Solid Fraction Determination at the Rigidity Point by Advanced Thermal Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
18.35.Mn − 6.16.Ti − 12.41.Zn − 3.62.Pb − 52.98.Sn + 161.77.Sr.
r2 = 0.81; Sey = 3.41
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornacchia, G.; Dioni, D.; Faccoli, M.; Gislon, C.; Solazzi, L.; Panvini, A.; Cecchel, S. Experimental and numerical study of an automotive component produced with innovative ceramic core in high pressure die casting (HPDC). Metals 2019, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Jolly, M.; Katgerman, L. Modelling of defects in aluminium cast products. Prog. Mater. Sci. 2022, 123, 1–39. [Google Scholar] [CrossRef]
- Bonollo, F.; Gramegna, N.; Timelli, G. High temperature behaviour of high pressure diecast. J. Miner. Met. Mater. Soc. 2015, 67, 901–908. [Google Scholar] [CrossRef]
- Bogagui, F.; Movahedi, M.; Kokabi, A.H.; Tavakoli, R. Effect of solid fraction, grain misorientation and grain boundary energy on solidification cracking in weld of Al–Cu aluminum alloys. Mater. Res. Express 2019, 6, 8. [Google Scholar]
- Voncina, M.; Mrvar, P.; Medved, J. Thermodynamic analysis of AlSi10Mg alloy. RMZ-Mater. Geoenviron. 2006, 52, 621–633. [Google Scholar]
- Chai, G.; Backerud, L.; Rolland, T.; Arnberg, L. Dendrite coherency during equiaxed solidification in binary aluminum alloys. Metall. Mater. Trans. A 1995, 26A, 965–970. [Google Scholar] [CrossRef]
- Hamadellah, A.; Bouayad, A. Study of hot tear of AlCu5MgTi by restraining casting shrinkage in green-sand mold. JMES 2017, 8, 3099–3105. [Google Scholar]
- Santhi, S. Calculation of shrinkage of sand cast aluminium alloys. Int. J. Appl. Eng. Res. 2018, 13, 8889–8893. [Google Scholar]
- Hamadellaha, A.; Bouayada, A.; Geromettaba, C. Hot tear characterization of AlCu5MgTi and AlSi9 casting alloys using an instrumented constrained six rods casting method. J. Mater. Process. Technol. 2017, 244, 282–288. [Google Scholar] [CrossRef]
- Chai, G.; Backerud, L.; Avnberg, L. Study of dendrite coherency in Al-Si alloys during equiaxed dendritic solidification. Int. J. Mat. Res. 1995, 86, 54–59. [Google Scholar] [CrossRef]
- Anglada, E.; Meléndez, A.; Vicario, I.; Arratibel, E.; Aguillo, I. Adjustment of a high pressure die casting simulation model against experimental data. Procedia Eng. 2015, 132, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Lu, H.; Hu, X.; Lin, F.; Li, X.; Zhu, Q. Current progress in rheoforming of wrought aluminum alloys: A review. Metals 2020, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Peti, F.; Strnad, G. The effect of squeeze pin dimension and operational parameters on material homogenity of aluminium high pressure die cast parts. Acta Marisiensis Ser. Technol. 2019, 16, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Djurdjevic, M.B.; Stockwell, T.; Sokolowski, J. The effect of strontium on the microstructure of the aluminium-silicon and aluminium-copper eutectics in the 319 aluminium alloy. Int. J. Cast Met. Res. 1999, 12, 67–73. [Google Scholar] [CrossRef]
- Vicario, I.; Villanueva, E.; Montero, J.; Djurdjevic, M.; Huber, G. The determination of dendrite coherency point characteristics using three new methods for aluminum alloys. Appl. Sci. 2018, 8, 1236. [Google Scholar] [CrossRef] [Green Version]
- Vicario, I.; Djurdjevic, G.; Villanueva, E.; Meléndez, A. Description of Al-Si10-Mg1 alloys by advanced thermal analysis based on their known chemical compositions. In Proceedings of the 72nd World Foundry Congress, Nagoya, Japan, 21–25 May 2016. [Google Scholar]
- Pelayo, G.; Sokolowski, J.H.; Lashkari, R.A. A case based reasoning aluminium thermal analysis platform for the prediction of W319 Al cast component characteristics. J. Achiev. Mater. Manuf. Eng. 2009, 36, 7–17. [Google Scholar]
- Djurdjevic, M.B.; Huber, G. Determination of rigidity point/temperature using thermal analysis method and mechanical technique. J. Alloys Compd. 2014, 590, 500–506. [Google Scholar] [CrossRef]
- Drezet, J.M.; Mireux, B.; Szaraz, Z.; Pirling, T. Determination of coherency and rigidity temperatures in Al-Cu alloys using in situ neutron diffraction during casting. JOM 2014, 66, 1425–1430. [Google Scholar] [CrossRef] [Green Version]
- Iron Thermal Analysis Thermal Arrests—Exothermic and Endothermic a More or Less Complete Guide to Arrests and Inflection Points in Iron. Available online: https://www.linkedin.com/pulse/iron-thermal-analysis-arrests-exothermic-endothermic-more-sparkman (accessed on 3 November 2021).
- Djurdjevic, M.B.; Vicario, I. Description of hypoeutectic Al-Si-Cu alloys based on their known chemical compositions. Rev. De Metal. 2013, 49, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, E.; Vicario, I.; Sánchez, J.M.; Crespo, I. Determination of solidification of rigidity point temperature using a new method. Appl. Sci. 2020, 10, 2472. [Google Scholar] [CrossRef] [Green Version]
- Gottardi, A.; Pola, A. Solid fraction determination via DSC analysis. Metall. Ital. 2015, 5, 11–16. [Google Scholar]
- Marchwica, P.; Sokolowski, J.H.; Kierkus, W.T. Fraction solid evolution characteristics of AlSiCu alloys—Dynamic baseline approach. J. Achiev. Mater. Manuf. Eng. 2011, 47, 115–136. [Google Scholar]
- Emadi, D. Comparison of Newtonian and Fourier thermal analysis techniques for calculation of latent heat and solid fraction of aluminum alloys. J. Metall. 2004, 10, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Djurdjevic, M.B. Impact of major alloying elements on the solidification parameters of cast hypoeutectic AlSi6Cu (1–4 wt.%) and AlSi8Cu (1−4 wt.%) alloys. Metall. Mater. Eng. 2014, 20, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Brùna, M.; Kucharík, L. A progressive method of porosity prediction for aluminium castings. Mater. Tehnol. 2014, 48, 949–952. [Google Scholar]
- Djurdjevic, M.B.; Manasijevic, S.; Odanovic, Z.; Radis, R. Analysis of the solidification path of Al-Si9-Cu(1–4) alloys using thermal analysis technique. Metall. Ital. 2014, 106, 23–28. [Google Scholar]
- Djurdjevic, M.B.; Sokolowski, J.H.; Odanovic, Z. Determination of dendrite coherency point characteristics using first derivative curve versus temperature. J. Therm. Anal. Calorim. 2012, 109, 875–882. [Google Scholar] [CrossRef]
- Richard, M. Atlas Métallographique des Alliages D´Aluminium de Fonderie; Centre Technique des Industries de la Fonderie: Paris, France, 1975. [Google Scholar]
- Atlas Métallographique de l´A-S5 U3, Éditions Techniques des industries de la Fonderie; Centre Technique des Industries de la Fonderie: Paris, France, 1980.
