CrossRef]
- Day-Lewis, F.D.; Slater, L.D.; Robinson, J.; Johnson, C.D.; Terry, N.; Werkema, D. An overview of geophysical technologies appropriate for characterization and monitoring at fractured-rock sites. J. Environ. Manag. 2017, 204, 709–720. [Google Scholar] [CrossRef]
- González, J.A.M.; Comte, J.C.; Legchenko, A.; Ofterdinger, U.; Healy, D. Quantification of groundwater storage heterogeneity in weathered/fractured basement rock aquifers using electrical resistivity tomography: Sensitivity and uncer-tainty associated with petrophysical modelling. J. Hydrol. 2021, 593, 125637. [Google Scholar] [CrossRef]
- Olsson, O.; Falk, L.; Forslund, O.; Lundmark, L.; Sandberg, E. Borehole radar applied to the characterization of hy-draulically conductive fracture zones in crystalline rock. Geophys. Prospect. 1992, 40, 109–142. [Google Scholar] [CrossRef]
- Vouillamoz, J.; Lawson, F.; Yalo, N.; Descloitres, M. The use of magnetic resonance sounding for quantifying specific yield and transmissivity in hard rock aquifers: The example of Benin. J. Appl. Geophys. 2014, 107, 16–24. [Google Scholar] [CrossRef]
- Wishart, D.N.; Slater, L.; Gates, A.E. Self potential improves characterization of hydraulically-active fractures from azimuthal geoelectrical measurements. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Wishart, D.N.; Slater, L.D.; Gates, A.E. Fracture anisotropy characterization in crystalline bedrock using field-scale azimuthal self potential gradient. J. Hydrol. 2008, 358, 35–45. [Google Scholar] [CrossRef]
- Hasan, M.; Shang, Y.-J.; Jin, W.-J.; Akhter, G. Investigation of fractured rock aquifer in South China using electrical resistivity tomography and self-potential methods. J. Mt. Sci. 2019, 16, 850–869. [Google Scholar] [CrossRef]
- Jackson, M.; Gulamali, M.Y.; Leinov, E.; Saunders, J.H.; Vinogradov, J. Spontaneous Potentials in Hydrocarbon Reservoirs During Waterflooding: Application to Water-Front Monitoring. SPE J. 2012, 17, 53–69. [Google Scholar] [CrossRef]
- Jackson, M.; Butler, A.P.; Vinogradov, J. Measurements of spontaneous potential in chalk with application to aquifer characterization in the southern UK. Q. J. Eng. Geol. Hydrogeol. 2012, 45, 457–471. [Google Scholar] [CrossRef]
- MacAllister, D.; Jackson, M.D.; Butler, A.P.; Vinogradov, J. Remote Detection of Saline Intrusion in a Coastal Aquifer Using Borehole Measurements of Self-Potential. Water Resour. Res. 2018, 54, 1669–1687. [Google Scholar] [CrossRef] [Green Version]
- Roubinet, D.; Linde, N.; Jougnot, D.; Irving, J. Streaming potential modeling in fractured rock: Insights into the identi-fication of hydraulically active fractures. Geophys. Res. Lett. 2016, 43, 4937–4944. [Google Scholar] [CrossRef] [Green Version]
- MacAllister, D.J.; Jackson, M.D.; Butler, A.P.; Vinogradov, J. Tidal influence on self-potential measurements. J. Geophys. Res. 2016, 121, 8432–8452. [Google Scholar] [CrossRef] [Green Version]
- Graham, M.T.; MacAllister, D.; Vinogradov, J.; Jackson, M.D.; Butler, A.P. Self-Potential as a Predictor of Seawater Intrusion in Coastal Groundwater Boreholes. Water Resour. Res. 2018, 54, 6055–6071. [Google Scholar] [CrossRef] [Green Version]
- Saunders, J.H.; Jackson, M.; Gulamali, M.; Vinogradov, J.; Pain, C. Streaming potentials at hydrocarbon reservoir conditions. Geophys. 2012, 77, E77–E90. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.D.; Leinov, E. On the validity of the “thin” and “thick” double-layer assumptions when calculating streaming currents in porous media. Int. J. Geophys. 2012, 2012, 897807. [Google Scholar] [CrossRef] [Green Version]
