Hypoxia Modulates Regenerative Potential of Fetal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Fetal NPC and SDSC Culture
2.2. Evaluation of Proliferation, Surface Markers, and Stemness Genes
2.2.1. Cell Proliferation
2.2.2. Surface Phenotypes of Expanded Cells
2.2.3. Reverse Transcription Quantitative Polymerase Chain Reaction
2.3. Three-Lineage Differentiation
2.3.1. Chondrogenic Induction and Evaluation
2.3.2. Adipogenic Differentiation and Evaluation
2.3.3. Osteogenic Differentiation
2.4. Statistical Analysis
3. Results
3.1. Effects of Hypoxia on Proliferation Potential, Surface Marker Expression, and Stemness Gene Expression of Fetal Cells
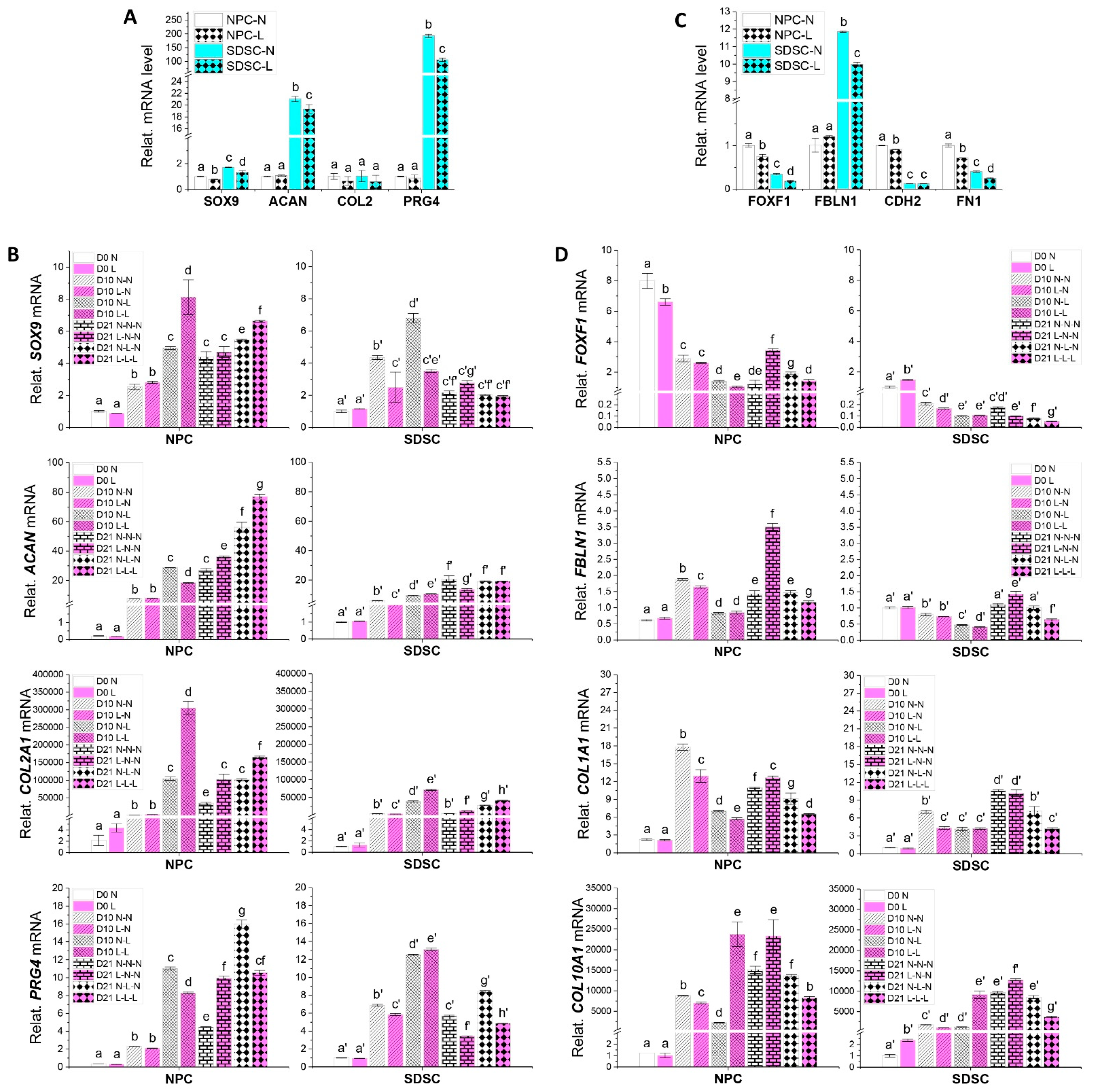
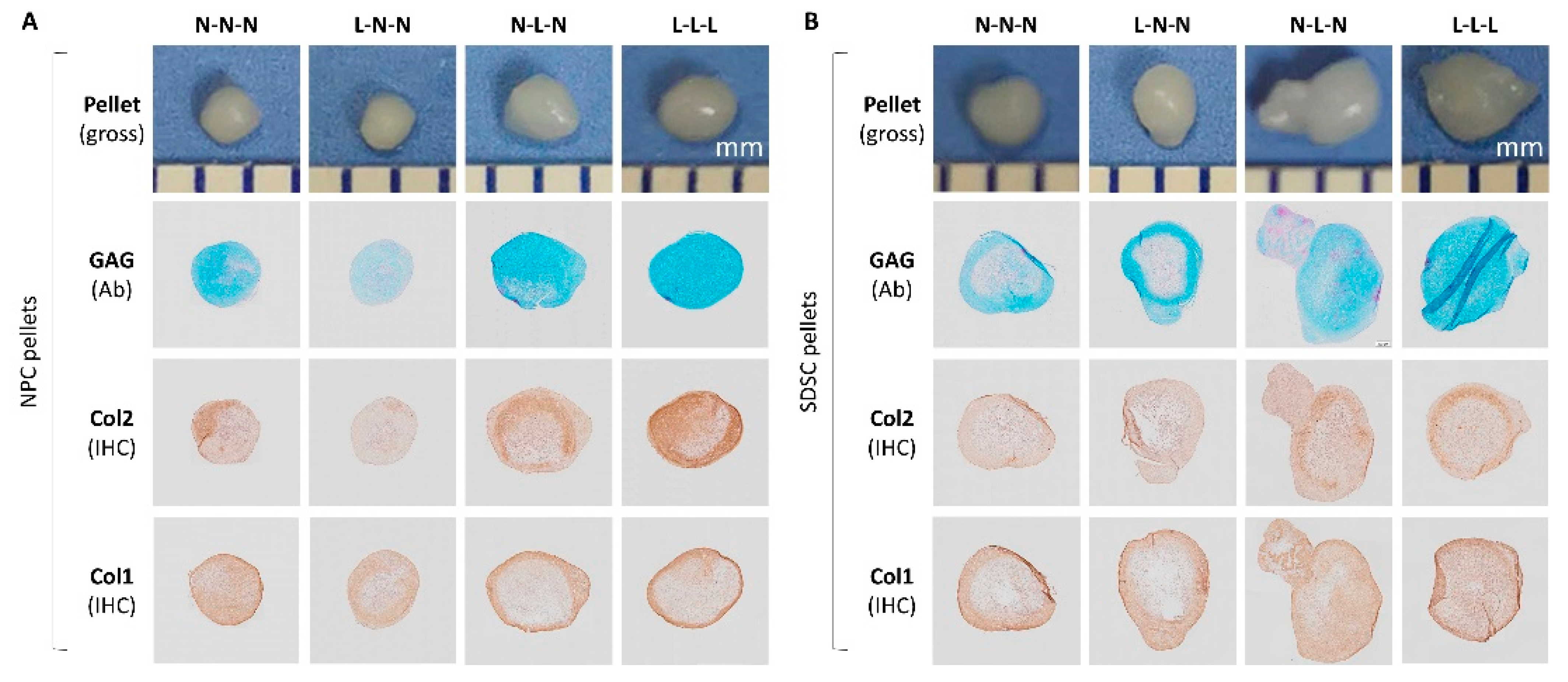
3.2. Effects of Hypoxia Pretreatment on Chondrogenic Capacity of Fetal Cells
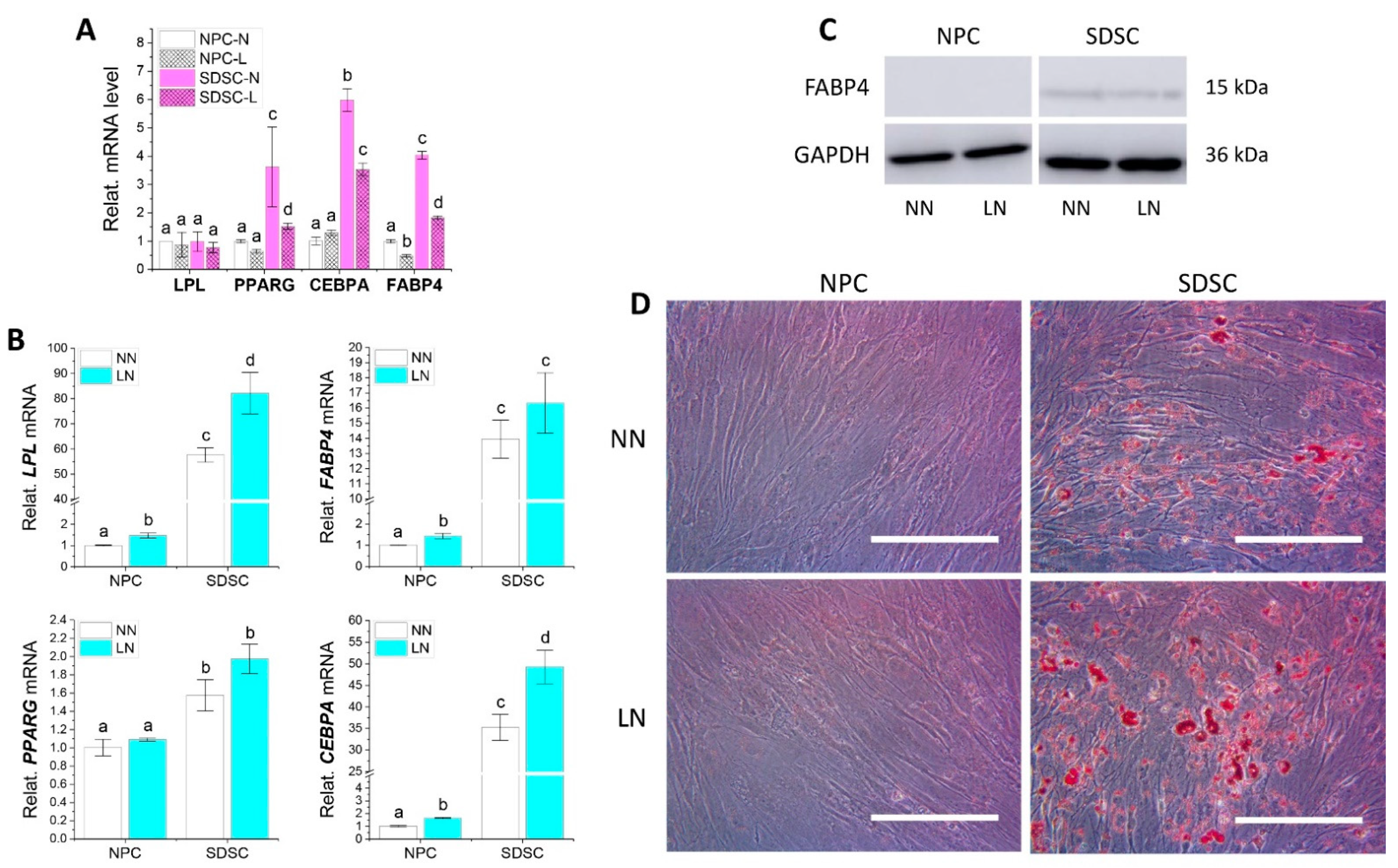
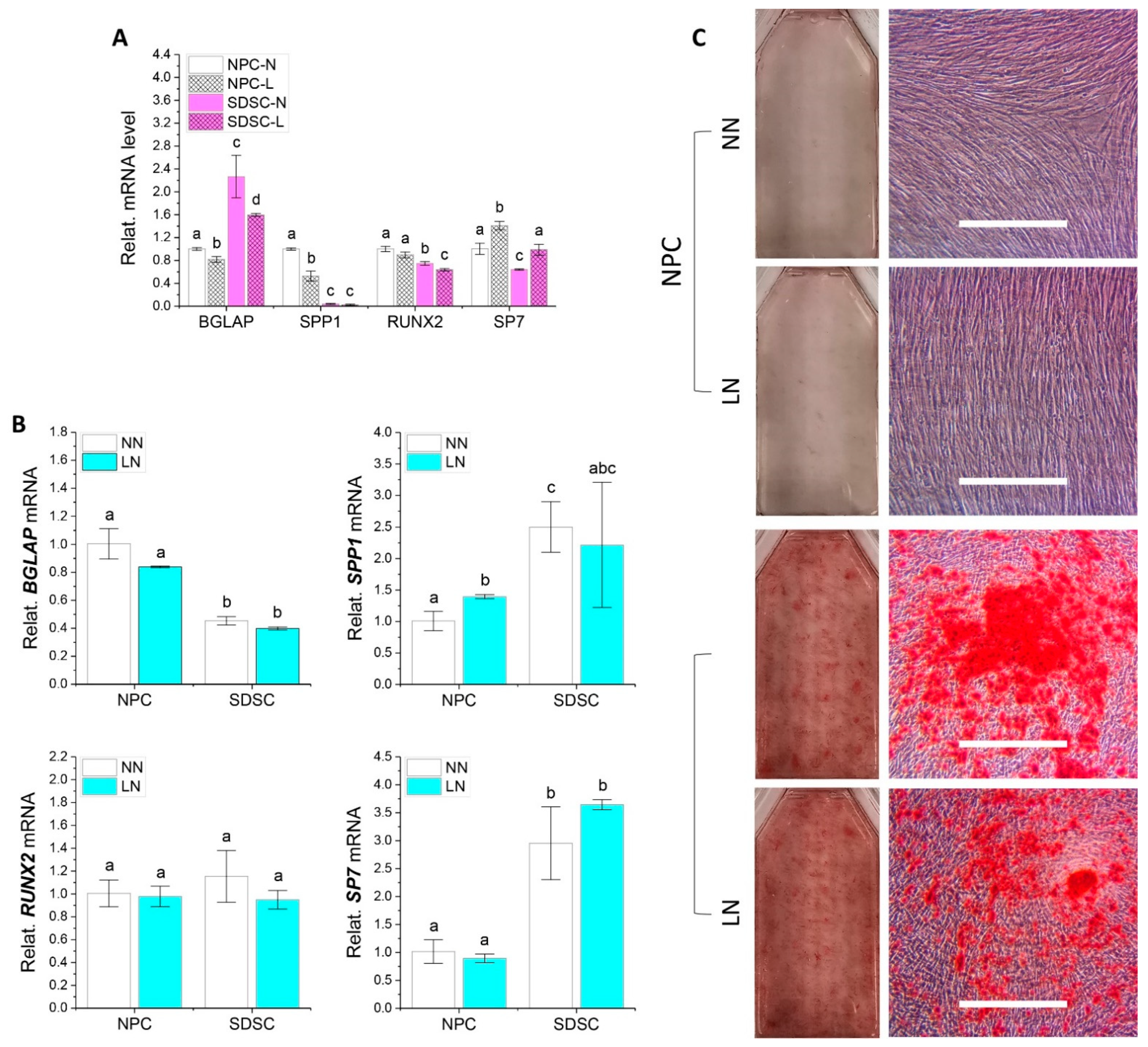
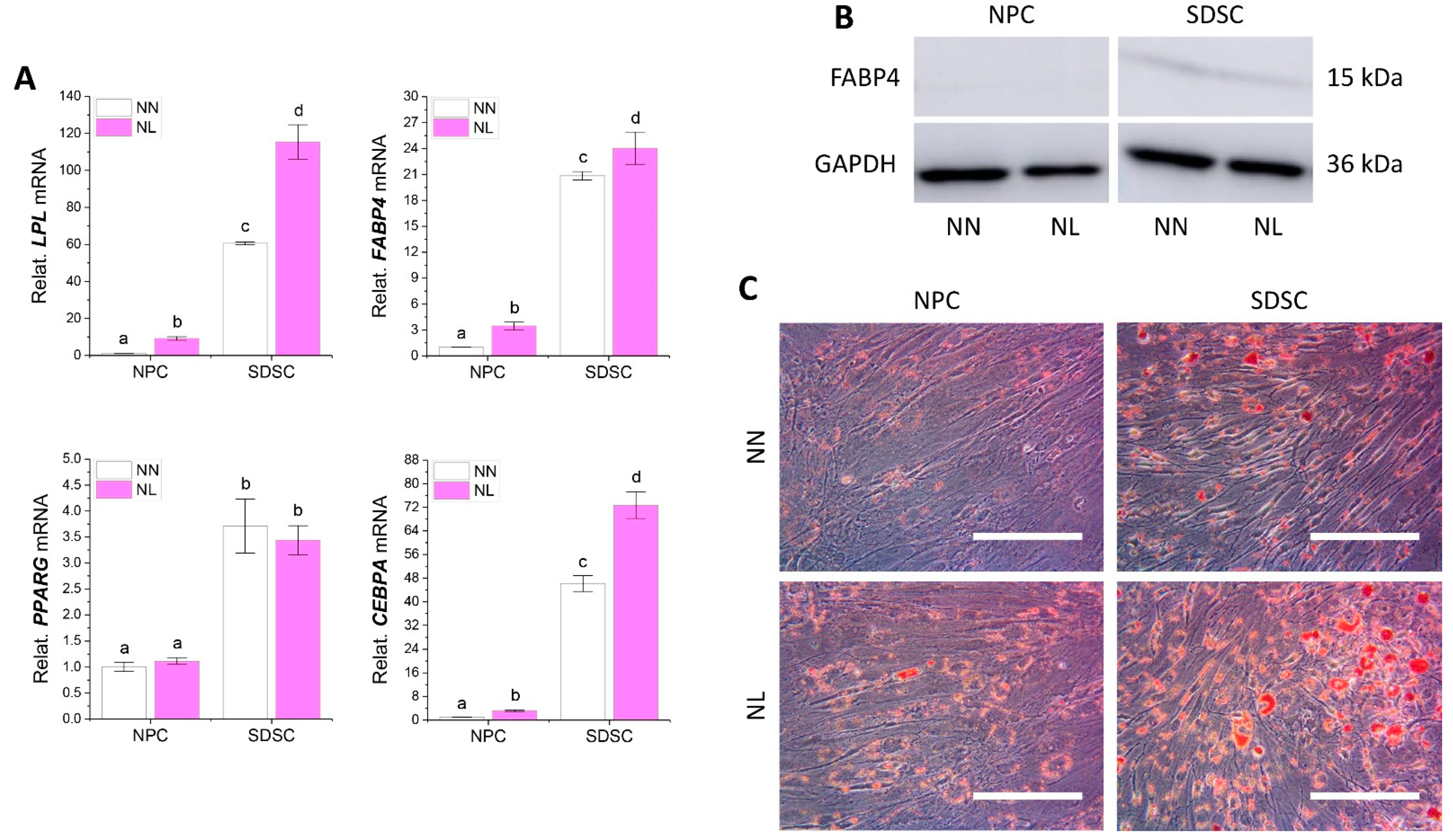
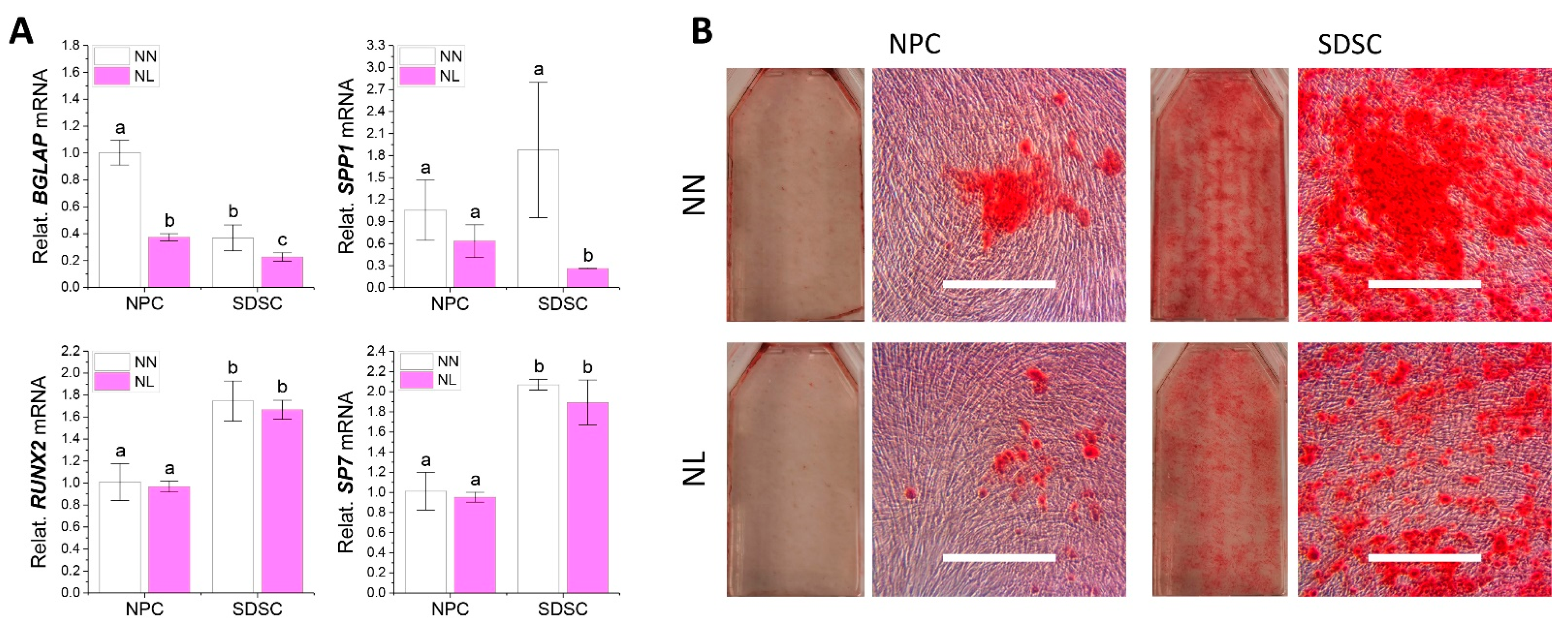
3.3. Effects of Hypoxia Pretreatment on Adipogenic and Osteogenic Potentials of Fetal Cells
3.4. Effects of Hypoxia on Chondrogenic Differentiation of Fetal Cells
3.5. Effects of Hypoxia on Adipogenic and Osteogenic Differentiation of Fetal Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Pei, M. Cell senescence: A challenge in cartilage engineering and regeneration. Tissue Eng. Part B Rev. 2012, 18, 270–287. [Google Scholar] [CrossRef]