- Atlas Métallographique de L´A-S13, Éditions Techniques des industries de la Fonderie; Centre Technique des Industries de la Fonderie: Paris, France, 1980.
- Vander, G. Metallography and Microstructures. In Metals Handbook, 9th ed.; ASM International: Novelty, OH, USA, 1999; Volume 9. [Google Scholar]
- Fabrizi, A.; Capuzzi, S.; Timelli, G. The influence of Sr, Mg and Cu addition on the microstructural properties of a secondary AlSi9Cu3(Fe) die casting alloy. Mater. Charact. 2013, 85, 13–25. [Google Scholar] [CrossRef]
- Timelli, G.; Bonollo, F. The influence of Cr content on the microstructure and mechanical properties of AlSi9Cu3(Fe) die-casting alloys. Mater. Sci. Eng. 2010, 528, 273–282. [Google Scholar] [CrossRef]
- Camicia, G.; Timelli, G. Grain refinement of gravity die cast AlSi7Cu3Mg alloys for automotoive cylinder heads. Trans. Nonferrous Met. Soc. China 2016, 26, 1211–1221. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Fabrizi, A.; Bonollo, F. Effect of V and Zr microalloying, and heat treatment on microstructure and mechanical properties of secondary Al7Si3Cu0.3Mg alloy. Int. J. Mater. Res. 2018, 109, 1099–2018. [Google Scholar] [CrossRef]
