Replacing Mineral Fertilisers for Bio-Based Fertilisers in Potato Growing on Sandy Soil: A Case Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
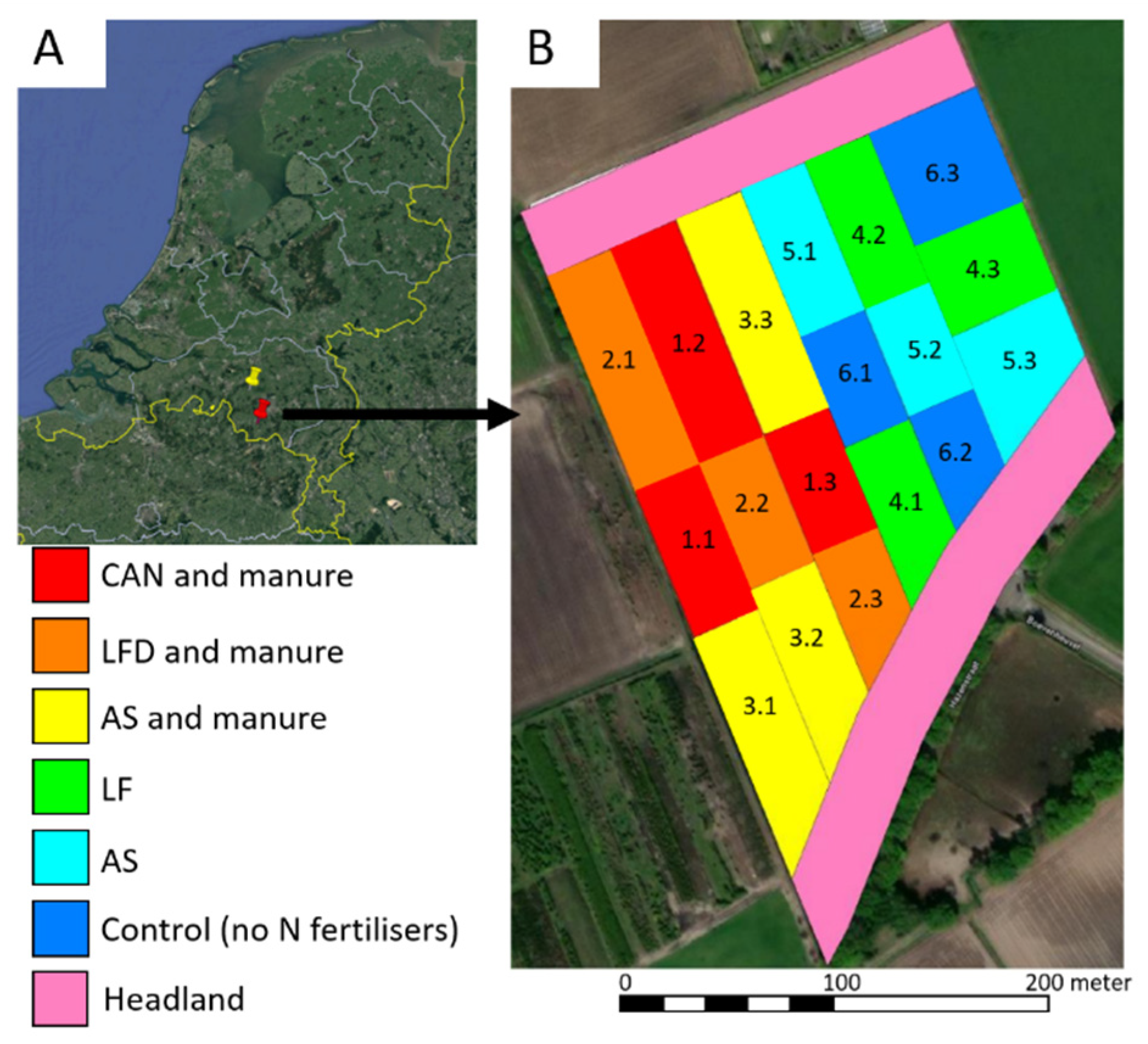
2.1. Study Area
2.1.1. Bio-Based Fertiliser Products
2.1.2. Bio-Based Fertiliser End User
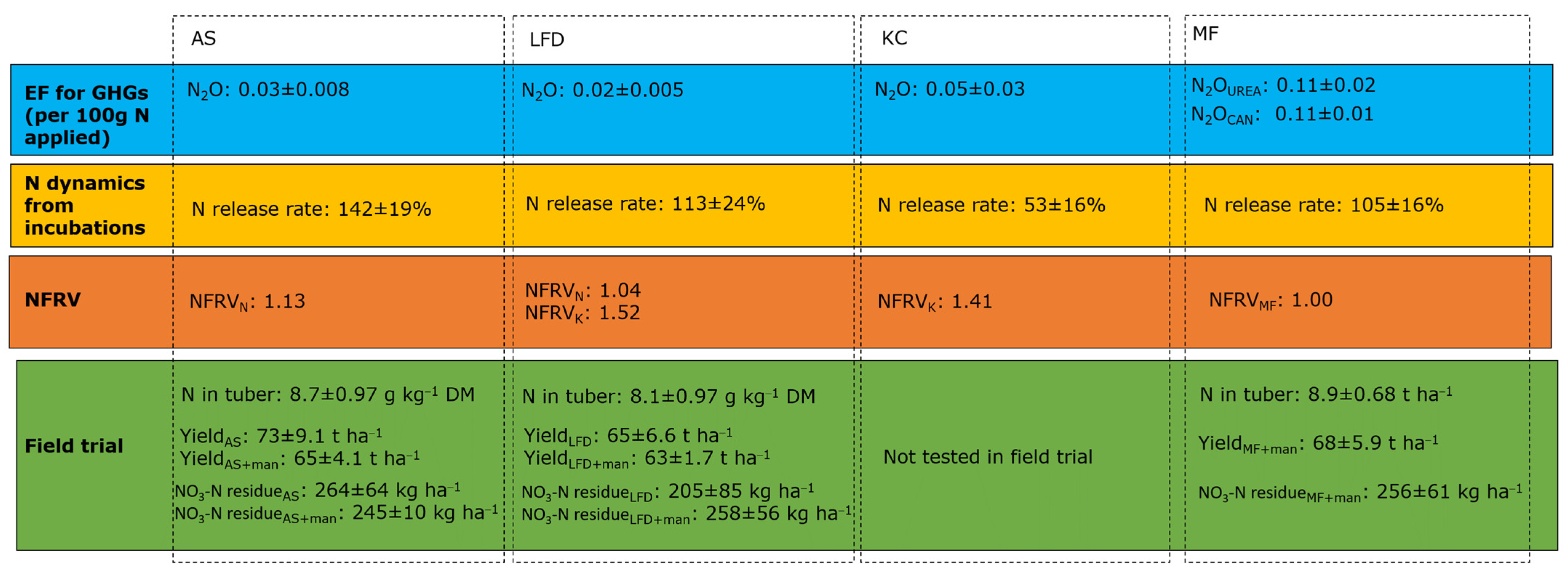
2.2. N Release Rate of Bio-Based Fertilisers
2.3. GHG Emissions of the Bio-Based Fertilisers after Soil Application
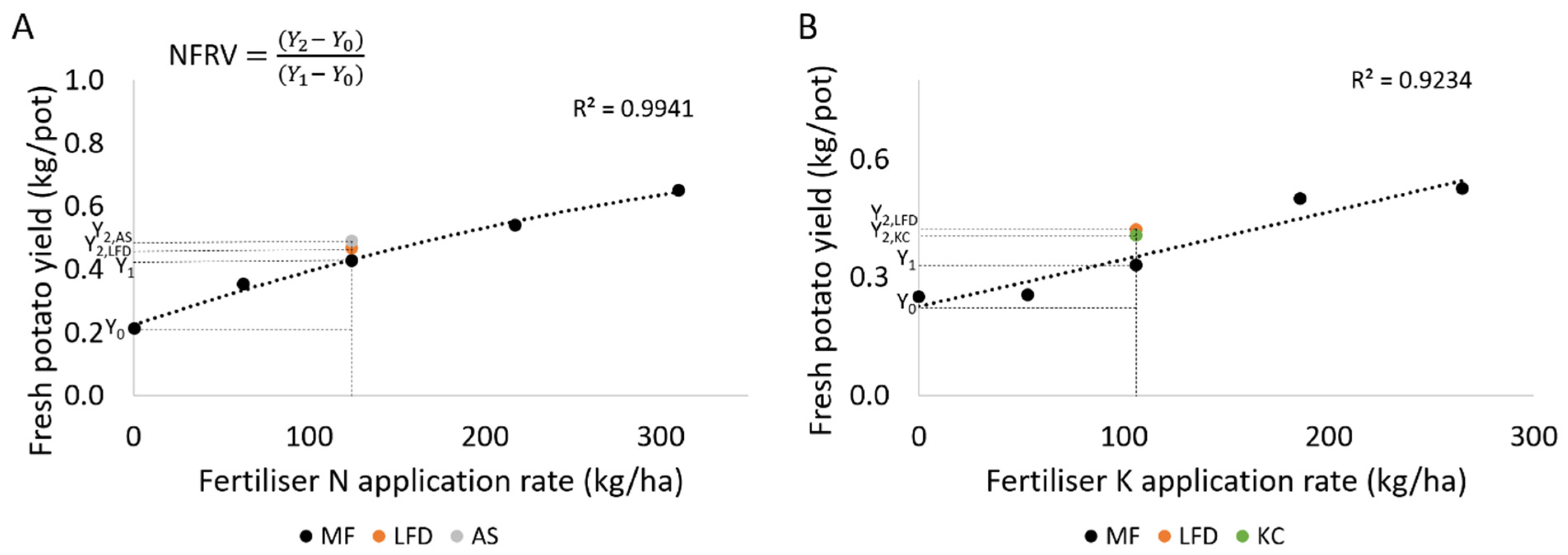
2.4. Nutrient Fertiliser Replacement Value
2.5. Applicability of Bio-Based Fertilisers in a Full-Scale Field Trial
2.6. Statistics
3. Results and Discussion
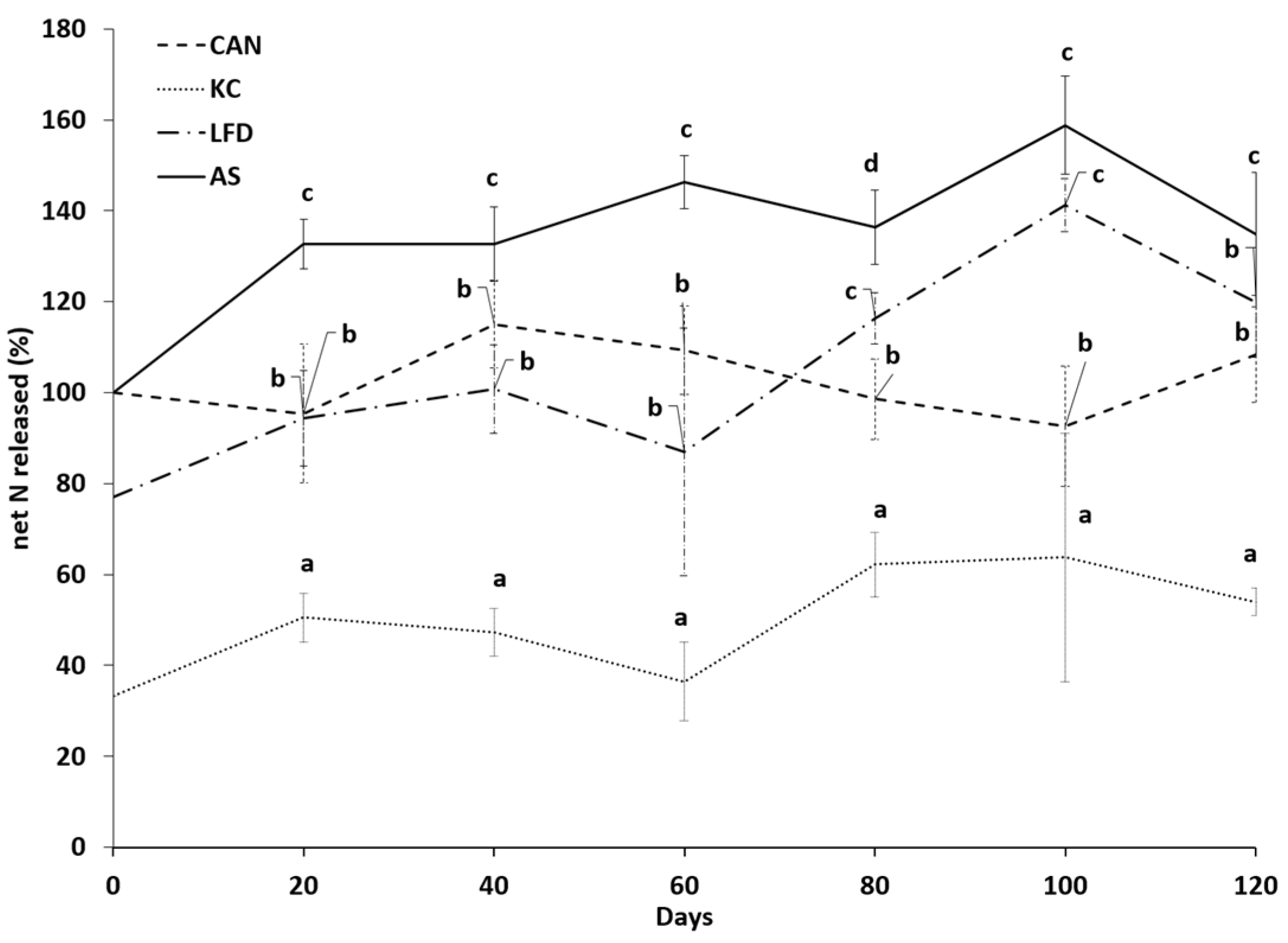
3.1. N Release Rate of Bio-Based Fertilisers
3.2. Emissions from the Bio-Based Fertilisers
3.3. Nutrient Fertiliser Replacement Value