- Pizzute, T.; Lynch, K.; Pei, M. Impact of tissue-specific stem cells on lineage-specific differentiation: A focus on the musculoskeletal system. Stem Cell Rev. Rep. 2015, 11, 119–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razi, F.; Tootee, A.; Larijani, B.; Esfahani, E.N. Transplantation of fetal stem cells: A new horizon for treatment of degenerative diseases. Iran J. Public Health 2015, 44 (Suppl. S2), 16–26. [Google Scholar]
- O’Donoghue, K.; Fisk, N.M. Fetal stem cells. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 853–875. [Google Scholar] [CrossRef] [PubMed]
- Watt, H. Ethical aspects of use of fetal/embryonic cells in treatment and research. Zentralbl. Neurochir. 2005, 66, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Li, J.T.; Shoukry, M.; Zhang, Y. A review of decellularized stem cell matrix: A novel cell expansion system for cartilage tissue engineering. Eur. Cell Mater. 2011, 22, 333–343; discussion 343. [Google Scholar] [CrossRef]
- Li, J.; Pei, M. Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering. Tissue Eng. Part A 2011, 17, 703–712. [Google Scholar] [CrossRef]
- Pei, M. Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential. Biomaterials 2017, 117, 10–23. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; He, F.; Pei, M. Chondrogenic priming of human fetal synovium-derived stem cells in an adult stem cell matrix microenvironment. Genes Dis. 2015, 2, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; He, F.; Pei, M. Creation of an in vitro microenvironment to enhance human fetal synovium-derived stem cell chondrogenesis. Cell Tissue Res. 2011, 345, 357–365. [Google Scholar] [CrossRef]
