Improved Bio-Oil Quality from Pyrolysis of Pine Biomass in Pressurized Hydrogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Raw Materials
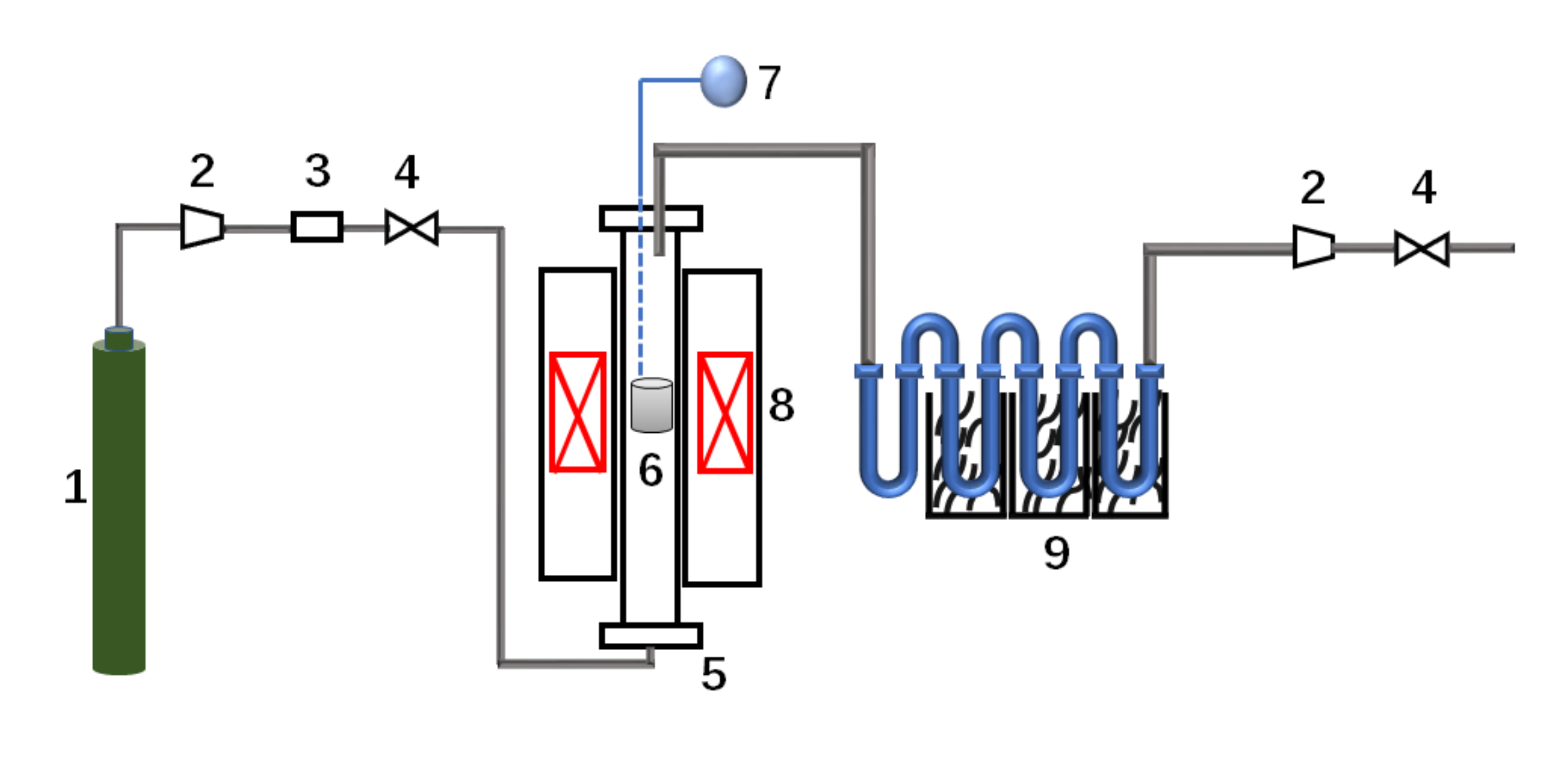
2.2. Experimental Device and Process
2.3. Detection and Characterization of Bio-Oil
2.3.1. Calculation of Yield
2.3.2. Elemental Analysis, Determination of H/C, and Heating Value
2.3.3. Thermogravimetry (TG) Analysis
2.3.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3. Results and Discussion
3.1. Yield of Pyrolysis Products
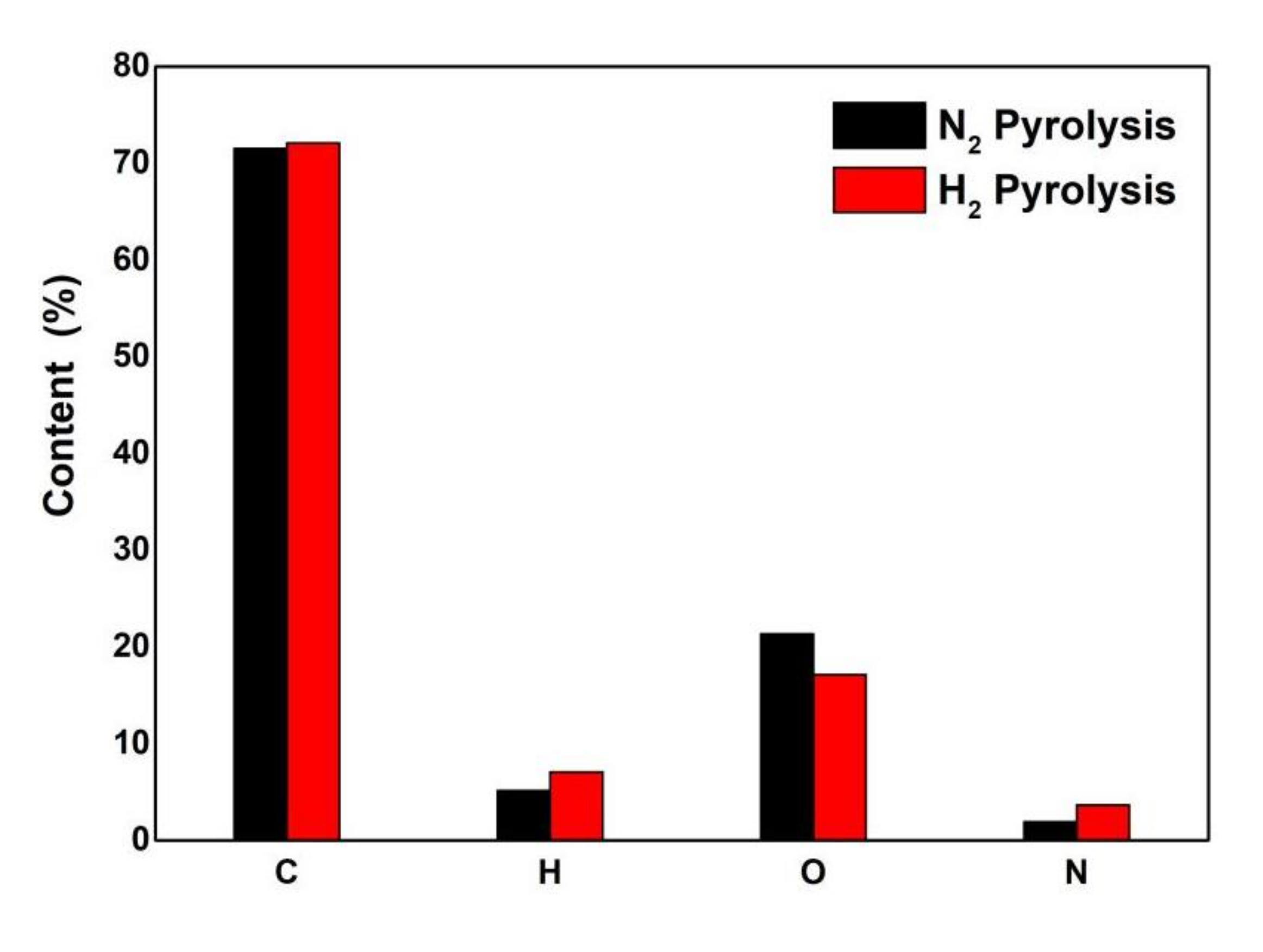
3.2. Elemental Analysis of Pyrolysis Oil
3.3. H/C and Heating Value
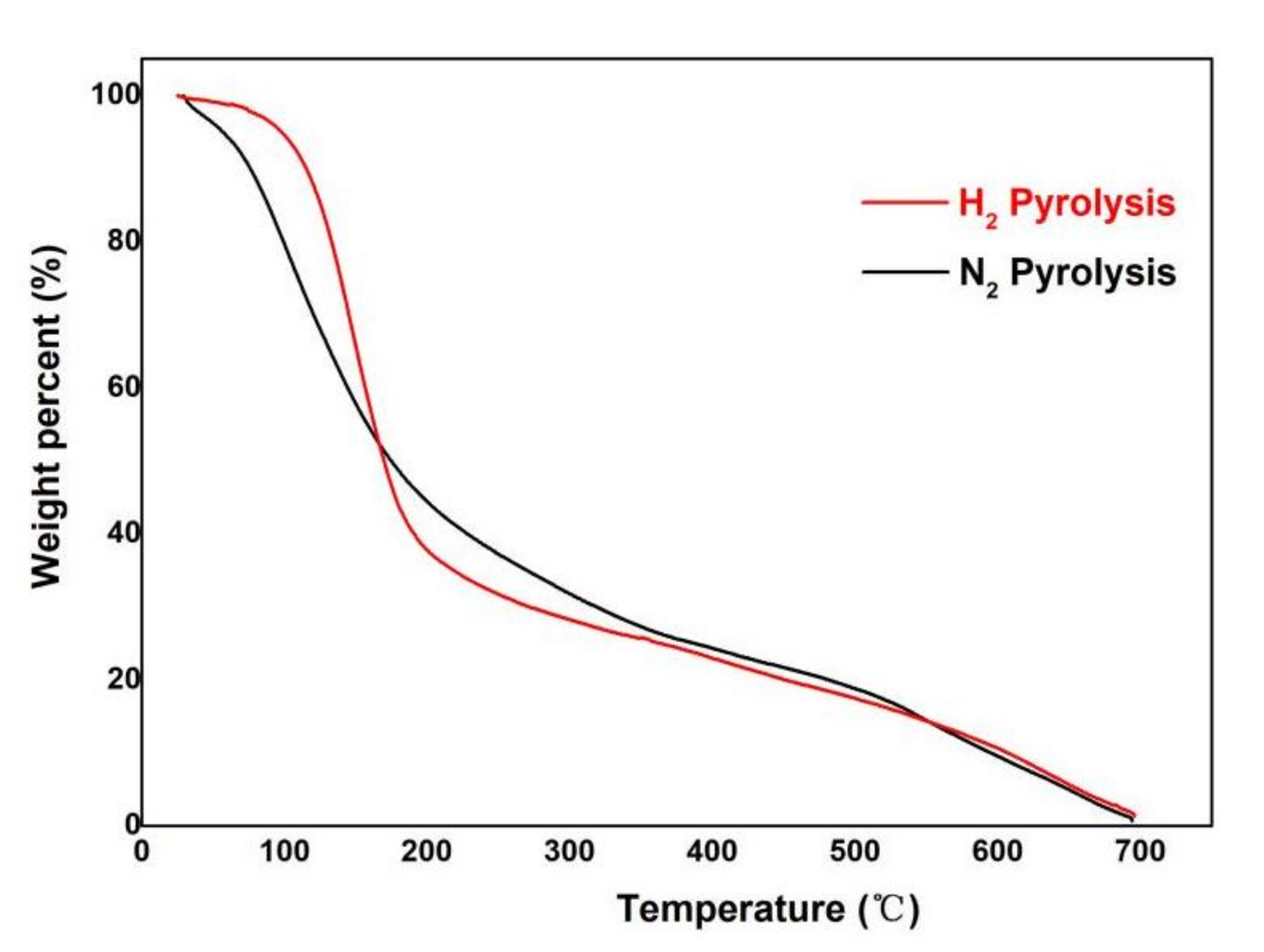
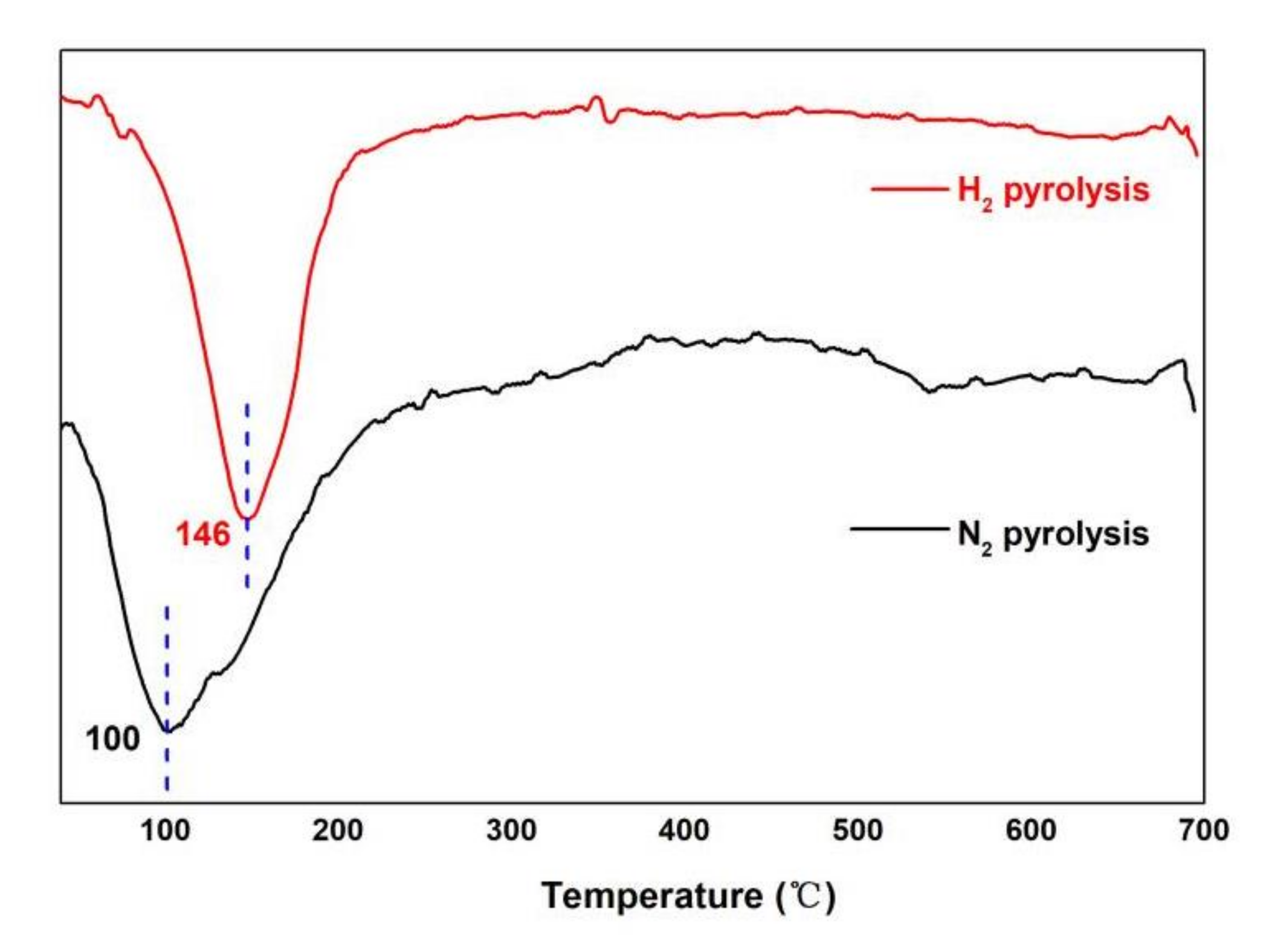
3.4. TGA Analysis of Bio-Oil
3.5. GC-MS Analysis of the Bio-Oil
4. Conclusions
- The yield of pyrolysis oil in a hydrogen atmosphere is 59.23%, which is higher than that (40.88%) in a nitrogen atmosphere, and the solid product is reduced from 26.02% in a nitrogen atmosphere to 20.41% in a hydrogen atmosphere. The carbon content of pyrolysis bio-oil obtained under hydrogen is basically unchanged. Oxygen decreased from 21.34% to 17.16%, hydrogen increased from 5.17% to 7.06%, the H/C ratio increased from 0.072 to 0.098, and the heating value increased from 27.75 MJ/kg to 31.40 MJ/kg;
- In the hydrogenated pyrolysis of bio-oil, the content of compounds with a boiling point of 0–200 °C is 63.21%, while in a nitrogen atmosphere, the content of components within the boiling point range is 55.11%. Therefore, the low boiling point fraction of pyrolysis bio-oil can be improved in a hydrogen atmosphere, which is conducive to improving the combustion characteristics of pyrolysis oil;