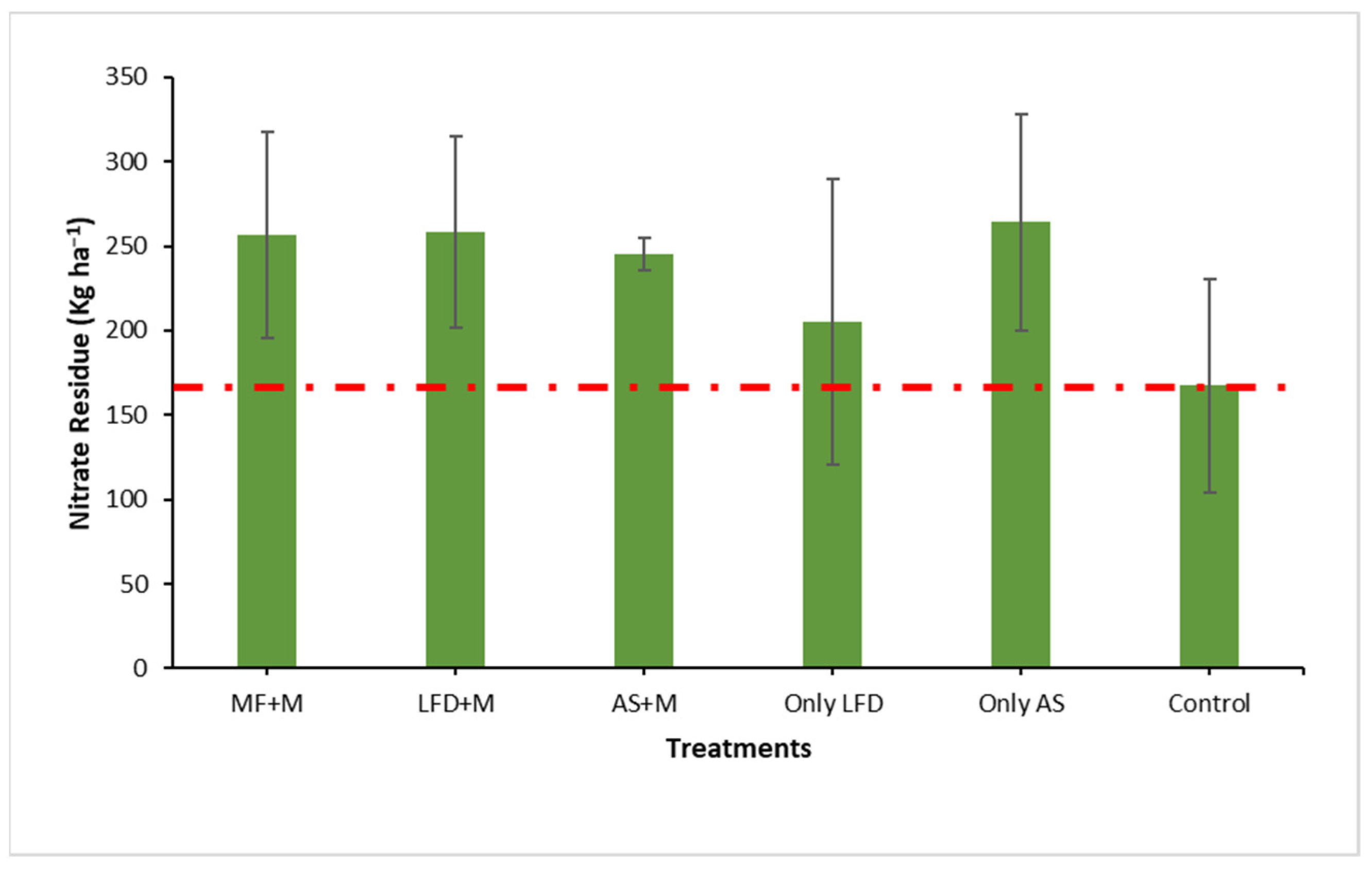
3.4. Applicability of Bio-Based Fertilisers in a Full-Scale Field Trial
3.5. Environmental and Agronomic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
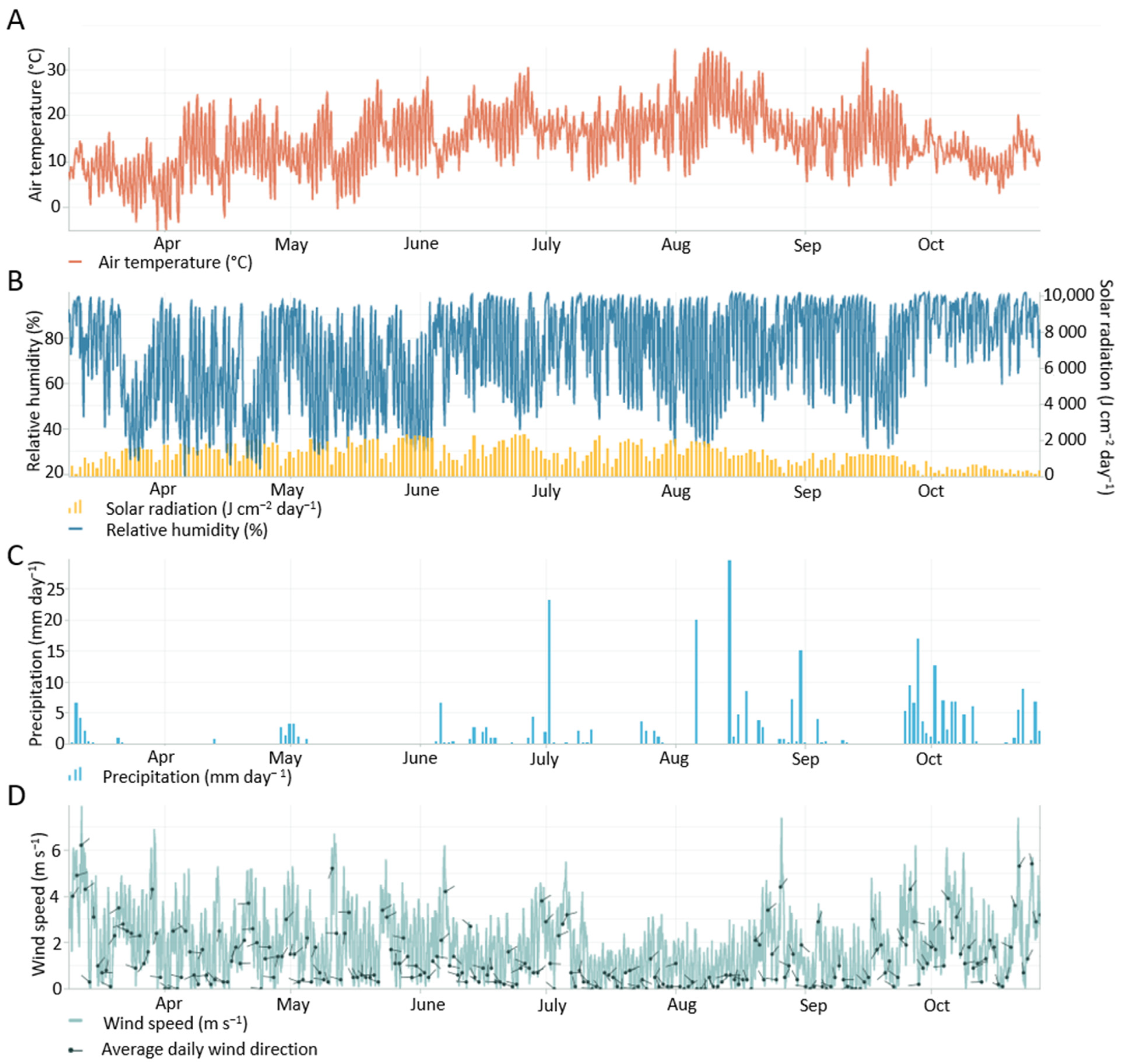
Appendix B
| Parameters | OC (g kg−1) | Ntot (g kg−1) | NH4-N (g kg−1) | C/Ntot | C/Organic N | NH4-N/Ntot | Organic N/Ntot | Total N-min (mg kg−1) | N Release (%) | N Mineralization (%) | Ntot Added (mg) | NH4-N Added (mg) | OC Added (mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OC (g kg−1) | - | ||||||||||||
| Ntot (g kg−1) | −0.558 | - | |||||||||||
| NH4-N (g kg−1) | −0.588 | 0.999 * | - | ||||||||||
| C/Ntot | 0.977 | −0.721 | −0.746 | - | |||||||||
| C/organic N | 0.711 | −0.98 | −0.987 | 0.844 | - | ||||||||
| NH4-N/Ntot | −0.996 | 0.633 | 0.661 | −0.993 | −0.774 | - | |||||||