- Rosová, I.; Dao, M.; Capoccia, B.; Link, D.; Nolta, J.A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008, 26, 2173–2182. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Fu, P.; Wu, H.; Pei, M. Meniscus, articular cartilage and nucleus pulposus: A comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017, 370, 53–70. [Google Scholar] [CrossRef]
- Jones, B.A.; Pei, M. Synovium-derived stem cells: A tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng. Part B Rev. 2012, 18, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Emery, S.E.; Pei, M. Coculture of synovium-derived stem cells and nucleus pulposus cells in serum-free defined medium with supplementation of transforming growth factor-beta1: A potential application of tissue-specific stem cells in disc regeneration. Spine (Phila Pa 1976) 2009, 34, 1272–1280. [Google Scholar] [CrossRef]
- Shang, J.; Liu, H.; Li, J.; Zhou, Y. Roles of hypoxia during the chondrogenic differentiation of mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2014, 9, 141–147. [Google Scholar] [CrossRef]
- Gibson, J.S.; Milner, P.I.; White, R.; Fairfax, T.P.A.; Wilkins, R.J. Oxygen and reactive oxygen species in articular cartilage: Modulators of ionic homeostasis. Pflugers Arch. Eur. J. Physiol. 2008, 455, 563–573. [Google Scholar] [CrossRef]