- By comparison, the biomass pyrolysis oil changed obviously in the hydrogen atmosphere. Ethyl hexadecanoate (peak area percentage 19.1%) and methyl octadecanoate (peak area percentage 15.42%) were obtained. The number of phenols decreased from 33.66% to 28.38%. Pyrolysis in a hydrogen atmosphere inhibited its aromatization and reduced the side chain reaction of the benzene ring. The content of esters increased significantly in the hydrogen atmosphere, and acids increased from 0.45% in the nitrogen atmosphere to 1.65% in the hydrogen atmosphere. It is mainly hexadecanoic acid, nineteenth acid, ethanol, and methanol. Therefore, esterification occurred in the pyrolysis process, resulting in the formation of long-chain esters and reducing the acidity of pyrolysis bio-oil.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Wat, J.; Liu, Z.; Wu, Y.; Yang, M. Elemental migration and transformation during hydrothermal liquefaction of biomass. J. Hazard. Mater. 2021, 423, 126961. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhou, M.; Li, W.; Wang, X.; Cui, M.; Yang, Y. Catalytic fast pyrolysis of biomass with noble metal-like catalysts to produce high-grade bio-oil. Analytical Py-GC/MS study. Catal. Today 2018, 302, 169–179. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.; Singh, R. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Q.; Wang, T.; Li, B.; Xu, Y.; Ma, L. Efficient upgrading process for production of low quality fuel from bio-oil. Fuel 2016, 179, 312–321. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.; Norbeck, J.; Park, C.S. A simple kinetic analysis of syngas during steam hydrogasification of biomass using a novel inverted batch reactor with instant high pressure feeding. Bioresour. Technol. 