| OrganicN/Ntot | 0.996 | −0.633 | −0.661 | 0.993 | 0.774 | −1.000 ** | - | ||||||
| Total N-min (mg kg−1) | −0.962 | 0.762 | 0.786 | −0.998 * | −0.875 | 0.984 | −0.984 | - | |||||
| N release (%) | −0.986 | 0.688 | 0.714 | −0.999 * | −0.818 | 0.997 * | −0.997 * | 0.994 | - | ||||
| N mineralization (%) | −0.96 | 0.769 | 0.792 | −0.997 * | −0.88 | 0.982 | −0.982 | 1.000 ** | 0.993 | - | |||
| Ntot added (mg) | 0.528 | −0.999 * | −0.997 * | 0.696 | 0.972 | −0.605 | 0.605 | −0.739 | −0.661 | −0.745 | - | ||
| NH4-N added (mg) | −0.996 | 0.629 | 0.657 | −0.992 | −0.77 | 1.000 ** | −1.000 ** | 0.983 | 0.997 * | 0.981 | −0.6 | - | |
| OC added (mg) | 0.935 | −0.227 | −0.263 | 0.839 | 0.415 | −0.898 | 0.898 | −0.803 | −0.863 | −0.797 | 0.192 | −0.9 | - |
References
- Liu, Y.; Pan, X.; Li, J. A 1961–2010 record of fertilizer use, pesticide application and cereal yields: A review. Agron. Sustain. Dev. 2015, 35, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Pinsard, C.; Martin, S.; Léger, F.; Accatino, F. Robustness to import declines of three types of European farming systems assessed with a dynamic nitrogen flow model. Agric. Syst. 2021, 193, 103215. [Google Scholar] [CrossRef]
- Duan, Y.-F.; Bruun, S.; Stoumann Jensen, L.; Van Gerven, L.; Hendriks, C.M.J.; Stokkermans, L.; Groenedijk, P.; Lesschen, J.P.; Prado, J.; Fangueiro, D. Nutri2Cycle: D1.5 Mapping and Characterization of CNP Flows and Their Stoichiometry in Main Farming Systems in Europe. 2020. Available online: https://www.nutri2cycle.eu/wp-content/uploads/2021/05/D1.5-Nutri2Cycle_Report-on-the-mapping-and-charcaterization-of-CNP-flows.pdf (accessed on 2 November 2021).
