Probiotics in Functional Foods: Survival Assessment and Approaches for Improved Viability
Abstract
:1. Introduction
2. Probiotics in Food Products
2.1. Selection and Identification of Probiotic Strains
2.2. Functionality of Probiotics
2.3. Survival Assessment and Viability
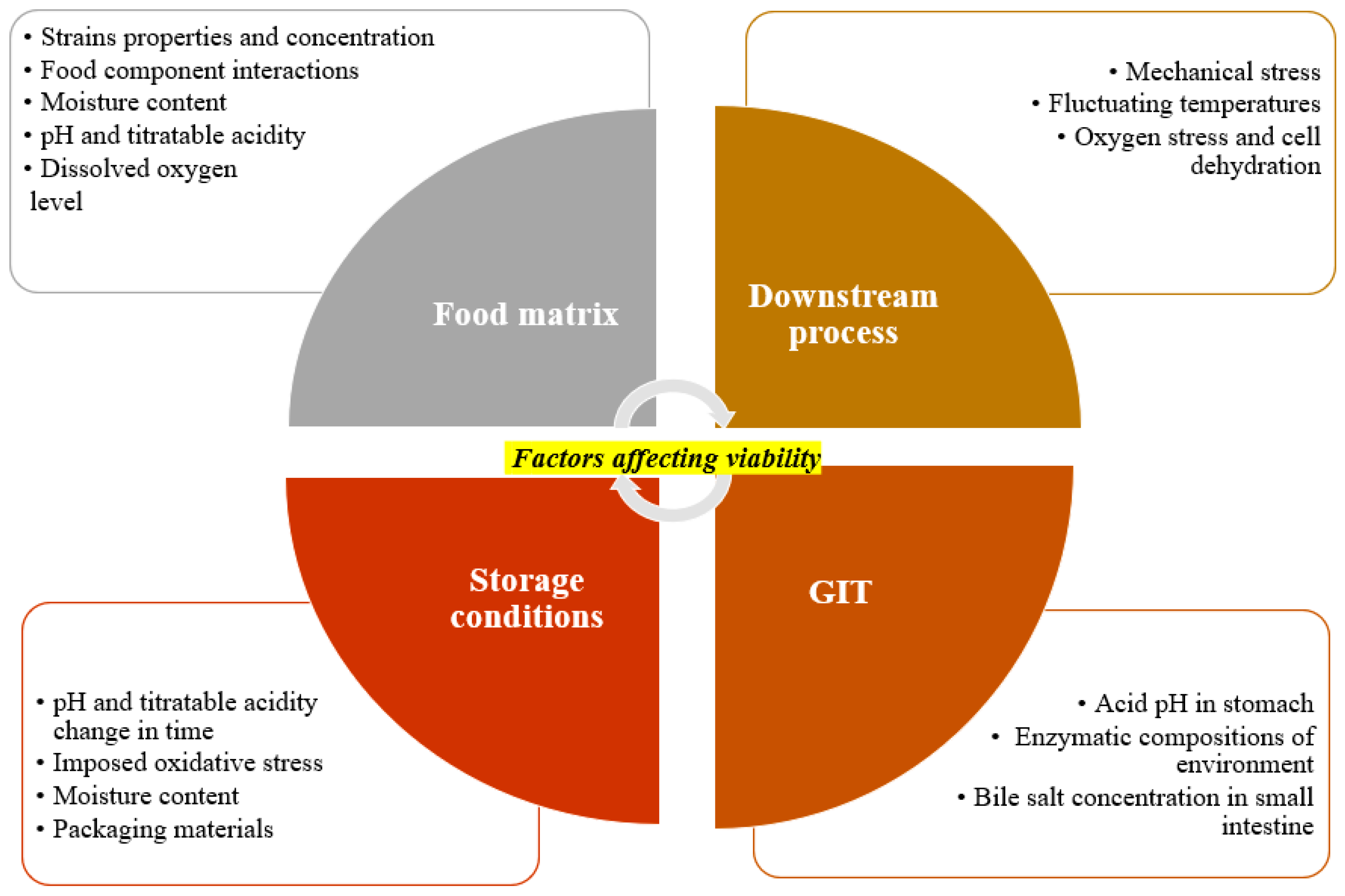
2.4. Role of Food Ingredients and Additives
| Name of Probiotic | Effect of Prebiotic | Probiotics Survival | References |
|---|---|---|---|
| Bifidobacterium species | Fructo and galacto oligosaccharides | Enhanced viability was observed upon the dosage level of prebiotics | [70] |
| Bifidobacterium infantis, B. pseudolongum, B. animalis and B. longum | Lactulose, raftilose, maize, and insulin | Retentive viability was observed up to 28 days with prebiotics addition | [3] |
| B. animalis and B. longum | Fructo oligosaccharide | The recommended level of viability was not achieved | [2] |
| Lactobacillus casei, L. zeae L. paracasei, L. rhamnosus | Lactulose, raftilose, Maize and insulin | Strain specific survival was observed | [71] |
| L. rhamnosus | Oligofructose and polydextrose mediated prebiotic substances | Resulted in a high rate of probiotic survival when substituted with prebiotics | [72] |
| L. acidophilus | Short and long chain inulin | Viable count was increased with L. acidophilus with addition of prebiotics | [73] |
| L. casei,L. acidophilus | Inulin and maize | The viability of probiotics has been improved with supplementation of yogurt | [35] |
| B. animalis | Beta glucan from two different cereals sources | Viability and stability were improved with yogurt supplementation. | [74] |
| L. acidophilus, B.animalis | Inulin and oligo fructose | Improved viability in the ice cream mix with minimum of 106 of B. animalis colony forming units of was maintained | [62] |
| L. rhamnosus | Combination of inulin mixed with yeast extract | The combined mixture positively influences on the growth of L. reuteri | [8] |
3. Progress to Enhance Viability
| Techniques | Probiotic | Size Range | Shape | Conditions | Remarks | Efficiency of Encapsulation | References |
|---|---|---|---|---|---|---|---|
| Freeze drying | L. plantarum P. acidilactici Pediococcus pentosaceus S. boulardii L. acidophilus L. casei L. rhamnosus P. acidilactici | >1 mm | Irregular | Vacuum -0.5 torr, lower condensation temperature preferred | Porous structure, expensive, | 98% (approximate maximum) | [79,80,81,82,83,84,85,86,87,88,89,90] |
| Extrusion | L. acidophilus Lactiplantibacillus plantarum Lacticaseibacillus paracasei Lactiplantibacillus pentosus L. casei | 3 mm | Beaded | Gelling 0.25% | Simple, cost effective, better viability, difficulty in scaling up | 97% (approximate maximum) | [91,92,93,94,95,96] |
| Emulsion | L. acidophilus L. plantarum L. casei Enterococcus faecium S. boulardii L. salivarius Bifidobacterium | 2 mm | Spherical | Speed of agitation should be controlled along with other general reaction parameters | High survival, 1 mm capsules produced | 84–97% (approximate maximum) | [96,97,98,99,100,101,102] |
| Spray drying | L. plantarum L. paracasei S. cerevisiae | 80 mm | Spherical | Nozzle determines the shape and concentration | Improved yield, low density particles | 96% (approximate maximum) | [92,103,104,105,106,107] |
| Refractance drying | L. plantarum | Greater than 1 mm | Flaky | Temperature 60 degree Celsius | Good quality, simple cost effective, better viability | 93% (approximate maximum) | [107,108,109,110] |