- MacAllister, D.; Graham, M.T.; Vinogradov, J.; Butler, A.P.; Jackson, M.D. Characterizing the Self-Potential Response to Concentration Gradients in Heterogeneous Subsurface Environments. J. Geophys. Res. Solid Earth 2019, 124, 7918–7933. [Google Scholar] [CrossRef] [Green Version]
- Vinogradov, J.; Jaafar, M.Z.; Jackson, M. Measurement of streaming potential coupling coefficient in sandstones saturated with natural and artificial brines at high salinity. J. Geophys. Res. Space Phys. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Walker, E.; Glover, P.W.J. Measurements of the Relationship Between Microstructure, pH, and the Streaming and Zeta Potentials of Sandstones. Transp. Porous Media 2017, 121, 183–206. [Google Scholar] [CrossRef] [Green Version]
- Hidayat, M.; Sarmadivaleh, M.; Derksen, J.; Vega-Maza, D.; Iglauer, S.; Vinogradov, J. Zeta potential of CO2-rich aqueous solutions in contact with intact sandstone sample at temperatures of 23 °C and 40 °C and pressures up to 10.0 MPa. J. Colloid Interface Sci. 2021, 607, 1226–1238. [Google Scholar] [CrossRef]
- Al Mahrouqi, D.; Vinogradov, J.; Jackson, M.D. Zeta potential of artificial and natural calcite in aqueous solution. Adv. Colloid Interface Sci. 2017, 240, 60–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosha, T.; Matsushima, N.; Ishido, T. Zeta potential measured for an intact granite sample at temperatures to 200 °C. Geophys. Res. Lett. 2003, 30, 1295. [Google Scholar] [CrossRef]
- BGS. Final report on groundwater investigations on Harris. In British Geological Survey Commissioned Report Cr/01/69; BGS: Nottingham, UK, 2001. [Google Scholar]
- Weaver, B.L.; Tarney, J. Lewisian gneiss geochemistry and Archaean crustal development models. Earth Planet. Sci. Lett. 1981, 55, 171–180. [Google Scholar] [CrossRef]
- Macdonald, J.M.; Magee, C.; Goodenough, K. Dykes as physical buffers to metamorphic overprinting: An example from the Archaean–Palaeoproterozoic Lewisian Gneiss Complex of NW Scotland. Scott. J. Geol. 2017, 53, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Alroudhan, A.; Vinogradov, J.; Jackson, M. Zeta potential of intact natural limestone: Impact of potential-determining ions Ca, Mg and SO4. Colloids Surf. A Physicochem. Eng. Asp. 2016, 493, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Vinogradov, J.; Jackson, M. Multiphase streaming potential in sandstones saturated with gas/brine and oil/brine during drainage and imbibition. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Jouniaux, L.; Pozzi, J.-P. Streaming potential and permeability of saturated sandstones under triaxial stress: Consequences for electrotelluric anomalies prior to earthquakes. J. Geophys. Res. Space Phys. 1995, 100, 10197–10209. [Google Scholar] [CrossRef]
- Jaafar, M.Z.; Vinogradov, J.; Jackson, M.D. Measurement of streaming potential coupling coefficient in sandstones saturated with high salinity NaCl brine. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef]
- Vinogradov, J.; Jackson, M.D.; Chamerois, M. Zeta potential in sandpacks: Effect of temperature, electrolyte pH, ionic strength and divalent cations. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 259–271. [Google Scholar] [CrossRef]