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving Crop Yield and Nutrient Use Efficiency via Biofertilization—A Global Meta-analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EUROSTAT. Agri-Environmental Indicator-Mineral Fertiliser Consumption. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agri-environmental_indicator_-_mineral_fertiliser_consumption (accessed on 15 October 2021).
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef] [Green Version]
- Carsel, R.F.; Smith, C.N.; Mulkey, L.A.; Dean, J.D.; Jowise, P. User’s Manual for the Pesticide Root Zone Model (PRZM): Release 1; Environmental Research Laboratory: Athens, GA, USA, 1984. [Google Scholar]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigurnjak, I.; Brienza, C.; Snauwaert, E.; De Dobbelaere, A.; De Mey, J.; Vaneeckhaute, C.; Michels, E.; Schoumans, O.; Adani, F.; Meers, E. Production and performance of bio-based mineral fertilizers from agricultural waste using ammonia (stripping-)scrubbing technology. Waste Manag. 2019, 89, 265–274. [Google Scholar] [CrossRef]
- Bamelis, L.; Blancke, S.; Camargo-Valero, M.A.; De Clercq, L.; Haumont, A.; De Keulenaere, B.; Delvigne, F.; Meers, E.; Michels, E.; Ramirez-Sosa, D.R.; et al. BIOREFINE: Recycling Inorganic Chemicals from Digestate Derivatives. 2011. Available online: https://www.biorefine.eu/wp-content/uploads/2021/03/wp2a5_gxabt_20151216_recovery_techniques_-_digestate.pdf (accessed on 17 December 2021).
- Regelink, I.; Van Puffelen, J.L.; Ehlert, P.A.I.; Schoumans, O.F. Evaluatie van Verwerkingsinstallaties voor Mest en Co-Vergiste Mest; Wageningen Environmental Research: Wageningen, The Netherlands, 2021. [Google Scholar]
- Huygens, D.; Orveillon, G.; Lugato, E.; Tavazzi, S.; Comero, S.; Jones, A.; Gawlik, B.; Saveyn, H. Technical Proposals for the Safe Use of Processed Manure above the Threshold Established for Nitrate Vulnerable Zones by the Nitrates Directive (91/676/EEC). 2020. Available online: https://op.europa.eu/en/publication-detail/-/publication/b135440f-f7c4-11ea-991b-01aa75ed71a1/language-en (accessed on 17 December 2021).
- Tur-Cardona, J.; Bonnichsen, O.; Speelman, S.; Verspecht, A.; Carpentier, L.; Debruyne, L.; Marchand, F.; Jacobsen, B.H.; Buysse, J. Farmers’ reasons to accept bio-based fertilizers: A choice experiment in seven different European countries. J. Clean. Prod. 2018, 197, 406–416. [Google Scholar] [CrossRef]
- Chesworth, W.; Camps Arbestain, M.; Macías, F.; Spaargaren, O.; Spaargaren, O.; Mualem, Y.; Morel-Seytoux, H.J.; Horwath, W.R.; Almendros, G.; Chesworth, W.; et al. Classification of Soils: World Reference Base (WRB) for soil resources. In Encyclopedia of Soil Science; Chesworth, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 120–122. ISBN 978-1-4020-3995-9. [Google Scholar]
- Ameloot, N.; Sleutel, S.; Das, K.C.; Kanagaratnam, J.; de Neve, S. Biochar amendment to soils with contrasting organic matter level: Effects on N mineralization and biological soil properties. GCB Bioenergy 2015, 7, 135–144. [Google Scholar] [CrossRef]
- Egene, C.E.; Sigurnjak, I.; Regelink, I.C.; Schoumans, O.F.; Adani, F.; Michels, E.; Sleutel, S.; Tack, F.M.G.; Meers, E. Solid fraction of separated digestate as soil improver: Implications for soil fertility and carbon sequestration. J. Soils Sediments 2021, 21, 678–688. [Google Scholar] [CrossRef]
- European Union. Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. L 1991, 375, 12. [Google Scholar]
- De Neve, S.; Hofman, G. Modelling N mineralization of vegetable crop residues during laboratory incubations. Soil Biol. Biochem. 1996, 28, 1451–1457. [Google Scholar] [CrossRef]
- Sigurnjak, I.; De Waele, J.; Michels, E.; Tack, F.M.G.; Meers, E.; De Neve, S. Nitrogen release and mineralization potential of derivatives from nutrient recovery processes as substitutes for fossil fuel-based nitrogen fertilizers. Soil Use Manag. 2017, 33, 437–446. [Google Scholar] [CrossRef]
- De Klein, C.; Novoa, R.S.A.; Ogle, S.; Smith, K.A.; Rochette, P.; Wirth, T.C.; McConkey, B.G.; Mosier, A.; Rypdal, K.; Walsh, M.; et al. Chapter 11: N2O Emissions from Managed Soils, and CO2 Emissions from Lime and Urea Applica. 2006. Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_11_Ch11_N2O&CO2.pdf (accessed on 17 December 2021).