| Electrohydrodynamic | L. plantarum B. lactis L. gasseri Bifidobacterium L. rhamnosus B. animalis lactis BB12 Streptococcus thermophilus | 150 nm | Fibers | Voltage and tip resistance | Reproducible fibers, good yield | 90% (approximate maximum) | [90,111,112,113,114] |
| Three-dimensional printing | mashed potatoes probiotics | - | Fibers | Nozzle diameter | Good viability | - | [115,116] |
| Microfluidics | gut bacteria | - | Droplet | Double emulsion | Deep functionalization of carriers and profiling of probiotics | - | [117,118] |
4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saguy, I.S.; Sirotinskaya, V. Challenges in exploiting open innovation’s full potential in the food industry with a focus on small and medium enterprises (SMEs). Trends Food Sci. Technol. 2014, 38, 136–148. [Google Scholar] [CrossRef]
- Savino, T.; Testa, S.; Messeni Petruzzelli, A. Researcher understanding of food innovations in Nordic and Southern European countries: A systematic literature review. Trends Food Sci. Technol. 2018, 77, 54–63. [Google Scholar] [CrossRef]
- Martin-Rios, C.; Demen-Meier, C.; Gössling, S.; Cornuz, C. Food waste management innovations in the foodservice industry. Waste Manag. 2018, 79, 196–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenssen, K.; Bast, A.; de Boer, A. Should botanical health claims be substantiated with evidence on traditional use? Reviewing the stakeholders’ arguments. PharmaNutrition 2020, 14, 100232. [Google Scholar] [CrossRef]
- Dinicola, S.; Proietti, S.; Cucina, A.; Bizzarri, M.; Fuso, A. Alpha-Lipoic Acid Downregulates IL-1β and IL-6 by DNA Hypermethylation in SK-N-BE Neuroblastoma Cells. Antioxidants 2017, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Inoue, A.; Taniguchi, S.; Yukutake, T.; Suyama, K.; Nose, T.; Maeda, I. Multifunctional biological activities of water extract of housefly larvae (Musca domestica). PharmaNutrition 2017, 5, 119–126. [Google Scholar] [CrossRef]
- Feng, K.; Zhai, M.-Y.; Zhang, Y.; Linhardt, R.J.; Zong, M.-H.; Li, L.; Wu, H. Improved Viability and Thermal Stability of the Probiotics Encapsulated in a Novel Electrospun Fiber Mat. J. Agric. Food Chem. 2018, 66, 10890–10897. [Google Scholar] [CrossRef]
- Yoha, K.S.; Nida, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Targeted Delivery of Probiotics: Perspectives on Research and Commercialization. Probiotics Antimicrob. Proteins 2021, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Dinkçi, N.; Akdeniz, V.; Akalin, A.S. Survival of probiotics in functional foods during shelf life. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–233. ISBN 978-0-12-817190-5. [Google Scholar]
- Chandrashekhar, P.; Minooei, F.; Arreguin, W.; Masigol, M.; Steinbach-Rankins, J.M. Perspectives on Existing and Novel Alternative Intravaginal Probiotic Delivery Methods in the Context of Bacterial Vaginosis Infection. AAPS J. 2021, 23, 66. [Google Scholar] [CrossRef]
- Mojaveri, S.J.; Hosseini, S.F.; Gharsallaoui, A. Viability improvement of Bifidobacterium animalis Bb12 by encapsulation in chitosan/poly(vinyl alcohol) hybrid electrospun fiber mats. Carbohydr. Polym. 2020, 241, 116278. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Huang, R.-M.; Wu, R.-Q.; Wei, Y.-S.; Zong, M.-H.; Linhardt, R.J.; Wu, H. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 2020, 310, 125977. [Google Scholar] [CrossRef] [PubMed]
- Mc Carthy, U.; Uysal, I.; Badia-Melis, R.; Mercier, S.; O’Donnell, C.; Ktenioudaki, A. Global food security—Issues, challenges and technological solutions. Trends Food Sci. Technol. 2018, 77, 11–20. [Google Scholar] [CrossRef]