- Vinogradov, J.; Jackson, M.D. Zeta potential in intact natural sandstones at elevated temperatures. Geophys. Res. Lett. 2015, 42, 6287–6294. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Collini, H.; Jackson, M.D. Anomalous Zeta Potential Trends in Natural Sandstones. Geophys. Res. Lett. 2018, 45, 11068–11073. [Google Scholar] [CrossRef]
- Reppert, P.M.; Morgan, F.D. Temperature-dependent streaming potentials: 2. Laboratory. J. Geophys. Res. Space Phys. 2003, 108. [Google Scholar] [CrossRef]
- Morgan, F.D.; Williams, E.R.; Madden, T.R. Streaming potential properties of westerly granite with applications. J. Geophys. Res. 1989, 94, 12449–12461. [Google Scholar] [CrossRef]
- Ishido, T.; Mizutani, H. Experimental and theoretical basis of electrokinetic phenomena in rock-water systems and its applications to geophysics. J. Geophys. Res. Solid Earth 1981, 86, 1763–1775. [Google Scholar] [CrossRef]
- Jackson, M.D.; Al-Mahrouqi, D.; Vinogradov, J. Zeta potential in oil-water-carbonate systems and its impact on oil recovery during controlled salinity water-flooding. Sci. Rep. 2016, 6, 37363. [Google Scholar] [CrossRef] [Green Version]
- Collini, H.; Li, S.; Jackson, M.D.; Agenet, N.; Rashid, B.; Couves, J. Zeta potential in intact carbonates at reservoir conditions and its impact on oil recovery during controlled salinity waterflooding. Fuel 2020, 266, 116927. [Google Scholar] [CrossRef]
- Heberling, F.; Trainor, T.P.; Lützenkirchen, J.; Eng, P.; Denecke, M.; Bosbach, D. Structure and reactivity of the calcite–water interface. J. Colloid Interface Sci. 2011, 354, 843–857. [Google Scholar] [CrossRef]
- Heberling, F.; Klačić, T.; Raiteri, P.; Gale, J.D.; Eng, P.J.; Stubbs, J.E.; Gil-Díaz, T.; Begović, T.; Lützenkirchen, J. Structure and Surface Complexation at the Calcite(104)–Water Interface. Environ. Sci. Technol. 2021, 55, 12403–12413. [Google Scholar] [CrossRef]
- Jackson, M.; Vinogradov, J.; Hamon, G.; Chamerois, M. Evidence, mechanisms and improved understanding of controlled salinity waterflooding part 1: Sandstones. Fuel 2016, 185, 772–793. [Google Scholar] [CrossRef] [Green Version]
- Nyabeze, W.; McFadzean, B. Adsorption of copper sulphate on PGM-bearing ores and its influence on froth stability and flotation kinetics. Miner. Eng. 2016, 92, 28–36. [Google Scholar] [CrossRef]
- Jie, Z.; Weiqing, W.; Jing, L.; Yang, H.; Qiming, F.; Hong, Z. Fe (III) as an activator for the flotation of spodumene, albite, and quartz minerals. Miner. Eng. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Nishimura, S.; Tateyama, H.; Tsunematsu, K.; Jinnai, K. Zeta potential measurement of muscovite mica basal plane-aqueous solution interface by means of plane interface technique. J. Colloid Interface Sci. 1992, 152, 359–367. [Google Scholar] [CrossRef]
- Bray, A.; Benning, L.G.; Bonneville, S.; Oelkers, E.H. Biotite surface chemistry as a function of aqueous fluid composition. Geochim. Cosmochim. Acta 2014, 128, 58–70. [Google Scholar] [CrossRef]
- Vinogradov, J.; Hidayat, M.; Sarmadivaleh, M.; Derksen, J.; Vega-Maza, D.; Iglauer, S.; Jougnot, D.; Azaroual, M.; Leroy, P. Predictive surface complexation model of the calcite-aqueous solution interface: The impact of high concentration and complex composition of brines. J. Colloid Interface Sci. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Zaucha, M.; Zembala, M. Zeta potential of mica covered by colloid particles: A streaming potential study. Langmuir 2010, 26, 9368–9377. [Google Scholar] [CrossRef] [PubMed]
- Gülgönül, I.; Karagüzel, C.; Çınar, M.; Çelik, M.S. Interaction of Sodium Ions with Feldspar Surfaces and Its Effect on the Selective Separation of Na- and K-Feldspars. Miner. Process. Extr. Met. Rev. 2012, 33, 233–245. [Google Scholar] [CrossRef]