2016, 200, 731–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, S.; Wang, W. Experimental and techno-economic studies of upgrading heavy pyrolytic oils from wood chips into valuable fuels. J. Clean. Prod. 2020, 277, 124136. [Google Scholar] [CrossRef]
- Figueirêdoa, M.; Venderboschb, R.; Heeresa, H.; Deuss, P. In-depth structural characterization of the lignin fraction of a pine-derived pyrolysis oil. J. Anal. Appl. Pyrol. 2020, 149, 104837. [Google Scholar] [CrossRef]
- Staš, M.; Auersvald, M.; Kejla, L.; Vrtiška, D.; Kroufek, J.; Kubička, D. Quantitative analysis of pyrolysis bio-oils. A review. TrAC Trends Anal. Chem. 2020, 126, 115857. [Google Scholar] [CrossRef]
- Jaffar, M.; Nahil, M.; Williams, P. Methane production from the pyrolysis-catalytic hydrogenation of waste biomass: Influence of process conditions and catalyst type. Energy Fuels 2019, 33, 7443–7457. [Google Scholar] [CrossRef]
- Ahamed, T.; Anto, S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Upgrading of bio-oil from thermochemical conversion of various biomass—Mechanism, challenges and opportunities. Fuel 2021, 287, 119329. [Google Scholar] [CrossRef]
- Han, D.; Yang, X.; Li, R.; Wu, Y. Environmental impact comparison of typical and resource-efficient biomass fast pyrolysis systems based on LCA and Aspen Plus simulation. J. Clean. Prod. 2019, 231, 254–267. [Google Scholar] [CrossRef]
- Chandler, D.; Resende, F. Comparison between catalytic fast pyrolysis and catalytic fast hydropyrolysis for the production of liquid fuels in a fluidized bed reactor. Energy Fuels 2019, 33, 3199–3209. [Google Scholar] [CrossRef]