- Hijbeek, R.; ten Berge, H.F.M.; Whitmore, A.P.; Barkusky, D.; Schröder, J.J.; van Ittersum, M.K. Nitrogen fertiliser replacement values for organic amendments appear to increase with N application rates. Nutr. Cycl. Agroecosyst. 2018, 110, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Schröder, J. Manure as a Suitable Component of Precise Nitrogen Nutrition. In Proceedings No. 574; International Fertiliser Society: York, UK, 2005; pp. 1–32. [Google Scholar]
- Delin, S. Fertilizer value of nitrogen in hen and broiler manure after application to spring barley using different application timing. Soil Use Manag. 2011, 27, 415–426. [Google Scholar] [CrossRef]
- Jensen, L.S. Animal Manure Fertiliser Value, Crop Utilisation and Soil Quality Impacts. In Animal Manure Recycling: Treatment and Management; Wiley: Hoboken, NJ, USA, 2013; pp. 295–328. ISBN 9781118676677. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Calderón, F.J.; McCarty, G.W.; Reeves, J.B. Analysis of manure and soil nitrogen mineralization during incubation. Biol. Fertil. Soils 2005, 41, 328–336. [Google Scholar] [CrossRef]
- Grigatti, M.; Di Girolamo, G.; Chincarini, R.; Ciavatta, C.; Barbanti, L. Potential nitrogen mineralization, plant utilization efficiency and soil CO2 emissions following the addition of anaerobic digested slurries. Biomass Bioenergy 2011, 35, 4619–4629. [Google Scholar] [CrossRef]
- Azeez, J.O.; Van Averbeke, W. Nitrogen mineralization potential of three animal manures applied on a sandy clay loam soil. Bioresour. Technol. 2010, 101, 5645–5651. [Google Scholar] [CrossRef] [PubMed]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilisers—Effects on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Bean, W.T.; Stafford, R.; Brashares, J.S. The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography 2012, 35, 250–258. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Velthof, G.L.; Rietra, R.P.J.J. Nitrogen Use Efficiency and Gaseous Nitrogen Losses from the Concentrated Liquid Fraction of Pig Slurries. Int. J. Agron. 2019, 2019, 9283106. [Google Scholar] [CrossRef]
- Velthof, G.L.; Rietra, R.P.J.J. Nitrous Oxide Emission from Agricultural Soils; Wageningen Environmental Research: Wageningen, The Netherlands, 2018. [Google Scholar]
- Ren, F.; Zhang, X.; Liu, J.; Sun, N.; Wu, L.; Li, Z.; Xu, M. A synthetic analysis of greenhouse gas emissions from manure amended agricultural soils in China. Sci. Rep. 2017, 7, 8123. [Google Scholar] [CrossRef]
- Shashank, T.; Shankar, S.J.; Pratap, S.D. Methanotrophs and CH4 sink: Effect of human activity and ecological perturbations. Clim. Change Environ. Sustain. 2015, 3, 35–50. [Google Scholar]
- Harty, M.A.; Forrestal, P.J.; Watson, C.J.; McGeough, K.L.; Carolan, R.; Elliot, C.; Krol, D.; Laughlin, R.J.; Richards, K.G.; Lanigan, G.J. Reducing nitrous oxide emissions by changing N fertiliser use from calcium ammonium nitrate (CAN) to urea based formulations. Sci. Total Environ. 2016, 563–564, 576–586. [Google Scholar] [CrossRef] [Green Version]
- de Klein, C.A.M.; Sherlock, R.R.; Cameron, K.C.; van der Weerden, T.J. Nitrous oxide emissions from agricultural soils in New Zealand—A review of current knowledge and directions for future research. J. R. Soc. N. Z. 2001, 31, 543–574. [Google Scholar] [CrossRef]