- Demir, C.; Bentli, I.; Gülgönül, I.; Çelik, M. Effects of bivalent salts on the flotation separation of Na-feldspar from K-feldspar. Miner. Eng. 2003, 16, 551–554. [Google Scholar] [CrossRef]
- Yukselen-Aksoy, Y.; Kaya, A. A study of factors affecting on the zeta potential of kaolinite and quartz powder. Environ. Earth Sci. 2010, 62, 697–705. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Neuzil, C.E.; Tracy, J.V. Flow through fractures. Water Resour. Res. 1981, 17, 191–199. [Google Scholar] [CrossRef]
- Thanh, L.; Jougnot, D.; Do, P.; Hue, D.; Thuy, T.; Tuyen, V. Predicting Electrokinetic Coupling and Electrical Conductivity in Fractured Media Using a Fractal Distribution of Tortuous Capillary Fractures. Appl. Sci. 2021, 11, 5121. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, Y.; Guo, Q.; Tan, L.; Zhuang, Y.; Liu, M.; Zhang, C.; Xiang, W.; Rohn, J. Experimental investigation of the hydraulic and heat-transfer properties of artificially fractured granite. Sci. Rep. 2017, 7, 39882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, P.; Devau, N.; Revil, A.; Bizi, M. Influence of surface conductivity on the apparent zeta potential of amor-phous silica nanoparticles. J. Colloid Interface Sci. 2013, 410, 81–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| AGW | LS | ASW | HS | |
|---|---|---|---|---|
| [Na+], M | 1.522 × 10−3 | 7.350 × 10−3 | 0.512 | 0.700 |
| [Ca2+], M | 1.612 × 10−3 | - | 0.013 | - |
| [Mg2+], M | 0.288 × 10−3 | - | 0.042 | - |
| [Cl−], M | 5.072 × 10−3 | 7.350 × 10−3 | 0.577 | 0.700 |
| [SO42−], M | 0.125 × 10−3 | - | 0.022 | - |
| pH | 6.0 ± 0.1 | 6.5 ± 0.1 | 6.2 ± 0.1 | 6.9 ± 0.1 |
| , S∙m−1 | 0.069 ± 0.001 | 0.097 ± 0.001 | 5.83 ± 0.01 | 6.13 ± 0.01 |
| Ionic strength, M | 7.350 × 10−3 | 7.350 × 10−3 | 0.700 | 0.700 |
| ID | P, MPa | , Pa·s | F·m−1 | k, mD | 2w, nm | ||
|---|---|---|---|---|---|---|---|
| LS | 4 | 9.33 × 10−4 | 7.00 × 10−10 | −86.4 | −11.12 | 51 ± 1 | 782 |
| LS | 7 | 9.33 × 10−4 | 7.00 × 10−10 | −89.5 | −11.51 | 43 ± 1 | 718 |
| HS | 4 | 9.89 × 10−4 | 6.97 × 10−10 | −1.21 | −10.52 | 51 ± 1 | 782 |
| HS | 7 | 9.89 × 10−4 | 6.97 × 10−10 | −1.23 | −10.69 | 43 ± 1 | 718 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradov, J.; Hidayat, M.; Kumar, Y.; Healy, D.; Comte, J.-C. Laboratory Measurements of Zeta Potential in Fractured Lewisian Gneiss: Implications for the Characterization of Flow in Fractured Crystalline Bedrock. Appl. Sci. 2022, 12, 180. https://doi.org/10.3390/app12010180
Vinogradov J, Hidayat M, Kumar Y, Healy D, Comte J-C. Laboratory Measurements of Zeta Potential in Fractured Lewisian Gneiss: Implications for the Characterization of Flow in Fractured Crystalline Bedrock. Applied Sciences. 2022; 12(1):180. https://doi.org/10.3390/app12010180
Chicago/Turabian StyleVinogradov, Jan, Miftah Hidayat, Yogendra Kumar, David Healy, and Jean-Christophe Comte. 2022. "Laboratory Measurements of Zeta Potential in Fractured Lewisian Gneiss: Implications for the Characterization of Flow in Fractured Crystalline Bedrock" Applied Sciences 12, no. 1: 180. https://doi.org/10.3390/app12010180
APA StyleVinogradov, J., Hidayat, M., Kumar, Y., Healy, D., & Comte, J.-C. (2022). Laboratory Measurements of Zeta Potential in Fractured Lewisian Gneiss: Implications for the Characterization of Flow in Fractured Crystalline Bedrock. Applied Sciences, 12(1), 180. https://doi.org/10.3390/app12010180