- Chen, W.; Zhuo, Y.; Duan, D.; Lu, M. Effects of hypoxia on differentiation of mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2020, 15, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Chen, S.; Jiang, Q.; Pei, M. Determinants of stem cell lineage differentiation toward chondrogenesis versus adipogenesis. Cell Mol. Life Sci. 2019, 76, 1653–1680. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Minogue, B.M.; Richardson, S.M.; Zeef, L.A.; Freemont, A.J.; Hoyland, J.A. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum. 2010, 62, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Schwarzbauer, J.E. Fibronectin matrix assembly is essential for cell condensation during chondrogenesis. J. Cell Sci. 2014, 127 Pt 20, 4420–4428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Fu, Y.; Yan, Z.; Zhang, X.B.; Pei, M. Impact of fibronectin knockout on proliferation and differentiation of human infrapatellar fat pad-derived stem cells. Front. Bioeng. Biotechnol. 2019, 7, 321. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.C.; Park, H.J.; Wang, S.Y.; Yang, H.R.; Lee, M.C.; Han, H.S. Hypoxic condition enhances chondrogenesis in synovium-derived mesenchymal stem cells. Biomater. Res. 2019, 23, 7. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [Green Version]
- Pattappa, G.; Johnstone, B.; Zellner, J.; Docheva, D.; Angele, P. The importance of physioxia in mesenchymal stem cell chondrogenesis and the mechanisms controlling its response. Int. J. Mol. Sci. 2019, 20, 484. [Google Scholar] [CrossRef] [Green Version]
- Amarilio, R.; Viukov, S.V.; Sharir, A.; Eshkar-Oren, I.; Johnson, R.S.; Zelzer, E. HIF1α regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development 2007, 134, 3917–3928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafont, J.E.; Talma, S.; Murphy, C.L. Hypoxia-inducible factor 2α is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007, 56, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Tang, Y.; Wu, J.H.; Yang, P.S.; Wang, D.L.; Ruan, D.K. Characteristics and potentials of stem cells derived from human degenerated nucleus pulposus: Potential for regeneration of the intervertebral disc. BMC Musculoskelet. Disord. 2017, 18, 242. [Google Scholar] [CrossRef]
- Minogue, B.M.; Richardson, S.M.; Zeef, L.A.; Freemont, A.J.; Hoyland, J.A. Transcriptional profiling of bovine intervertebral disc cells: Implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res. Ther. 2010, 12, R22. [Google Scholar] [CrossRef] [Green Version]
- Quintin, A.; Schizas, C.; Scaletta, C.; Jaccoud, S.; Applegate, L.A.; Pioletti, D.P. Plasticity of fetal cartilaginous cells. Cell Transpl. 2010, 19, 1349–1357. [Google Scholar] [CrossRef] [Green Version]
- Hung, S.P.; Ho, J.H.; Shih, Y.R.V.; Lo, T.; Lee, O.K. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J. Orthop. Res. 2012, 30, 260–266. [Google Scholar] [CrossRef]