- Bélanger, G.; Ziadi, N.; Walsh, J.R.; Richards, J.E.; Milburn, P.H. Residual Soil Nitrate after Potato Harvest. J. Environ. Qual. 2003, 32, 607–612. [Google Scholar] [CrossRef]
- Joshi, M.; Fogelman, E.; Belausov, E.; Ginzberg, I. Potato root system development and factors that determine its architecture. J. Plant Physiol. 2016, 205, 113–123. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Romeo, F.; Mallamaci, C.; Muscolo, A. Digestate Application on Two Different Soils: Agricultural Benefit and Risk. Waste Biomass Valorization 2021, 12, 4341–4353. [Google Scholar] [CrossRef]
- Reuland, G.; Sigurnjak, I.; Dekker, H.; Michels, E.; Meers, E. The Potential of Digestate and the Liquid Fraction of Digestate as Chemical Fertiliser Substitutes under the RENURE Criteria. Agronomy 2021, 11, 1374. [Google Scholar] [CrossRef]
- Velthof, G.L. Mineral Concentrate from Processed Manure as Fertiliser; Wageningen Environmental Research: Wageningen, The Netherlands, 2015. [Google Scholar]


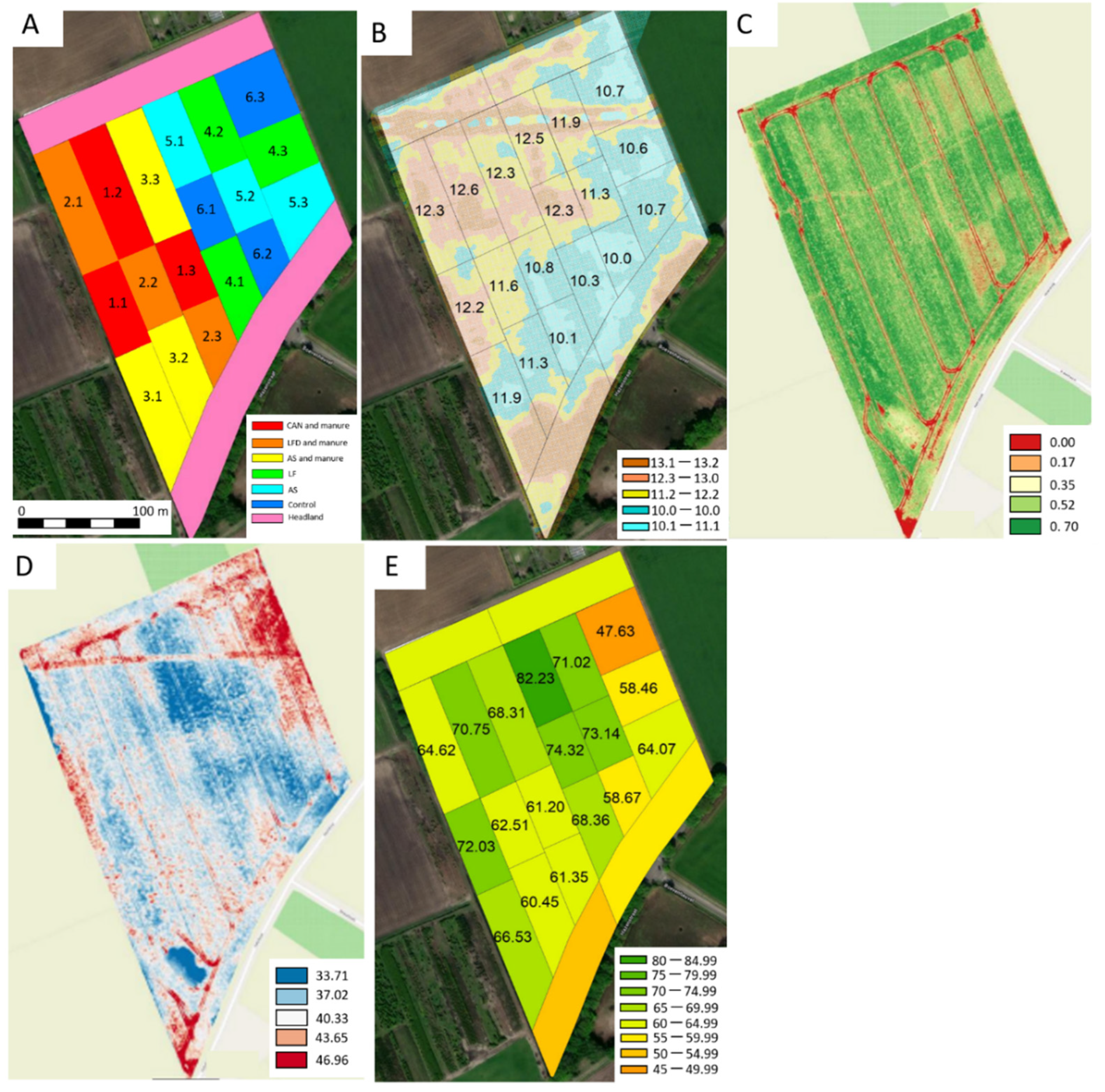
| Dry Matter % | OM g kg−1 | N g kg−1 | NH4+-N g kg−1 | Organic N g kg−1 | Total C g kg−1 | TOC g L−1 | C/N Ratio | P g kg−1 | K g kg−1 | S g kg−1 | pH | EC mS cm−1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AS | 36 | N/A | 81.6 | 81.6 | N/A | 0.3 | 0.1 | N/A | 0.1 | N/A | 11.9 | 2.8 | 278 |
| KC | 12 | 69 | 6.4 | 2.5 | 3.9 | 41.4 | 27.8 | 6.5 | 4.9 | 20.7 | 3 | 7.4 | 59 |
| LFD | 3 | 9 | 4.5 | 3.7 | 0.8 | 9.2 | 2.8 | 2 | 0.7 | 6.9 | 0.1 | 8.0 | 44 |
| Man | 3.8 | 2.4 | 4.0 |
| Soil Characteristics | Fertiliser Recommendation | ||||
|---|---|---|---|---|---|
| Potato Field Soil | K-Poor Soil | Potato Field Soil | K-Poor Soil | ||
| N total stock (kg ha−1) | 4030 | 3930 | |||
| N-supply capacity (kg ha−1) | 60 | 45 | N kg ha−1 yr−1 | 310 | 310 |
| C/N-ratio | 13 | 17 | |||
| Plant available S (kg ha−1) | 4 | 161 | SO3 kg ha−1 yr−1 | 23 | 23 |
| Plant available P (kg ha−1) | 6.5 | 5.4 | P2O5 kg ha−1 yr−1 | 0 | 60 |
| Plant available K (kg ha−1) | 500 | 115 | K2O kg ha−1 yr−1 | 70 | 265 |
| Plant available Ca (kg ha−1) | 190 | 55 | CaO kg ha−1 yr−1 | 75 | 75 |
| Plant available Mg (kg ha−1) | 300 | 115 | MgO kg ha−1 yr−1 | 0 | 21 |
| Plant available Na (kg ha−1) | <20 | <20 | |||
| pH | 5.8 | 6 | |||
| C-organic (%) | 1.5 | 2 | |||
| OM (%) | 3.1 | 3.6 | Effective OM kg ha−1 yr−1 | 1020 | 1095 |
| Clay (%) | 2 | 2 | |||
| Silt (%) | 12 | 6 | |||
| Sand (%) | 83 | 88 | |||
| The Total Application Rate On each Pot | Equivalent to Elements Applied to the Field | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | CAN | Kali60 | MgSO4 | TSP | LFD | AS | N | K2O | P2O5 | SO3 |
| g pot−1 | g pot−1 | g pot−1 | g pot−1 | g pot−1 | g pot−1 | kg ha−1 | kg ha−1 | kg ha−1 | kg ha−1 | |
| CAN—0% of the advised amount N | 0 | 0.7 | 0.3 | 0 | 0 | 0 | 0 | 70 | 0 | 23 |
| CAN—20% of the advised amount N | 1.4 | 0.7 | 0.3 | 0 | 0 | 0 | 62 | 70 | 0 | 23 |
| CAN—40% of the advised amount N | 2.8 | 0.7 | 0.3 | 0 | 0 | 0 | 124 | 70 | 0 | 23 |
| CAN—70% of the advised amount N | 4.9 | 0.7 | 0.3 | 0 | 0 | 0 | 217 | 70 | 0 | 23 |
| CAN—100% of the advised amount N | 7.1 | 0.7 | 0.3 | 0 | 0 | 0 | 310 | 70 | 0 | 23 |
| LFD—40% of the advised amount N | 0 | 0 | 0.2 | 0 | 5.1 | 0 | 124 | 190 | 19 | 23 |