- Su, G.L.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Barlow, S.; Chesson, A.; Collins, J.D.; Dybing, E.; Flynn, A.; Fruijtier-, C.; Hardy, A.; Knaap, A.; Kuiper, H.; Neindre, P.L.; et al. Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA—Opinion of the Scientific Committee. EFSA J. 2007, 5, 587. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Generally recognized as safe (GRAS): History and description. Toxicol. Lett. 2004, 150, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. An overview of microencapsulation in the food industry: Opportunities, challenges, and innovations. Eur. Food Res. Technol. 2020, 246, 1371–1382. [Google Scholar] [CrossRef]
- Teneva-Angelova, T.; Hristova, I.; Pavlov, A.; Beshkova, D. Chapter 4—Lactic Acid Bacteria—From Nature Through Food to Health. In Handbook of Food Bioengineering; Grumezescu, A., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 91–133. ISBN 978-0-12-811443-8. [Google Scholar]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Vijaya Kumar, B.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calinoiu, L.F.; Vodnar, D.; Precup, G. A Review: The Probiotic Bacteria Viability under Different Conditions. Bull. UASVM Food Sci. Technol. 2016, 73, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Akalın, A.S.; Kesenkas, H.; Dinkci, N.; Unal, G.; Ozer, E.; Kınık, O. Enrichment of probiotic ice cream with different dietary fibers: Structural characteristics and culture viability. J. Dairy Sci. 2018, 101, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Akalın, A.S.; Karagözlü, C.; Ünal, G. Rheological properties of reduced-fat and low-fat ice cream containing whey protein isolate and inulin. Eur. Food Res. Technol. 2008, 227, 889–895. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foligne, B.; Nutten, S.; Grangette, C.; Dennin, V.; Goudercourt, D.; Poiret, S.; Dewulf, J.; Brassart, D.; Mercenier, A.; Pot, B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J. Gastroenterol. 2007, 13, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grangette, C.; Nutten, S.; Palumbo, E.; Morath, S.; Hermann, C.; Dewulf, J.; Pot, B.; Hartung, T.; Hols, P.; Mercenier, A. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc. Natl. Acad. Sci. USA 2005, 102, 10321–10326. [Google Scholar] [CrossRef] [Green Version]
- Speranza, B.; Campaniello, D.; Petruzzi, L.; Altieri, C.; Sinigaglia, M.; Bevilacqua, A.; Rosaria Corbo, M. The Inoculation of Probiotics In Vivo Is a Challenge: Strategies to Improve Their Survival, to Avoid Unpleasant Changes, or to Enhance Their Performances in Beverages. Beverages 2020, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of Probiotic Cells for Food Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmo, C.; Giordano, I.; Mauriello, G. Effect of Microencapsulation on Survival at Simulated Gastrointestinal Conditions and Heat Treatment of a Non Probiotic Strain, Lactiplantibacillus plantarum 48M, and the Probiotic Strain Limosilactobacillus reuteri DSM 17938. Foods 2021, 10, 217. [Google Scholar] [CrossRef]
- Heller, K.J. Probiotic bacteria in fermented foods: Product characteristics and starter organisms. Am. J. Clin. Nutr. 2001, 73, 374S–379S. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Abu-Ghannam, N. Probiotic fermentation of plant based products: Possibilities and opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, V.H.; Ötles, S. Properties of probiotics and encapsulated probiotics in food. Acta Sci. Pol. Technol. Aliment. 2014, 13, 413–424. [Google Scholar] [CrossRef] [Green Version]