- Huber, G.; Djurdjevic, M.B.; Manasijevic, S. Quantification of feeding regions of hypoeutectic Al-(5, 7, 9)Si-(0-4)Cu (wt.%) alloys using cooling curve analysis. In Mass Production Processes; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Djurdjevic, M.B.; Mnasijevic, S.; Odanovic, Z.; Radisa, R. Influence of different contents of Si and Cu on the solidification pathways of cast hypoeutecticAl-(5–9)Si-(1–4)Cu (wt.%) alloy. Int. J. Mater. Res. 2013, 104, 865–873. [Google Scholar] [CrossRef]
- Mahfoud, M.; Prasada, A.K.; Emadi, D. The role of thermal analysis in detecting impurity levels during aluminum recycling. J. Therm. Anal. Calorim. 2010, 100, 847–851. [Google Scholar] [CrossRef]
- Reddy, N.S.; Prasada, A.K.; Chakraborty, M.; Murty, B.S. Prediction of grain size of Al–7Si Alloy by neural network. Mater. Sci. Eng. 2005, 391, 131–140. [Google Scholar] [CrossRef]
- Seifeddine, S.; Svensson, I.L. The influence of Fe and Mn content and cooling rate on the microstructure and mechanical properties of A380-die casting alloys. Metall. Sci. Technol. 2009, 27, 11–20. [Google Scholar]
- Khalajzadeh, V.; Beckerman, C. Simulation of shrinkage porosity formation during alloy solidification. Metall. Mater. Trans. 2020, 51, 2239–2254. [Google Scholar] [CrossRef] [Green Version]