- Saidi, M.; Moradi, P. Catalytic hydrotreatment of lignin-derived pyrolysis bio-oils using Cu/γ-Al2O3 catalyst. Reaction network development and kinetic study of anisole upgrading. Int. J. Energy Res. 2021, 45, 8267–8284. [Google Scholar] [CrossRef]
- Chaihad, N.; Situmorang, Y.; Anniwaer, A.; Kurnia, I.; Karnjanakom, S.; Kasai, Y.; Abudula, A.; Reubroycharoen, P.; Guan, G. Preparation of various hierarchical HZSM-5 based catalysts for in-situ fast upgrading of bio-oil. Renew. Energy 2021, 169, 283–292. [Google Scholar] [CrossRef]
- Arazo, R.; Capareda, S.; Ofrasio, B.; Ido, A.; Chen, W.; Luna, M. Low-temperature catalytic conversion of alkaline sewage sludge bio-oil to biodiesel: Product characteristics and reaction mechanisms. Environ. Technol. Innov. 2021, 21, 101266. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, F.; Djandja, J.; Zhang, S.; Xu, Y.; Duan, P. Hydrothermal liquefaction of crop straws: Effect of feedstock composition. Fuel 2020, 265, 116946. [Google Scholar] [CrossRef]
- Yang, X.; Han, D.; Zhao, Y.; Li, R.; Wu, Y. Environmental evaluation of a distributed-centralized biomass pyrolysis system. Total Environ. 2020, 716, 136915. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Zhang, J.; Wang, J. Parametric study of two-stage hydropyrolysis of lignocellulosic biomass for production of gaseous and light aromatic hydrocarbons. Bioresour. Technol. 2017, 244, 142–150. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, N.; Wang, J. Comparative investigation of rice husk, thermoplastic bituminous coal and liquid products during hydropyrolysis/co-hydropyrolysis. Bioresour. Technol. 