- Buriti, F.C.A.; Saad, S.M.I. Chilled milk-based desserts as emerging probiotic and prebiotic products. Crit. Rev. Food Sci. Nutr. 2014, 54, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Schaafsma, G.J. Probiotics. Int. J. Food Microbiol. 1998, 39, 237–238. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Shah, N.P. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Mantegazza, C.; Molinari, P.; D’Auria, E.; Sonnino, M.; Morelli, L.; Zuccotti, G.V. Probiotics and antibiotic-associated diarrhea in children: A review and new evidence on Lactobacillus rhamnosus GG during and after antibiotic treatment. Pharmacol. Res. 2018, 128, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hojsak, I.; Szajewska, H.; Canani, R.B.; Guarino, A.; Indrio, F.; Kolacek, S.; Orel, R.; Shamir, R.; Vandenplas, Y.; Van Goudoever, J.B.; et al. Probiotics for the prevention of nosocomial diarrhea in children. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gotova, I.; Dimitrov, Z.; Najdenski, H. Selected lactobacillus bulgaricus and streptococcus thermophilus strains from bulgarian yogurt demonstrate significant anti-inflammatory potential. Acta Microbiol. Bulg. 2017, 33, 137–142. [Google Scholar]
- Kostelac, D.; Gerić, M.; Gajski, G.; Markov, K.; Domijan, A.M.; Čanak, I.; Jakopović, Ž.; Svetec, I.K.; Žunar, B.; Frece, J. Lactic acid bacteria isolated from equid milk and their extracellular metabolites show great probiotic properties and anti-inflammatory potential. Int. Dairy J. 2021, 112, 104828. [Google Scholar] [CrossRef]
- Maria-Aggeliki, K.S.; Nikolaos, K.L.; Kyrias, G.M.; Vassilis, K.E. The Potential Clinical Impact of Probiotic Treatment for the Prevention and/or Anti-Inflammatory Therapeutic Effect Against Radiation Induced Intestinal Mucositis. A Review. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Perna, S.; Giacosa, A.; Peroni, G.; Castellazzi, A.M. Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes 2017, 8, 521–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdellatif, B.; McVeigh, C.; Bendriss, G.; Chaari, A. The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 4159. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti Neto, M.P.; de Souza Aquino, J.; Romão da Silva, L.d.F.; de Oliveira Silva, R.; de Lima Guimarães, K.S.; de Oliveira, Y.; de Souza, E.L.; Magnani, M.; Vidal, H.; de Brito Alves, J.L. Gut microbiota and probiotics intervention: A potential therapeutic target for management of cardiometabolic disorders and chronic kidney disease? Pharmacol. Res. 2018, 130, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Szántó, M.; Dózsa, A.; Antal, D.; Szabó, K.; Kemény, L.; Bai, P. Targeting the gut-skin axis—Probiotics as new tools for skin disorder management? Exp. Dermatol. 2019, 28, 1210–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramirez-Jirano, L.J.; Morales, J.A.; Bitzer-Quintero, O.K. From Probiotics to Psychobiotics: Live Beneficial Bacteria Which Act on the Brain-Gut Axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.d.A.F.; Shah, N.P. Probiotic Dairy Products as Functional Foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Rzepkowska, A.; Szydłowska, A.; Kołożyn-Krajewska, D. Trends and Possibilities of the Use of Probiotics in Food Production. In Handbook of Food Bioengineering; Holban, A.M., Grumezescu, A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 65–94. ISBN 978-0-12-811446-9. [Google Scholar]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Mocchiutti, P.; Reinheimer, J.A. Interactions among lactic acid starter and probiotic bacteria used for fermented dairy products. J. Dairy Sci. 2002, 85, 721–729. [Google Scholar] [CrossRef]