- Rana, R.S.; Purohit, R.; Das, S. Reviews on the influences of alloying elements on the microstructure and mechanical properties of aluminum alloys and aluminum alloy composites. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Caliari, D.; Timelli, G.; Bonollo, F. Fluidity of aluminiun foundry alloys: Development of a testing procedure. Metall. Ital. 2015, 5, 17–24. [Google Scholar]
- Timelli, G.; Capuzzi, S.; Fabrizi, A. Precipitation of primary Fe-rich compounds in secondary AlSi9Cu3(Fe) alloys. J. Therm. Anal. Calorim. 2016, 123, 249–262. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Bonollo, F.; Arnberg, L. Influence of grain refiner addition on the precipitation of Fe-rich phases in secondary AlSi7Cu3Mg alloys. Int. J. Met. 2017, 11, 294–304. [Google Scholar] [CrossRef]
- Fabrizi, A.; Timelli, G. The influence of cooling rate and Fe/Cr content on AlSiCu diecasting alloy. Conf. Ser. Mater. Sci. Eng. 2016, 117, 1–6. [Google Scholar]
- Fabrizi, A.; Timelli, G.; Ferrano, S.; Bonollo, F. Evolution of Fe-rich compounds in a secondary AlSiCu alloy: Influence of cooling rate. Int. J. Mater. Res. 2015, 106, 719–724. [Google Scholar] [CrossRef]
- Timelli, G.; Fabrizi, A.; Vezzu, S.; Mori, A.D. Design of wear resistant diecast AlSi9Cu3(Fe) for high temperature components. Metals 2020, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Girad, E.; Suéry, M.; Adrien, J.; Maire, E.; Corest, M. Hot