2018, 268, 445–453. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Zhang, L.; Liu, Z.; Wu, Y. Catalytic hydrothermal liquefaction of microalgae over mesoporous silicabased materials with site-separated acids and bases. Fuel 2020, 279, 118–127. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J.; Bhaskar, T. Utilization of lignin: A sustainable and eco-friendly approach. J. Energy Inst. 2020, 93, 235–271. [Google Scholar] [CrossRef]
- Wan, S.; Ru, B.; Lin, H.; Sun, W.; Luo, Z. Pyrolysis behaviors of four lignin polymers isolated from the same pine wood. Bioresour. Technol. 2015, 182, 120–127. [Google Scholar]
- Zhou, D.; Zhang, S.; Fu, H.; Chen, J. Liquefaction of macroalgae enteromorpha prolifera in sub-/supercritical alcohols: Direct production of ester compounds. Energy Fuels 2012, 26, 2342–2351. [Google Scholar] [CrossRef]
- Neutelings, G. Lignin variability in plant cell walls: Contribution of new models. Plant Sci. 2011, 181, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Haruo, K.; Sunao, H.; Shiro, S. Effects of side-chain hydroxyl groups on pyrolytic β-ether cleavage of phenolic lignin model dimer. J. Wood Sci. 2007, 53, 268–271. [Google Scholar]



| Elemental Analysis (%) | Proximate Analysis (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | Moisture | Volatiles | Fixed Carbon | Ash Content |
| 47.10 | 5.89 | 0.36 | 0.01 | 43.93 | 2.03 | 71.41 | 19.21 | 2.72 |
| Bio-Oil in Nitrogen Atmosphere | Bio-Oil in Hydrogen Atmosphere | ||||
|---|---|---|---|---|---|
| Time/Min | Component | Peak Area % | Time/Min | Component | Peak Area % |
| 3.07 | Aceticacid, hydrazide | 9.91 | 3.24 | Toluene | 5.42 |