- Boylston, T.D.; Vinderola, C.G.; Ghoddusi, H.B.; Reinheimer, J.A. Incorporation of bifidobacteria into cheeses: Challenges and rewards. Int. Dairy J. 2004, 14, 375–387. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Harmstorf, I.; Phillips, M. Survival of Lactobacillus acidophilus and Bifidobacterium animalis ssp. lactis in stirred fruit yogurts. LWT-Food Sci. Technol. 2008, 41, 1317–1322. [Google Scholar] [CrossRef]
- Gursoy, O.; Kinik, O. Incorporation of adjunct cultures of Enterococcus faecium, Lactobacillus paracasei subsp. paracasei and Bifidobacterium bifidum into white cheese. J. Food Agric. Environ. 2010, 8, 107–112. [Google Scholar]
- Mohammadi, R.; Mortazavian, A.M.; Khosrokhavar, R.; da Cruz, A.G. Probiotic ice cream: Viability of probiotic bacteria and sensory properties. Ann. Microbiol. 2011, 61, 411–424. [Google Scholar] [CrossRef]
- Wilkinson, M.G. Flow cytometry as a potential method of measuring bacterial viability in probiotic products: A review. Trends Food Sci. Technol. 2018, 78, 1–10. [Google Scholar] [CrossRef]
- Davey, H.M. Life, death, and in-between: Meanings and methods in microbiology. Appl. Environ. Microbiol. 2011, 77, 5571–5576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Shojaee-Aliabadi, S.; Koushki, M.R. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT 2018, 87, 54–60. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi-Varkani, A.; Emam-Djomeh, Z.; Askari, G. Physicochemical and microstructural properties of a novel edible film synthesized from Balangu seed mucilage. Int. J. Biol. Macromol. 2018, 108, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Burgain, J.; Scher, J.; Francius, G.; Borges, F.; Corgneau, M.; Revol-Junelles, A.M.; Cailliez-Grimal, C.; Gaiani, C. Lactic acid bacteria in dairy food: Surface characterization and interactions with food matrix components. Adv. Colloid Interface Sci. 2014, 213, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Ly, M.H.; Covarrubias-Cervantes, M.; Dury-Brun, C.; Bordet, S.; Voilley, A.; Le, T.M.; Belin, J.-M.; Waché, Y. Retention of aroma compounds by lactic acid bacteria in model food media. Food Hydrocoll. 2008, 22, 211–217. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagga, D.; Reichert, J.L.; Koschutnig, K.; Aigner, C.S.; Holzer, P.; Koskinen, K.; Moissl-Eichinger, C.; Schöpf, V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018, 9, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Langa, S.; van den Bulck, E.; Peirotén, A.; Gaya, P.; Schols, H.A.; Arqués, J.L. Application of lactobacilli and prebiotic oligosaccharides for the development of a synbiotic semi-hard cheese. LWT 2019, 114, 108361. [Google Scholar] [CrossRef]
- Tarrah, A.; de Castilhos, J.; Rossi, R.C.; Duarte, V. da S.; Ziegler, D.R.; Corich, V.; Giacomini, A. In vitro Probiotic Potential and Anti-cancer Activity of Newly Isolated Folate-Producing Streptococcus thermophilus Strains. Front. Microbiol. 2018, 9, 2214. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Gallego, J.; Ferrocino, I.; Botta, C.; Ercolini, D.; Cocolin, L.; Rantsiou, K. Probiotic potential of a Lactobacillus rhamnosus cheese isolate and its effect on the fecal microbiota of healthy volunteers. Food Res. Int. 2019, 119, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genomics. Proteom. Bioinform. 2018, 16, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Caligiuri, S.; Brown, D.; Pierce, G. Clinical trials using functional foods provide unique challenges. J. Funct. Foods 2018, 45, 233–238. [Google Scholar] [CrossRef]