tearing sensitivity of Al-Mg-Si alloys evaluated by X-ray microtomography after constrained solidification at high cooling rate. In Hot Cracking Phenomena in Welds III; Böllinghaus, T., Lippold, J., Cross, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic-Krstic, J. Impact of Casting Parameters and Chemical Composition on the Solidification Behaviour of Al-Si-Cu Hypoeutectic Alloy. Ph.D. Thesis, Fakultät für Maschinenbau, Universität Magdeburg, Magdeburg, Germany, 2010. [Google Scholar]
- Timelli, G.; Fabrizi, A.; Capuzzi, S.; Bonollo, F.; Ferraro, S. The role of Cr additions and Fe-rich compounds on microstructural features and impact toughness of AlSi9Cu3(Fe) diecasting alloys. Mater. Sci. Eng. A 2014, 603, 58–68. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Zhou, H. Effects of pouring temperature on microstructure and mechanical properties of the A356 aluminum alloy die castings. Mater. Res. 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Sanna, A.F.; Fabrizi, S.; Ferraro, G.; Timelli, P.; Bonollo, F. Multiscale characterisation of AlSi9Cu3(Fe) die casting alloys after Cu, Mg, Zn and Sr addition. Metall. Ital. 2013, 4, 13–24. [Google Scholar]

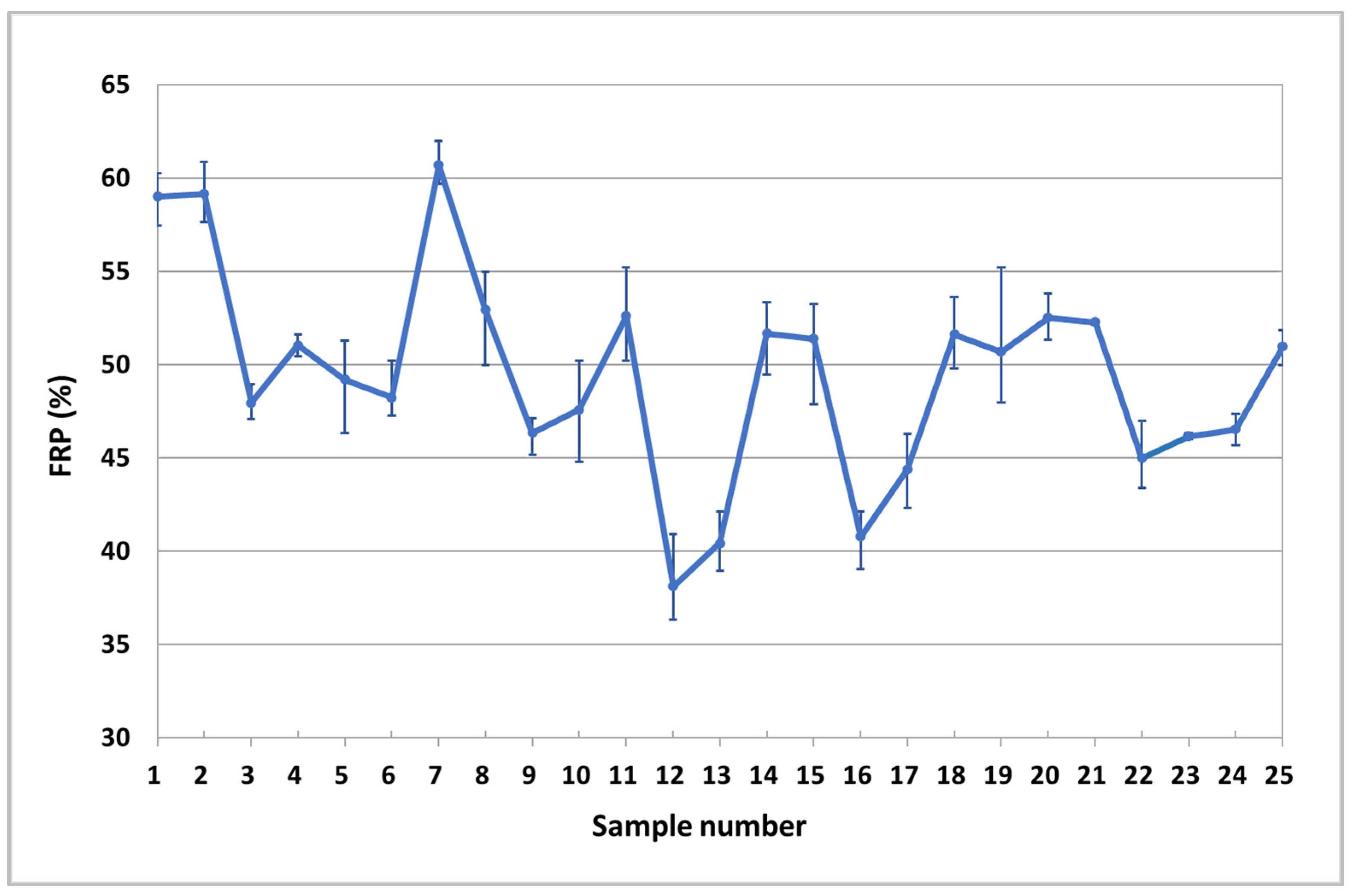
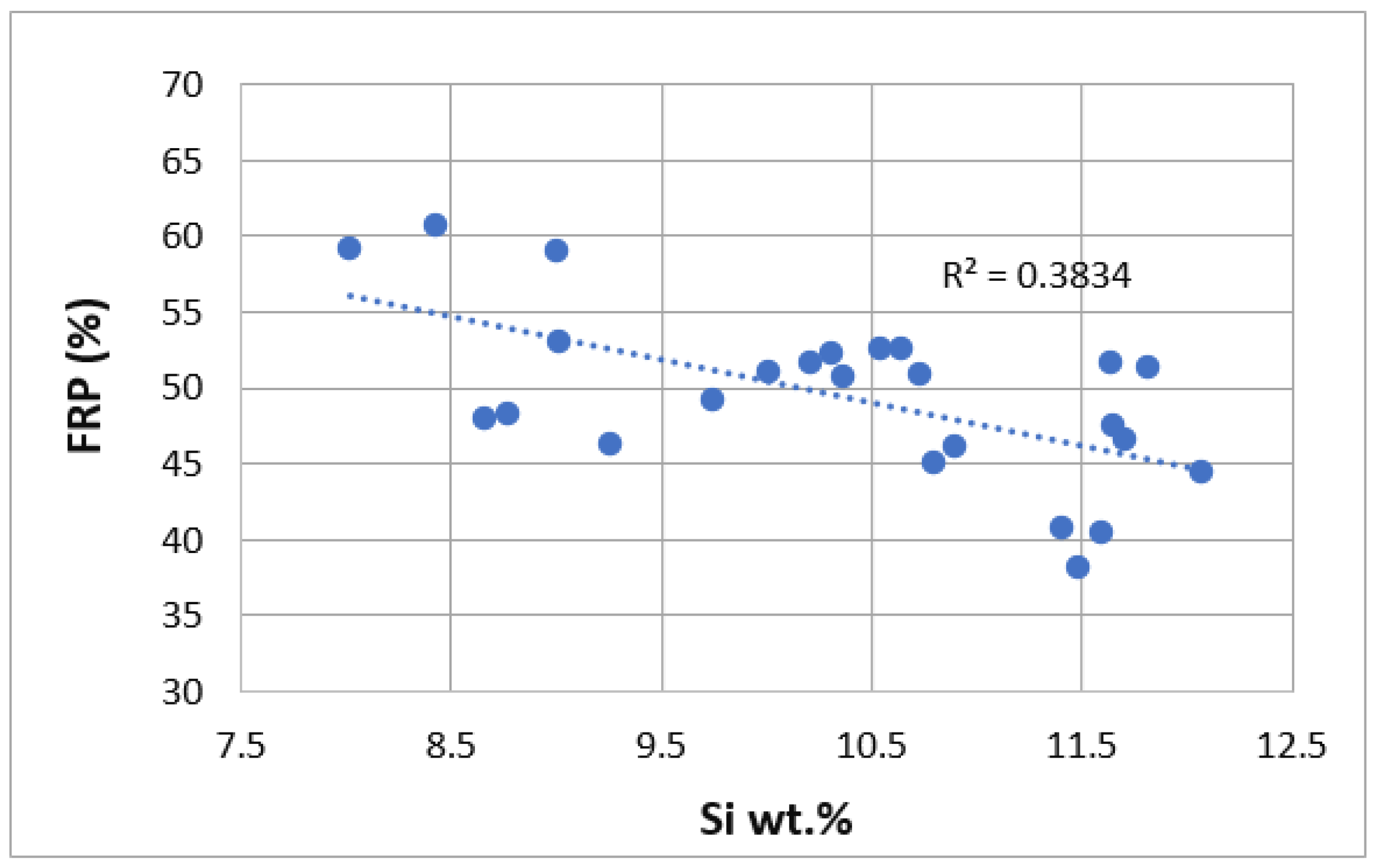