| 3.18 | Butanoicacid, 2-methyl-, methylester | 1.83 | 4.88 | Furfural | 1.05 |
| 4.03 | 2-Pentanone, 4-hydroxy-4-methyl- | 0.48 | 5.64 | Ethylbenzene | 1.78 |
| 4.79 | Styrene | 0.98 | 5.85 | Benzene, 1,3-dimethyl- | 1.89 |
| 5.78 | 2(3H)-Furanone, dihydro-5-methyl- | 1.41 | 8.16 | Benzene, 1,2,3-trimethyl- | 1.11 |
| 5.99 | 2-Cyclopenten-1-one, 3-methyl- | 0.36 | 8.77 | Phenol | 4.19 |
| 6.15 | Phenol | 12.7 | 9.27 | Butanoicacid, anhydride | 0.96 |
| 6.49 | 3-Phenyl-1-propanol, acetate | 0.55 | 9.32 | Ethanol, 2,2-oxybis-, diacetate | 0.65 |
| 6.55 | Benzofuran | 0.83 | 9.76 | 1,2-Cyclopentanedione, 3-methyl- | 1.01 |
| 7.36 | Indene | 2.27 | 9.88 | Indane | 0.72 |
| 7.40 | Phenol, 2-methyl- | 3.5 | 10.36 | Phenol, 2-methyl- | 1.83 |
| 7.57 | Aceticacid, phenylester | 0.79 | 10.83 | Phenol, 3-methyl- | 3.96 |
| 7.73 | Phenol, 2-methyl- | 6.79 | 11.09 | Phenol, 2-methoxy- | 3.09 |
| 8.32 | Cinnamaldehyde | 0.58 | 11.42 | 3-Pentanol, 2,2,4,4-tetramethyl- | 0.51 |
| 8.93 | Phenol, 2,3-dimethyl- | 1.4 | 12.33 | Lacticacid, 2-methyl-, monoanhydride | 2.14 |
| 9.06 | 1H-Indene, 3-methyl- | 0.47 | 12.37 | Phenol, 2,5-dimethyl- | 1.01 |
| 9.63 | Azulene | 7.84 | 12.71 | Phenol, 4-ethyl- | 0.41 |
| 9.85 | Benzofuran, 2,3-dihydro- | 1.03 | 12.76 | Phenol, 2,3-dimethyl- | 1.31 |
| 9.98 | Benzofuran, 2,3-dihydro- | 0.69 | 13.10 | Naphthalene | 6.81 |
| 11.28 | Naphthalene, 1-methyl- | 1.97 | 13.24 | Creosol | 2.87 |
| 11.54 | Bicyclo(4.4.1) undeca-1,3,5,7,9-pentae | 2.8 | 13.42 | Catechol | 2.59 |
| 12.35 | 1,2-Benzenediol, diacetate | 0.49 | 14.90 | Phenol, 4-ethyl-2-methoxy- | 1.79 |
| 12.45 | Biphenyl | 0.55 | 15.12 | 1,2-Benzenediol, 4-methyl- | 1.2 |