- Chan, S.C.; Tekari, A.; Bennekerm, L.M.; Heini, P.F.; Gantenbein, B. Osteogenic differentiation of bone marrow stromal cells is hindered by the presence of intervertebral disc cells. Arthritis Res. Ther. 2016, 18, 29. [Google Scholar] [CrossRef] [Green Version]
- Valorani, M.G.; Montelatici, E.; Germani, A.; Biddle, A.; D’Alessandro, D.; Strollo, R.; Patrizi, M.P.; Lazzari, L.; Nye, E.; Otto, W.R.; et al. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012, 45, 225–238. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.H.; Song, S.Y.; Kim, W.S.; Song, S.U.; Yi, T.; Jeon, M.S.; Chung, H.M.; Xia, Y.; Sung, J.H. Hypoxia induces adipocyte differentiation of adipose-derived stem cells by triggering reactive oxygen species generation. Cell Biol. Int. 2014, 38, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Fink, T.; Abildtrup, L.; Fogd, K.; Abdallah, B.M.; Kassem, M.; Ebbesen, P.; Zachar, V. Induction of adipocyte-like phenotype in human mesenchymal stem cells by hypoxia. Stem Cells 2004, 22, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Sun, J.; Dai, Y.; Cao, P.; Zhang, L.; Peng, S.; Zhou, Y.; Li, G.; Tang, J.; Xiang, J. HIF-1A and C/EBPs transcriptionally regulate adipogenic differentiation of bone marrow-derived MSCs in hypoxia. Stem Cell Res. Ther. 2015, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotherstrom, C.; Ringden, O.; Tammik, C.; Zetterberg, E.; Westgren, M.; Le Blanc, K. Immunologic properties of human fetal mesenchymal stem cells. Am. J. Obstet. Gynecol. 