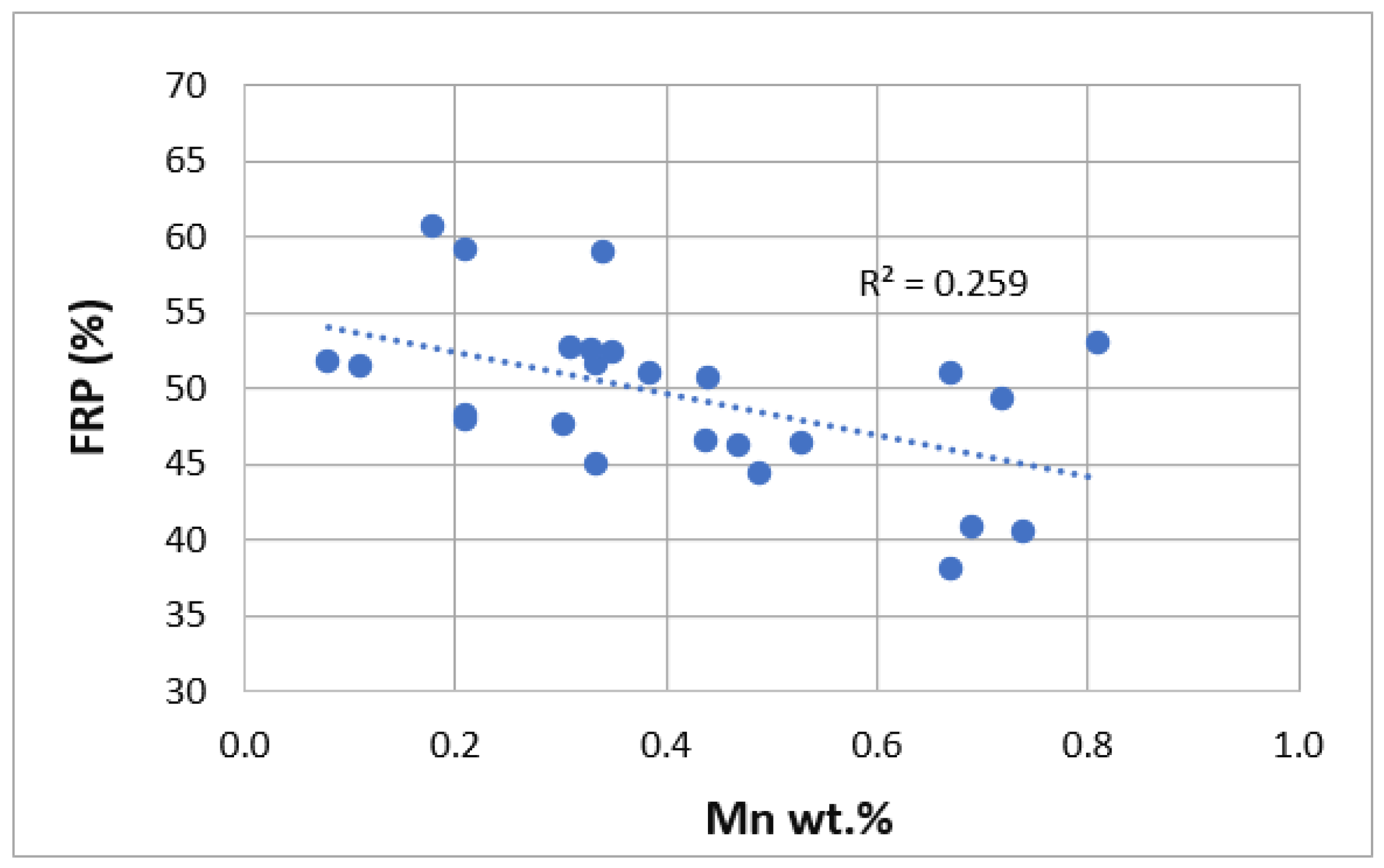
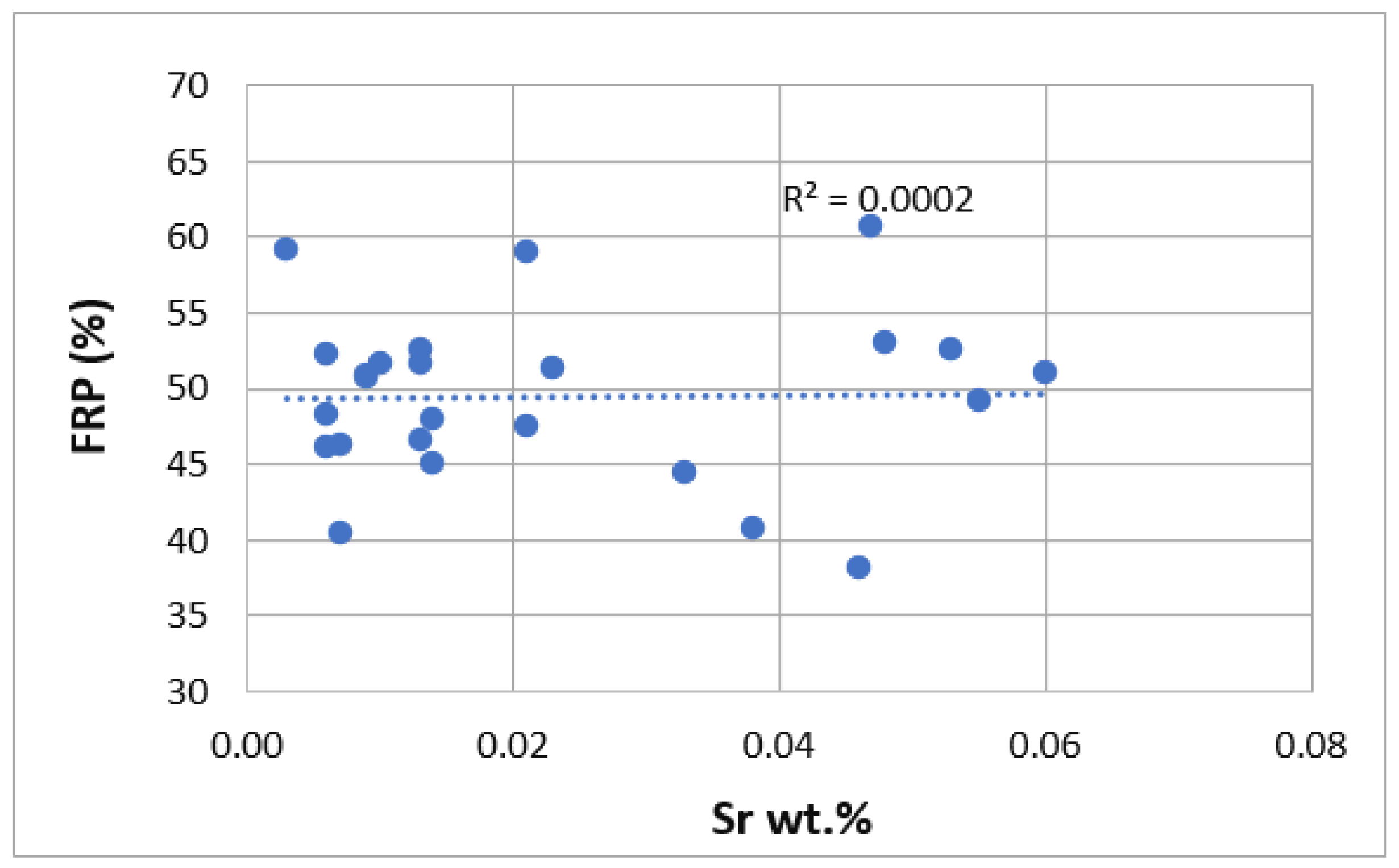
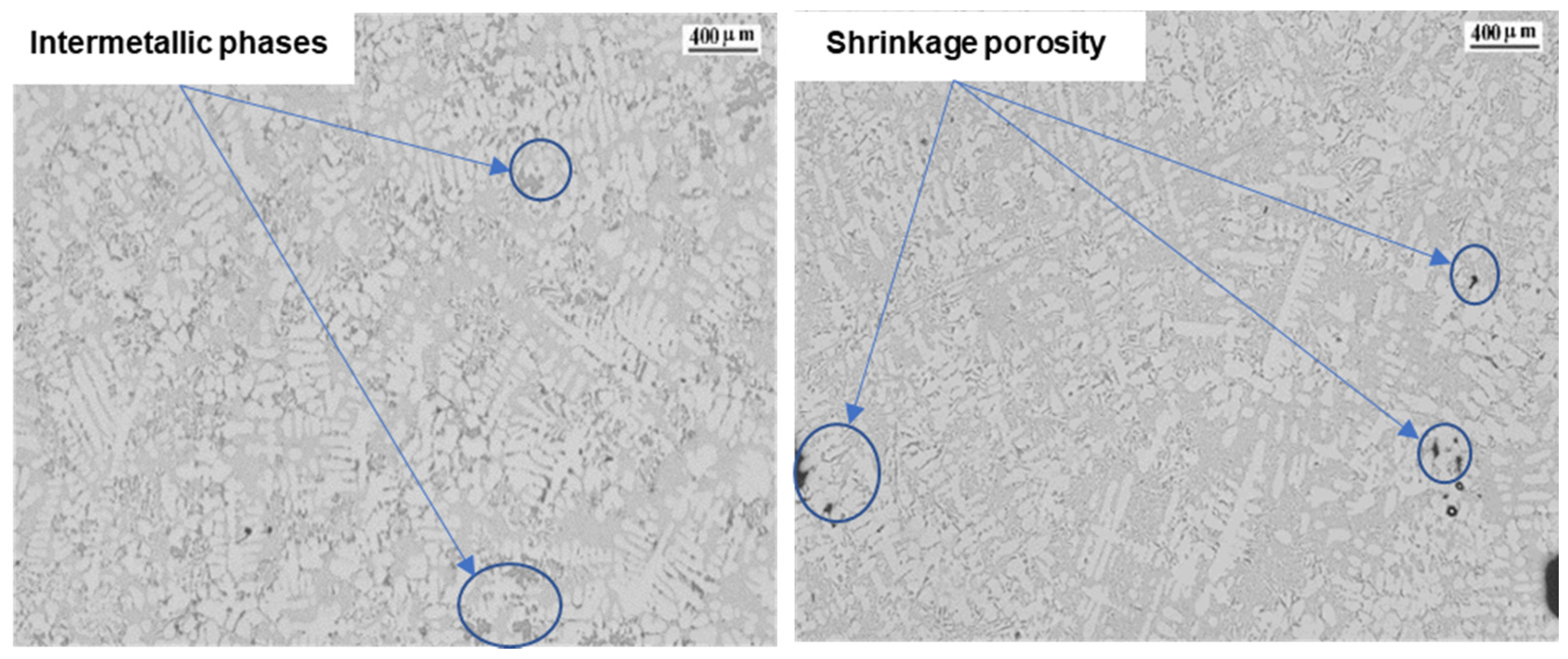


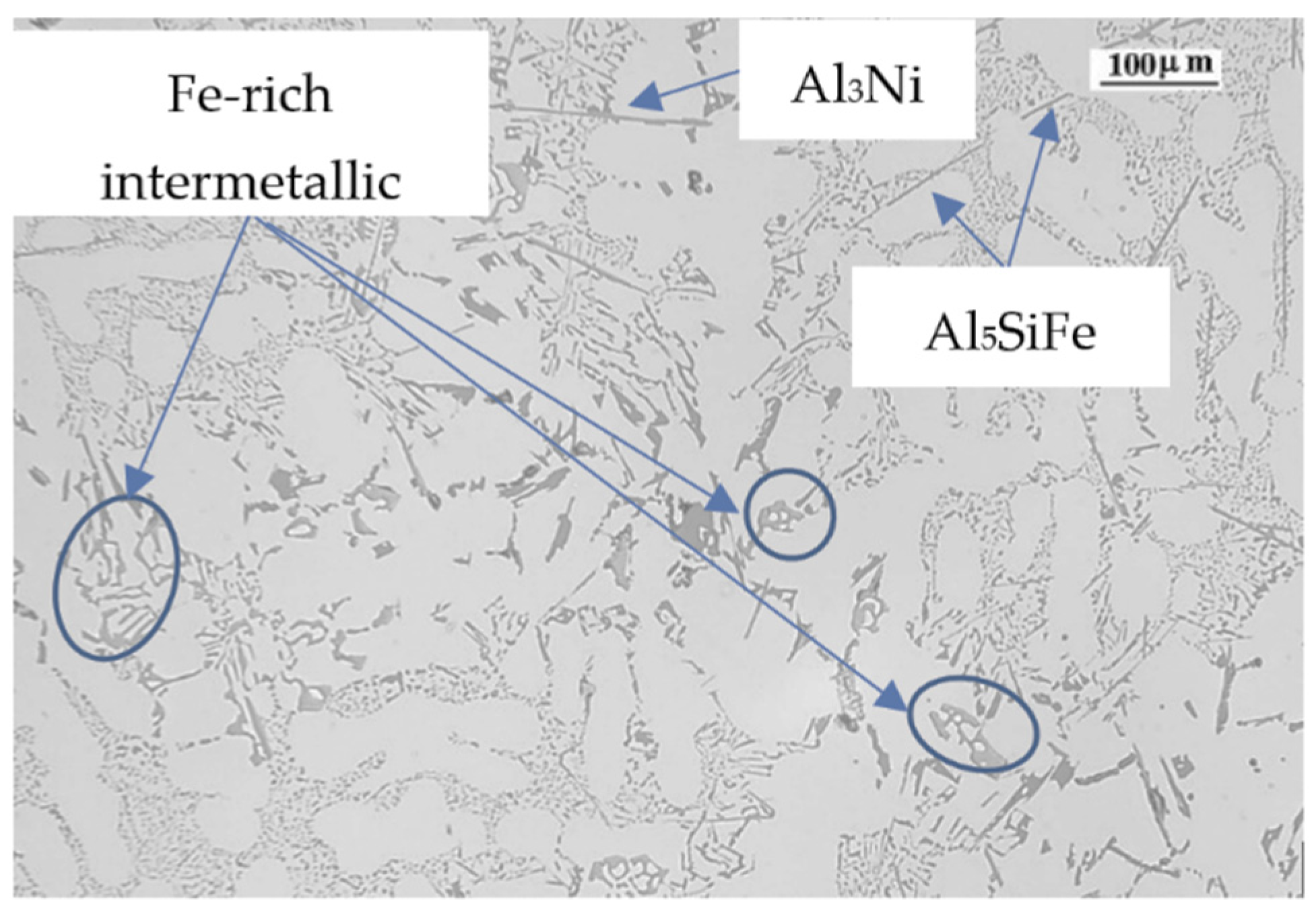
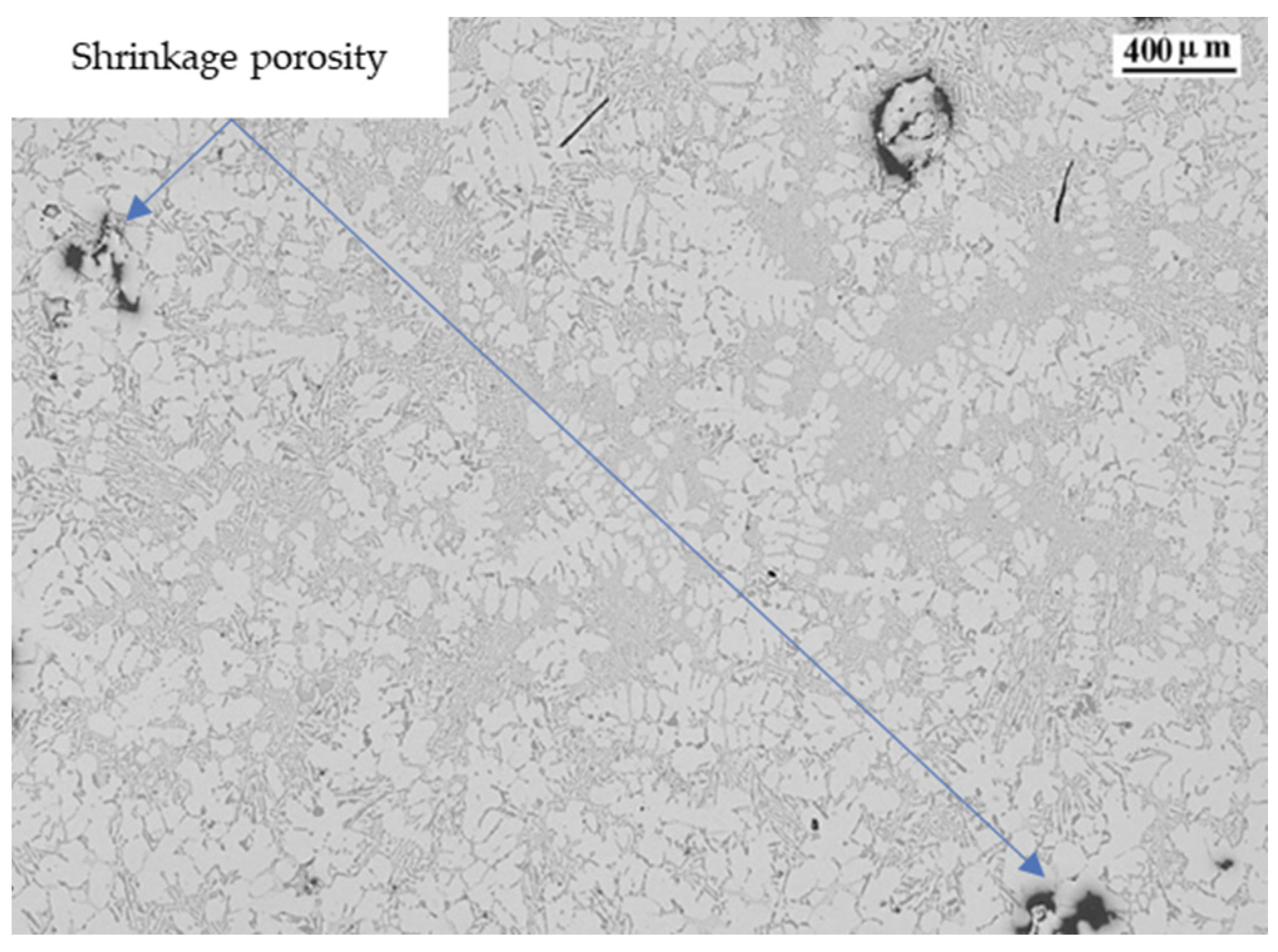
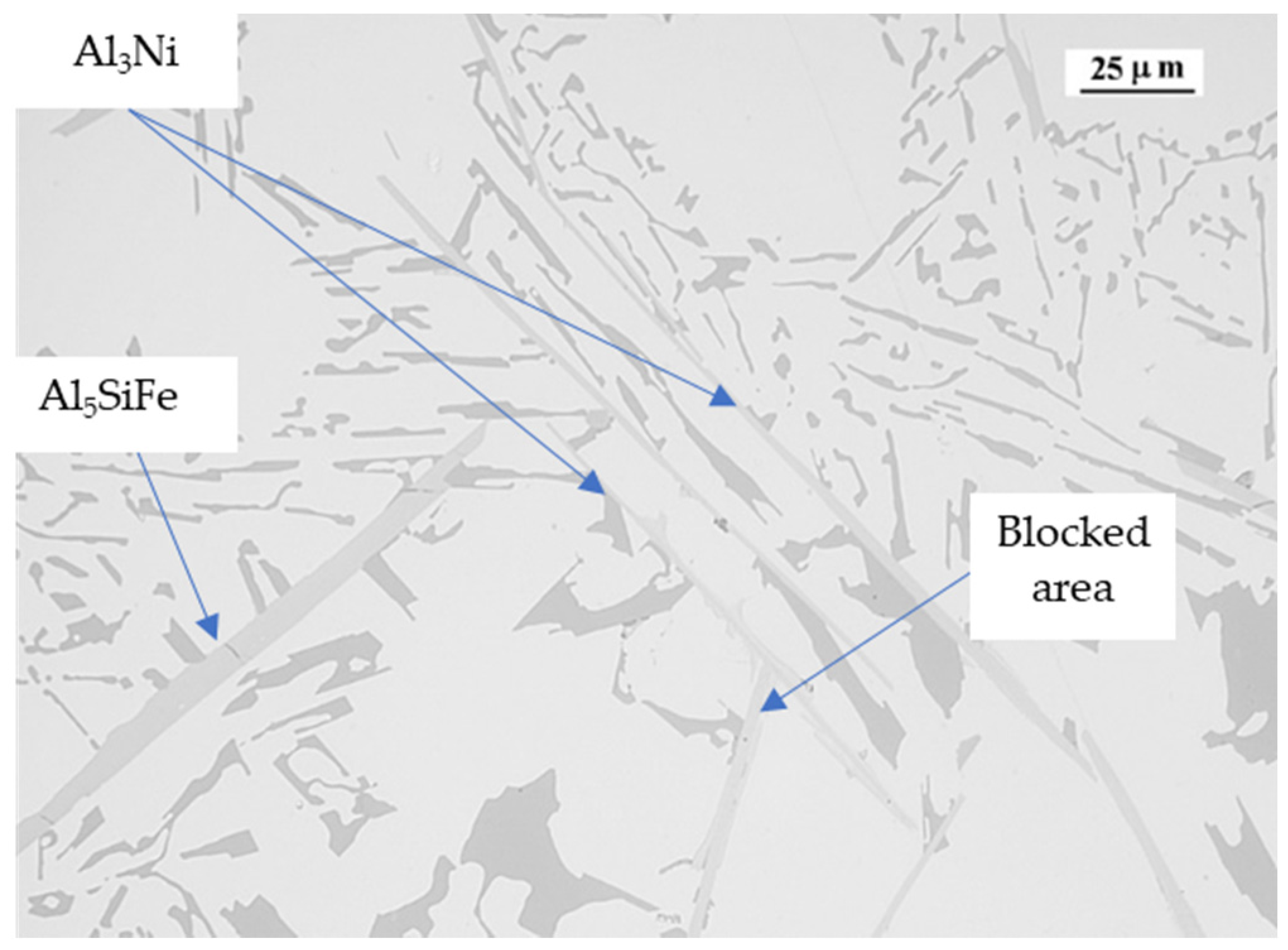
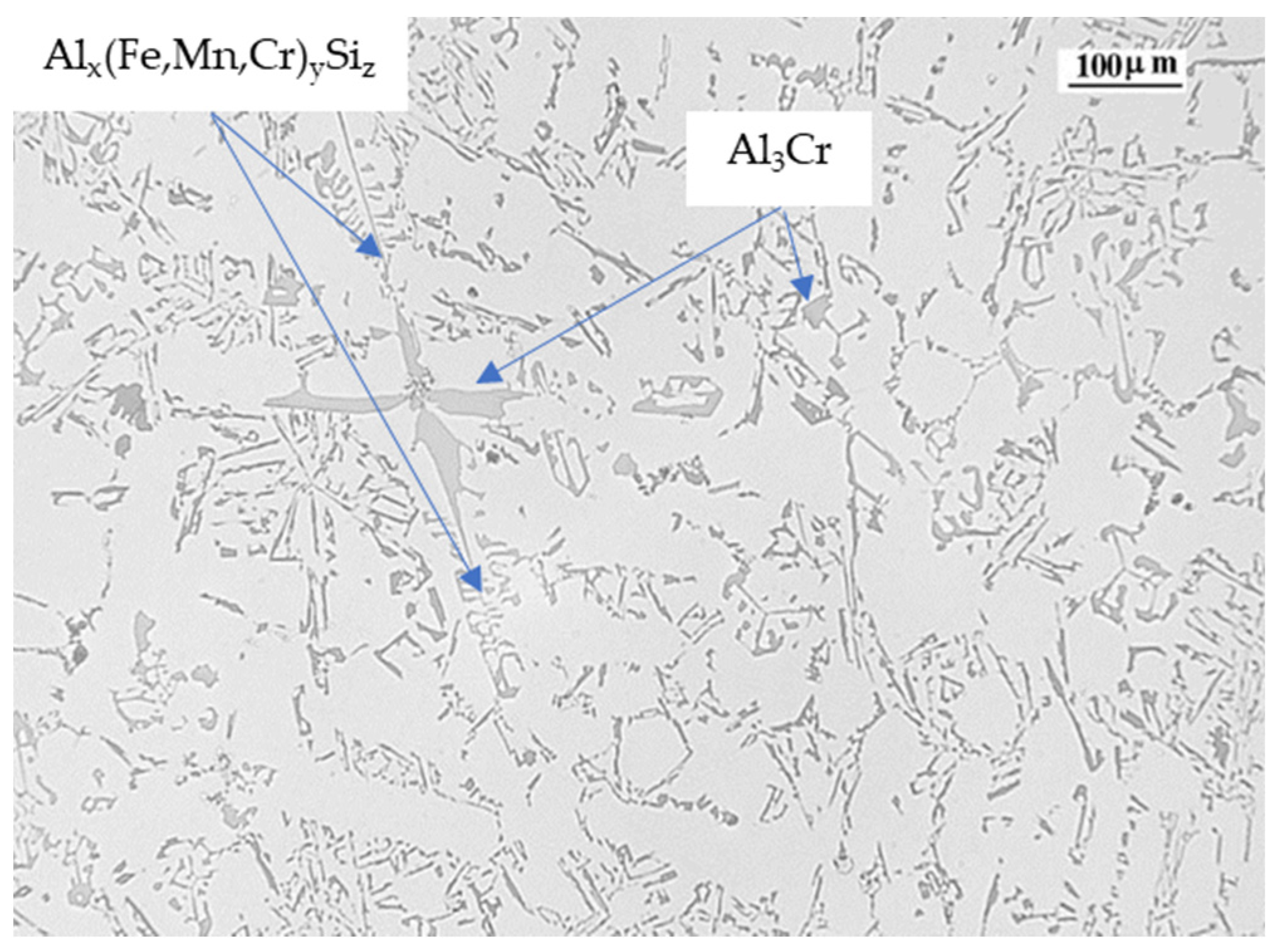
| Ref. | Si | Mg | Fe | Cu | Ni | Cr | Mn | Ti | Zn | Pb | Sn | Sr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9.00 | 0.30 | 0.38 | 0.03 | 0.00 | 0.01 | 0.34 | 0.02 | 0.01 | 0.00 | 0.002 | 0.021 |
| 2 | 8.02 | 0.19 | 0.29 | 0.02 | 0.00 | 0.01 | 0.21 | 0.01 | 0.00 | 0.00 | 0.003 | 0.003 |
| 3 | 8.66 | 0.14 | 0.3 | 0.02 | 0.00 | 0.01 | 0.21 | 0.2 | 0.29 | 0.27 | 0.039 | 0.014 |
| 4 | 10.01 | 0.69 | 0.34 | 0.02 | 0.23 | 0.15 | 0.67 | 0.02 | 0.01 | 0.00 | 0.002 | 0.060 |
| 5 | 9.75 | 0.68 | 0.34 | 0.02 | 0.23 | 0.15 | 0.72 | 0.12 | 0.35 | 0.14 | 0.064 | 0.055 |
| 6 | 8.77 | 0.15 | 0.85 | 0.19 | 0.21 | 0.16 | 0.21 | 0.12 | 0.16 | 0.21 | 0.073 | 0.006 |
| 7 | 8.43 | 0.11 | 0.91 | 0.19 | 0.19 | 0.14 | 0.18 | 0.19 | 0.18 | 0.19 | 0.066 | 0.047 |
| 8 | 9.02 | 0.38 | 1.05 | 0.29 | 0.21 | 0.07 | 0.81 | 0.17 | 0.06 | 0.21 | 0.019 | 0.048 |
| 9 | 9.26 | 0.56 | 0.73 | 0.09 | 0.00 | 0.07 | 0.53 | 0.02 | 0.21 | 0.01 | 0.002 | 0.007 |
| 10 | 11.65 | 0.58 | 0.34 | 0.20 | 0.20 | 0.02 | 0.3 | 0.24 | 0.03 | 0.07 | 0.032 | 0.021 |