| 13.01 | Naphthalene, 1,3-dimethyl- | 0.36 | 15.23 | Naphthalene, 2-methyl- | 1.54 |
| 13.14 | Acenaphthene | 1.25 | 15.54 | Naphthalene, 2-methyl- | 0.66 |
| 13.44 | Biphenylene | 2.52 | 16.50 | Phenol, 2-methoxy-4-propyl- | 0.52 |
| 15.07 | Hexane, 3,3-dimethyl- | 0.52 | 16.75 | Biphenyl | 0.87 |
| 15.12 | Fluorene | 0.61 | 17.92 | trans-Isoeugenol | 0.76 |
| 15.64 | Fluorene | 0.37 | 20.17 | Fluorene | 1.52 |
| 17.42 | Anthracene | 2.34 | 23.12 | Anthracene | 2.11 |
| 17.53 | 9H-Fluorene, 9-methylene- | 0.63 | 25.34 | n-Hexadecanoicacid | 1.14 |
| 19.12 | 1,2-Benzenedicarboxylicacid | 0.29 | 25.80 | Hexadecanoicacid, ethylester | 19.1 |
| 20.54 | Fluoranthene | 0.28 | 27.78 | Octadecanoicacid | 0.51 |
| 20.84 | Fluoranthene | 0.51 | 28.19 | Octadecanoicacid, 17-methyl-, methyl | 15.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, S.; Lu, J.; Yang, M.; Wu, Y. Improved Bio-Oil Quality from Pyrolysis of Pine Biomass in Pressurized Hydrogen. Appl. Sci. 2022, 12, 46. https://doi.org/10.3390/app12010046
Wang J, Wang S, Lu J, Yang M, Wu Y. Improved Bio-Oil Quality from Pyrolysis of Pine Biomass in Pressurized Hydrogen. Applied Sciences. 2022; 12(1):46. https://doi.org/10.3390/app12010046
Chicago/Turabian StyleWang, Jingliang, Shanshan Wang, Jianwen Lu, Mingde Yang, and Yulong Wu. 2022. "Improved Bio-Oil Quality from Pyrolysis of Pine Biomass in Pressurized Hydrogen" Applied Sciences 12, no. 1: 46. https://doi.org/10.3390/app12010046
APA StyleWang, J., Wang, S., Lu, J., Yang, M., & Wu, Y. (2022). Improved Bio-Oil Quality from Pyrolysis of Pine Biomass in Pressurized Hydrogen. Applied Sciences, 12(1), 46. https://doi.org/10.3390/app12010046