2004, 190, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Montjovent, M.O.; Bocelli-Tyndall, C.; Scaletta, C.; Scherberich, A.; Mark, S.; Martin, I.; Applegate, L.A.; Pioletti, D.P. In vitro characterization of immune-related properties of human fetal bone cells for potential tissue engineering applications. Tissue Eng. Part A 2009, 15, 1523–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ast, T.; Mootha, V.K. Oxygen and mammalian cell culture: Are we repeating the experiment of Dr. Ox? Nat. Metab. 2019, 1, 858–860. [Google Scholar] [CrossRef]



| Gene Name | Full Name | Assay ID |
|---|---|---|
| Stemness related genes | ||
| BMI1 | B lymphoma Mo-MLV insertion region 1 homolog | Hs00180411_m1 |
| KLF4 | Kruppel-like factor 4 | Hs00358836_m1 |
| MYC | MYC proto-oncogene | Hs00153408_m1 |
| NANOG | Nanog Homeobox | Hs02387400_g1 |
| NES | Nestin | Hs04187831_g1 |
| NOV | Nephroblastoma overexpressed | Hs00159631_m1 |
| POU5F1 | POU class 5 homeobox 1 | Hs04260367_gH |
| SOX2 | SRY-box 2 | Hs01053049_s1 |
| Chondrogenesis related genes | ||
| ACAN | Aggrecan | Hs00153936_m1 |
| CDH2 | Cadherin 2 | Hs00983056_m1 |
| COL2A1 | Type II collagen | Hs00156568_m1 |
| COL1A1 | Type I collagen | Hs00164004_m1 |
| COL10A1 | Type X collagen | Hs00166657_m1 |
| PRG4 | Proteoglycan 4 | Hs00981633_m1 |
| SOX9 | SRY-Box 9 | Hs00165814_m1 |