| 11 | 10.54 | 0.52 | 0.34 | 0.16 | 0.15 | 0.02 | 0.31 | 0.17 | 0.23 | 0.26 | 0.026 | 0.053 |
| 12 | 11.49 | 0.40 | 0.91 | 0.42 | 0.00 | 0.14 | 0.67 | 0.23 | 0.15 | 0.18 | 0.040 | 0.046 |
| 13 | 11.6 | 0.46 | 0.83 | 0.18 | 0.00 | 0.18 | 0.74 | 0.02 | 0.19 | 0.23 | 0.003 | 0.007 |
| 14 | 11.64 | 0.53 | 0.96 | 0.08 | 0.08 | 0.16 | 0.08 | 0.27 | 0.13 | 0.08 | 0.033 | 0.010 |
| 15 | 11.82 | 0.52 | 0.96 | 0.11 | 0.11 | 0.14 | 0.11 | 0.11 | 0.18 | 0.11 | 0.046 | 0.023 |
| 16 | 11.41 | 0.35 | 0.95 | 0.27 | 0.30 | 0.09 | 0.69 | 0.25 | 0.09 | 0.25 | 0.026 | 0.038 |
| 17 | 12.07 | 0.28 | 0.83 | 0.13 | 0.17 | 0.03 | 0.49 | 0.08 | 0.02 | 0.16 | 0.055 | 0.033 |
| 18 | 10.21 | 0.28 | 0.43 | 0.05 | 0.00 | 0.07 | 0.33 | 0.02 | 0.08 | 0.00 | 0.002 | 0.013 |
| 19 | 10.37 | 0.28 | 0.50 | 0.11 | 0.00 | 0.14 | 0.44 | 0.02 | 0.01 | 0.00 | 0.002 | 0.009 |
| 20 | 10.64 | 0.63 | 0.41 | 0.05 | 0.00 | 0.07 | 0.33 | 0.02 | 0.10 | 0.00 | 0.001 | 0.013 |
| 21 | 10.31 | 0.29 | 0.54 | 0.09 | 0.00 | 0.11 | 0.35 | 0.01 | 0.01 | 0.00 | 0.002 | 0.006 |
| 22 | 10.8 | 0.52 | 0.48 | 0.05 | 0.00 | 0.06 | 0.34 | 0.03 | 0.10 | 0.00 | 0.002 | 0.014 |
| 23 | 10.9 | 0.43 | 0.51 | 0.10 | 0.00 | 0.11 | 0.47 | 0.01 | 0.02 | 0.00 | 0.005 | 0.006 |
| 24 | 11.71 | 0.44 | 0.57 | 0.07 | 0.00 | 0.08 | 0.44 | 0.02 | 0.04 | 0.00 | 0.002 | 0.013 |