| AS—40% of the advised amount N | 0 | 0.7 | 0.04 | 0 | 0 | 3.4 | 124 | 70 | 0 | 23 |
| The Total Application Rate on Each Pot | Equivalent to Elements Applied to the Field | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | CAN | KCL Solution | MgSO4 | TSP | LFD | KC | N | K2O | P2O5 | SO3 |
| g pot−1 | g pot−1 | g pot−1 | g pot−1 | kg ha−1 | g pot−1 | kg ha−1 | kg ha−1 | kg ha−1 | g pot−1 | |
| KCl solution at 0% of advised amount K | 7.1 | 0 | 0.3 | 0.8 | 0 | 0 | 310 | 0 | 60 | 23 |
| KCl solution at 20% of advised amount K | 7.1 | 0.5 | 0.3 | 0.8 | 0 | 0 | 310 | 53 | 60 | 23 |
| KCl solution at 40% of advised amount K | 7.1 | 1.1 | 0.3 | 0.8 | 0 | 0 | 310 | 106 | 60 | 23 |
| KCl solution at 70% of advised amount K | 7.1 | 1.9 | 0.3 | 0.8 | 0 | 0 | 310 | 186 | 60 | 23 |
| KCl solution at 100% of advised amount K | 7.1 | 2.7 | 0.3 | 0.8 | 0 | 0 | 310 | 265 | 60 | 23 |
| LFD at 40% of advised amount K | 5.2 | 0 | 0.3 | 0.7 | 2.8 | 0 | 310 | 106 | 60 | 23 |
| KC at 40% of advised amount K | 6.9 | 0 | 0.1 | 0.5 | 0 | 3.8 | 310 | 106 | 60 | 23 |
| Fertiliser Type | N2O | CO2 | CH4 * |
|---|---|---|---|
| per 100g N Applied | |||
| Liquid fraction of digestate | 0.02 ± 0.005 | 53 ± 9 | −0.11 |
| Potassium concentrate from evaporator | 0.05 ± 0.03 | 58 ± 9 | −0.06 |
| Ammonium sulphate solution | 0.03 ± 0.008 | −20 ± 12 | −0.14 |
| Calcium ammonium nitrate | 0.11 ± 0.01 | −20 ± 7 | −0.06 |
| Urea | 0.11 ± 0.02 | 20 ± 6 | −0.08 |
| Quantity Required | Product Costs | Application Costs | Total Costs | |
|---|---|---|---|---|
| (kg/ha) | (€ kg−1 N Application ha−1) | (€ kg−1 N Application ha−1) | (€ kg−1 N Application ha−1) | |
| Manure + CAN | Manure: 80,000 | 5.7 | 30 | 35.7 |
| MF: 200 | ||||
| Manure + LFD | Manure: 80,000 | −4.4 | 87.7 * | 83.3 * |
| LFD: 10,900 | ||||
| Manure + AS | Manure: 80,000 | −3.9 | 4.8 | 0.9 |
| AS: 600 | ||||
| LFD | LFD: 64,778 | −2.5 | 321.8 * | 319.3 * |
| AS | AS: 3,582 | −1.5 | 25.7 | 24.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendriks, C.M.J.; Shrivastava, V.; Sigurnjak, I.; Lesschen, J.P.; Meers, E.; Noort, R.v.; Yang, Z.; Rietra, R.P.J.J. Replacing Mineral Fertilisers for Bio-Based Fertilisers in Potato Growing on Sandy Soil: A Case Study. Appl. Sci. 2022, 12, 341. https://doi.org/10.3390/app12010341
Hendriks CMJ, Shrivastava V, Sigurnjak I, Lesschen JP, Meers E, Noort Rv, Yang Z, Rietra RPJJ. Replacing Mineral Fertilisers for Bio-Based Fertilisers in Potato Growing on Sandy Soil: A Case Study. Applied Sciences. 2022; 12(1):341. https://doi.org/10.3390/app12010341
Chicago/Turabian StyleHendriks, Chantal M. J., Vaibhav Shrivastava, Ivona Sigurnjak, Jan Peter Lesschen, Erik Meers, Rembert van Noort, Zhongchen Yang, and Rene P. J. J. Rietra. 2022. "Replacing Mineral Fertilisers for Bio-Based Fertilisers in Potato Growing on Sandy Soil: A Case Study" Applied Sciences 12, no. 1: 341. https://doi.org/10.3390/app12010341
APA StyleHendriks, C. M. J., Shrivastava, V., Sigurnjak, I., Lesschen, J. P., Meers, E., Noort, R. v., Yang, Z., & Rietra, R. P. J. J. (2022). Replacing Mineral Fertilisers for Bio-Based Fertilisers in Potato Growing on Sandy Soil: A Case Study. Applied Sciences, 12(1), 341. https://doi.org/10.3390/app12010341