| Adipogenesis related genes | ||
| CEBPA | CCAAT/enhancer-binding protein alpha | Hs00269972_s1 |
| FABP4 | Fatty acid-binding protein 4 | Hs01086177_m1 |
| LPL | Lipoprotein lipase | Hs00173425_m1 |
| PPARG | Peroxisome Proliferator Activated Receptor Gamma | Hs01115513_m1 |
| Osteogenesis related genes | ||
| BGLAP | Osteocalcin | Hs01587814_g1 |
| SPP1 | Osteopontin | Hs00959010_m1 |
| SP7 | Osterix | Hs01866874_s1 |
| RUNX2 | Runt-related transcription factor 2 | Hs00231692_m1 |
| Other related genes | ||
| FBLN1 | Fibulin 1 | Hs00972609_m1 |
| FN1 | Fibronectin 1 | Hs01549976_m1 |
| FOXF1 | Forkhead Box F1 | Hs00230962_m1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Hs02758991_g1 |
| HSPG2 | Heparan Sulfate Proteoglycan 2 | Hs01078536_m1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.A.; Pei, M. Hypoxia Modulates Regenerative Potential of Fetal Stem Cells. Appl. Sci. 2022, 12, 363. https://doi.org/10.3390/app12010363
Pei YA, Pei M. Hypoxia Modulates Regenerative Potential of Fetal Stem Cells. Applied Sciences. 2022; 12(1):363. https://doi.org/10.3390/app12010363
Chicago/Turabian StylePei, Yixuan Amy, and Ming Pei. 2022. "Hypoxia Modulates Regenerative Potential of Fetal Stem Cells" Applied Sciences 12, no. 1: 363. https://doi.org/10.3390/app12010363
APA StylePei, Y. A., & Pei, M. (2022). Hypoxia Modulates Regenerative Potential of Fetal Stem Cells. Applied Sciences, 12(1), 363. https://doi.org/10.3390/app12010363