| 25 | 10.73 | 0.36 | 0.60 | 0.10 | 0.00 | 0.09 | 0.38 | 0.02 | 0.10 | 0.00 | 0.002 | 0.009 |
| Si | Mg | Fe | Cu | Ni | Cr | Mn | Ti | Zn | Pb | Sn | Sr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.95 | 0.19 | 1.03 | 0.77 | 0.12 | 0.10 | 3.19 | 0.41 | 0.7 | 0.2 | 0.81 | 2.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanueva, E.; Vicario, I.; Sánchez, J.M.; Albizuri, J.; Montero, J. Solid Fraction Determination at the Rigidity Point by Advanced Thermal Analysis. Appl. Sci. 2022, 12, 237. https://doi.org/10.3390/app12010237
Villanueva E, Vicario I, Sánchez JM, Albizuri J, Montero J. Solid Fraction Determination at the Rigidity Point by Advanced Thermal Analysis. Applied Sciences. 2022; 12(1):237. https://doi.org/10.3390/app12010237
Chicago/Turabian StyleVillanueva, Ester, Iban Vicario, Jon Mikel Sánchez, Joseba Albizuri, and Jessica Montero. 2022. "Solid Fraction Determination at the Rigidity Point by Advanced Thermal Analysis" Applied Sciences 12, no. 1: 237. https://doi.org/10.3390/app12010237
APA StyleVillanueva, E., Vicario, I., Sánchez, J. M., Albizuri, J., & Montero, J. (2022). Solid Fraction Determination at the Rigidity Point by Advanced Thermal Analysis. Applied Sciences, 12(1), 237. https://doi.org/10.3390/app12010237