- Yu, H.; Liu, W.; Li, D.; Liu, C.; Feng, Z.; Jiang, B. Targeting Delivery System for Lactobacillus Plantarum Based on Functionalized Electrospun Nanofibers. Polymers 2020, 12, 1565. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Knøchel, S.; Nielsen, D.S. Low-moisture food matrices as probiotic carriers. FEMS Microbiol. Lett. 2019, 366, fnz006. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, H. Recent Advances in Probiotics Encapsulation by Electrospinning. ES Food Agrofor. 2020, 2, 3–12. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Padma, S. Encapsulation of probiotics by spray drying. In Spray Drying Techniques for Food Ingredient Encapsulation; Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 101–125. [Google Scholar]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Gul, O.; Atalar, I. Different stress tolerance of spray and freeze dried Lactobacillus casei Shirota microcapsules with different encapsulating agents. Food Sci. Biotechnol. 2019, 28, 807–816. [Google Scholar] [CrossRef]
- Chavarri, M.; Maranon, I.; Carmen, M. Encapsulation Technology to Protect Probiotic Bacteria. In Probiotics; Marañón, I., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.S.; Anandharamakrishnan, C. Effect of whey protein—Alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- Sakai, T.; Moteki, Y.; Takahashi, T.; Shida, K.; Kiwaki, M.; Shimakawa, Y.; Matsui, A.; Chonan, O.; Morikawa, K.; Ohta, T.; et al. Probiotics into outer space: Feasibility assessments of encapsulated freeze-dried probiotics during 1 month’s storage on the International Space Station. Sci. Rep. 2018, 8, 10687. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B.; Vaidyanathan, M.; Radhakrishnan, K.; Raichur, A.M. Enhanced viability of probiotic Saccharomyces boulardii encapsulated by layer-by-layer approach in pH responsive chitosan–dextran sulfate polyelectrolytes. J. Food Eng. 2014, 136, 1–8. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B. 18—Properties and applications of different probiotic delivery systems. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Garti, N., McClements, D.J., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 541–594. ISBN 978-0-85709-124-6. [Google Scholar]
- Yao, M.; Li, B.; Ye, H.; Huang, W.; Luo, Q.; Xiao, H.; McClements, D.J.; Li, L. Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles. Food Hydrocoll. 2018, 83, 246–252. [Google Scholar] [CrossRef]
- Massounga Bora, A.F.; Li, X.; Zhu, Y.; Du, L. Improved Viability of Microencapsulated Probiotics in a Freeze-Dried Banana Powder During Storage and Under Simulated Gastrointestinal Tract. Probiotics Antimicrob. Proteins 2019, 11, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Heidebach, T.; Först, P.; Kulozik, U. Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J. Food Eng. 2010, 98, 309–316. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Patarroyo, J.L.; Florez-Rojas, J.; Pradilla, D.; Valderrama-Rincon, J.; Cruz, J.; Reyes, L. Formulation and Characterization of Gelatin-Based Hydrogels for the Encapsulation of Kluyveromyces lactis—Applications in Packed-Bed Reactors and Probiotics Delivery in Humans. Polymers 2020, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Vishali, D.A.; Monisha, J.; Sivakamasundari, S.K.; Moses, J.A.; Anandharamakrishnan, C. Spray freeze drying: Emerging applications in drug delivery. J. Control. Release 2019, 300, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.; Agnol, W.; Dallé, D.; Serpa, V.; Jachetti Maciel, M.; Lehn, D.; Souza, C. Development of alginate-pectin microparticles with dairy whey using vibration technology: Effects of matrix composition on the protection of Lactobacillus spp. from adverse conditions. Food Res. Int. 2018, 113, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Poletto, G.; Raddatz, G.; Cichoski, A.; Zepka, L.; Jacob-Lopes, E.; Barin, J.; Wagner, R.; Menezes, C. Study of viability and storage stability of Lactobacillus acidophillus when encapsulated with the prebiotics rice bran, inulin and Hi-maize. Food Hydrocoll. 2019, 95, 238–244. [Google Scholar] [CrossRef]
- Gul, O.; Dervisoglu, M. Application of multicriteria decision technique to determine optimum sodium alginate concentration for microencapsulation of Lactobacillus casei Shirota by extrusion and emulsification. J. Food Process Eng. 2017, 40, e12481. [Google Scholar] [CrossRef]
- Qi, W.; Liang, X.; Yun, T.; Guo, W. Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. J. Food Sci. Technol. 2019, 56, 1398–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gbassi, G.K.; Vandamme, T. Probiotic encapsulation technology: From microencapsulation to release into the gut. Pharmaceutics 2012, 4, 149–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida Paula, D.; Martins, E.M.F.; de Almeida Costa, N.; de Oliveira, P.M.; de Oliveira, E.B.; Ramos, A.M. Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. Int. J. Biol. Macromol. 2019, 133, 722–731. [Google Scholar] [CrossRef]
- Holkem, A.T.; Raddatz, G.C.; Nunes, G.L.; Cichoski, A.J.; Jacob-Lopes, E.; Ferreira Grosso, C.R.; de Menezes, C.R. Development and characterization of alginate microcapsules containing Bifidobacterium BB-12 produced by emulsification/internal gelation followed by freeze drying. LWT-Food Sci. Technol. 2016, 71, 302–308. [Google Scholar] [CrossRef]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Lotfipour, F.; Mirzaeei, S.; Maghsoodi, M. Evaluation of the effect of CaCl2 and alginate concentrations and hardening time on the characteristics of Lactobacillus acidophilus loaded alginate beads using response surface analysis. Adv. Pharm. Bull. 2012, 2, 71–78. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Spray freeze drying method for microencapsulation of Lactobacillus plantarum. J. Food Eng. 2015, 166, 95–103. [Google Scholar] [CrossRef]
- Semyonov, D.; Ramon, O.; Kaplun, Z.; Levin-Brener, L.; Gurevich, N.; Shimoni, E. Microencapsulation of Lactobacillus paracasei by spray freeze drying. Food Res. Int. 2010, 43, 193–202. [Google Scholar] [CrossRef]
- Levine, H.; Slade, L. Another view of trehalose for drying and stabilizing biological materials. BioPharm 1992, 5, 36–40. [Google Scholar]
- Solanki, H.K.; Pawar, D.D.; Shah, D.A.; Prajapati, V.D.; Jani, G.K.; Mulla, A.M.; Thakar, P.M. Development of microencapsulation delivery system for long-term preservation of probiotics as biotherapeutics agent. BioMed Res. Int. 2013, 2013, 620719. [Google Scholar] [CrossRef]
- Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Effect of encapsulation methods on the physicochemical properties and the stability of Lactobacillus plantarum (NCIM 2083) in synbiotic powders and in-vitro digestion conditions. J. Food Eng. 2020, 283, 110033. [Google Scholar] [CrossRef]
- Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Conductive hydro drying through refractance window drying—An alternative technique for drying of Lactobacillus plantarum (NCIM 2083). Dry Technol. 2020, 38, 610–620. [Google Scholar] [CrossRef]
- Raghavi, L.M.; Moses, J.A.; Anandharamakrishnan, C. Refractance window drying of foods: A review. J. Food Eng. 2018, 222, 267–275. [Google Scholar] [CrossRef]
- Bernaert, N.; Van Droogenbroeck, B.; Van Pamel, E.; De Ruyck, H. Innovative refractance window drying technology to keep nutrient value during processing. Trends Food Sci. Technol. 2019, 84, 22–24. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Pandeya, D.R.; Khil, M.-S.; Hwang, I.H. Classy non-wovens based on animate L. gasseri-inanimate poly(vinyl alcohol): Upstream application in food engineering. Appl. Microbiol. Biotechnol. 2013, 97, 4523–4531. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.; García-Moreno, P.J.; Mendes, A.C.; Mateiu, R.V.; Chronakis, I.S. Use of Electrohydrodynamic Processing for Encapsulation of Sensitive Bioactive Compounds and Applications in Food. Annu. Rev. Food Sci. Technol. 2018, 9, 525–549. [Google Scholar] [CrossRef] [PubMed]
- López-Rubio, A.; Sanchez, E.; Wilkanowicz, S.; Sanz, Y.; Lagaron, J.M. Electrospinning as a useful technique for the encapsulation of living bifidobacteria in food hydrocolloids. Food Hydrocoll. 2012, 28, 159–167. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Zhang, L.; Lou, Y.; Schutyser, M.A.I. 3D printing of cereal-based food structures containing probiotics. Food Struct. 2018, 18, 14–22. [Google Scholar] [CrossRef]
- Yoha, K.S.; Anukiruthika, T.; Anila, W.; Moses, J.A.; Anandharamakrishnan, C. 3D printing of encapsulated probiotics: Effect of different post-processing methods on the stability of Lactiplantibacillus plantarum (NCIM 2083) under static in vitro digestion conditions and during storage. LWT 2021, 146, 111461. [Google Scholar] [CrossRef]
- Chen, J.; Vestergaard, M.; Shen, J.; Solem, C.; Dufva, M.; Jensen, P. Droplet-based microfluidics as a future tool for strain improvement in lactic acid bacteria. FEMS Microbiol. Lett. 2018, 365, fny258. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.; Bloom, R.; Silverman, J.; Durand, H.; Jiang, S.; Wu, A.; Huang, S.; You, L.; David, L. High-Throughput Isolation and Culture of Human Gut Bacteria with Droplet Microfluidics. 2019. Available online: https://www.semanticscholar.org/paper/High-throughput-isolation-and-culture-of-human-gut-Villa-Bloom/7d750443ffa753680b11d8980d7ea5fd92e93a50 (accessed on 12 October 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palanivelu, J.; Thanigaivel, S.; Vickram, S.; Dey, N.; Mihaylova, D.; Desseva, I. Probiotics in Functional Foods: Survival Assessment and Approaches for Improved Viability. Appl. Sci. 2022, 12, 455. https://doi.org/10.3390/app12010455
Palanivelu J, Thanigaivel S, Vickram S, Dey N, Mihaylova D, Desseva I. Probiotics in Functional Foods: Survival Assessment and Approaches for Improved Viability. Applied Sciences. 2022; 12(1):455. https://doi.org/10.3390/app12010455
Chicago/Turabian StylePalanivelu, Jeyanthi, Sundaram Thanigaivel, Sundaram Vickram, Nibedita Dey, Dasha Mihaylova, and Ivelina Desseva. 2022. "Probiotics in Functional Foods: Survival Assessment and Approaches for Improved Viability" Applied Sciences 12, no. 1: 455. https://doi.org/10.3390/app12010455







