Stable Isotope and Radiocarbon Analysis for Diet, Climate and Mobility Reconstruction in Agras (Early Iron Age) and Edessa (Roman Age), Northern Greece
Abstract
:1. Introduction
Historical Background
2. Material and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Diet Reconstruction
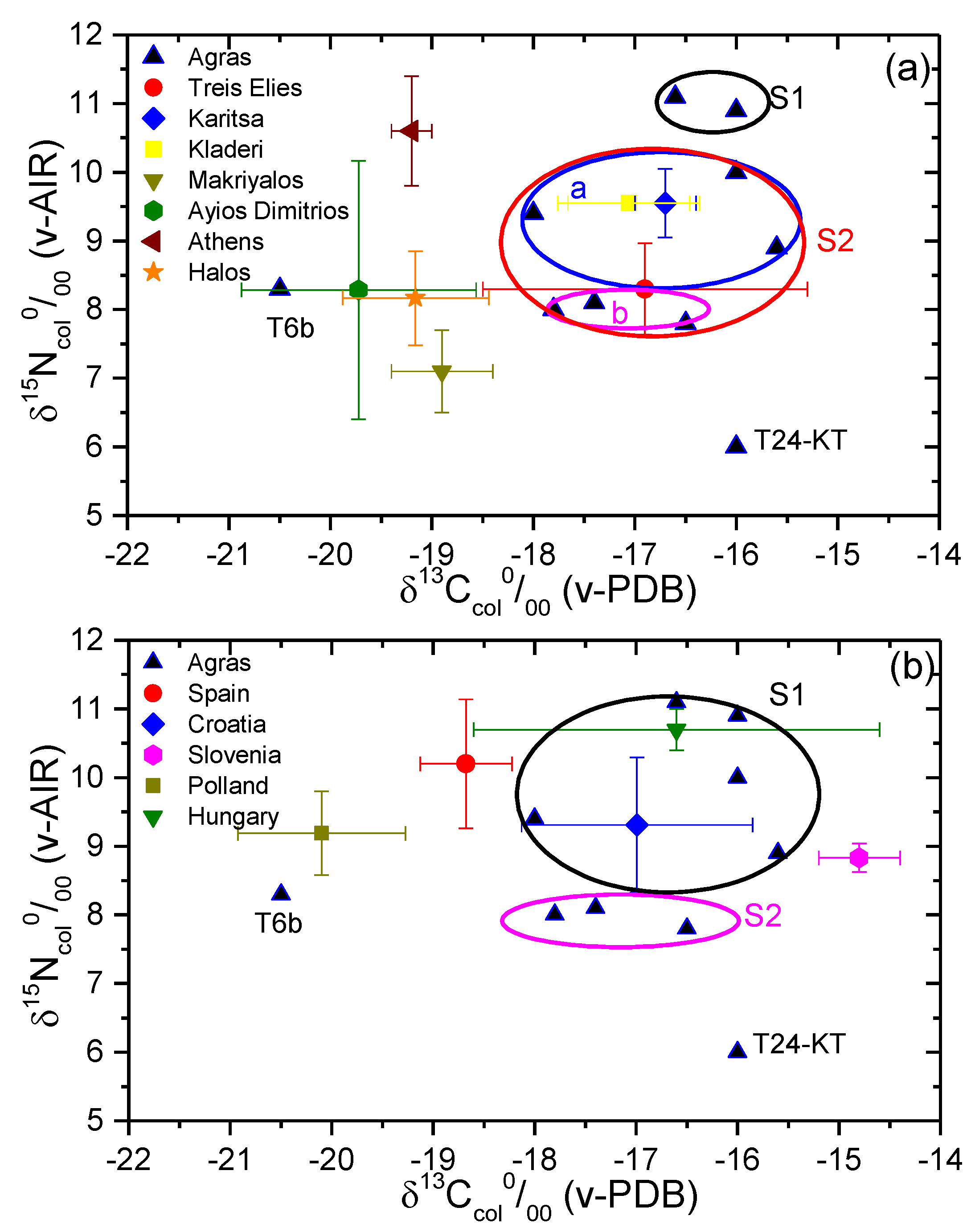
3.2. Comparison with Other Regions
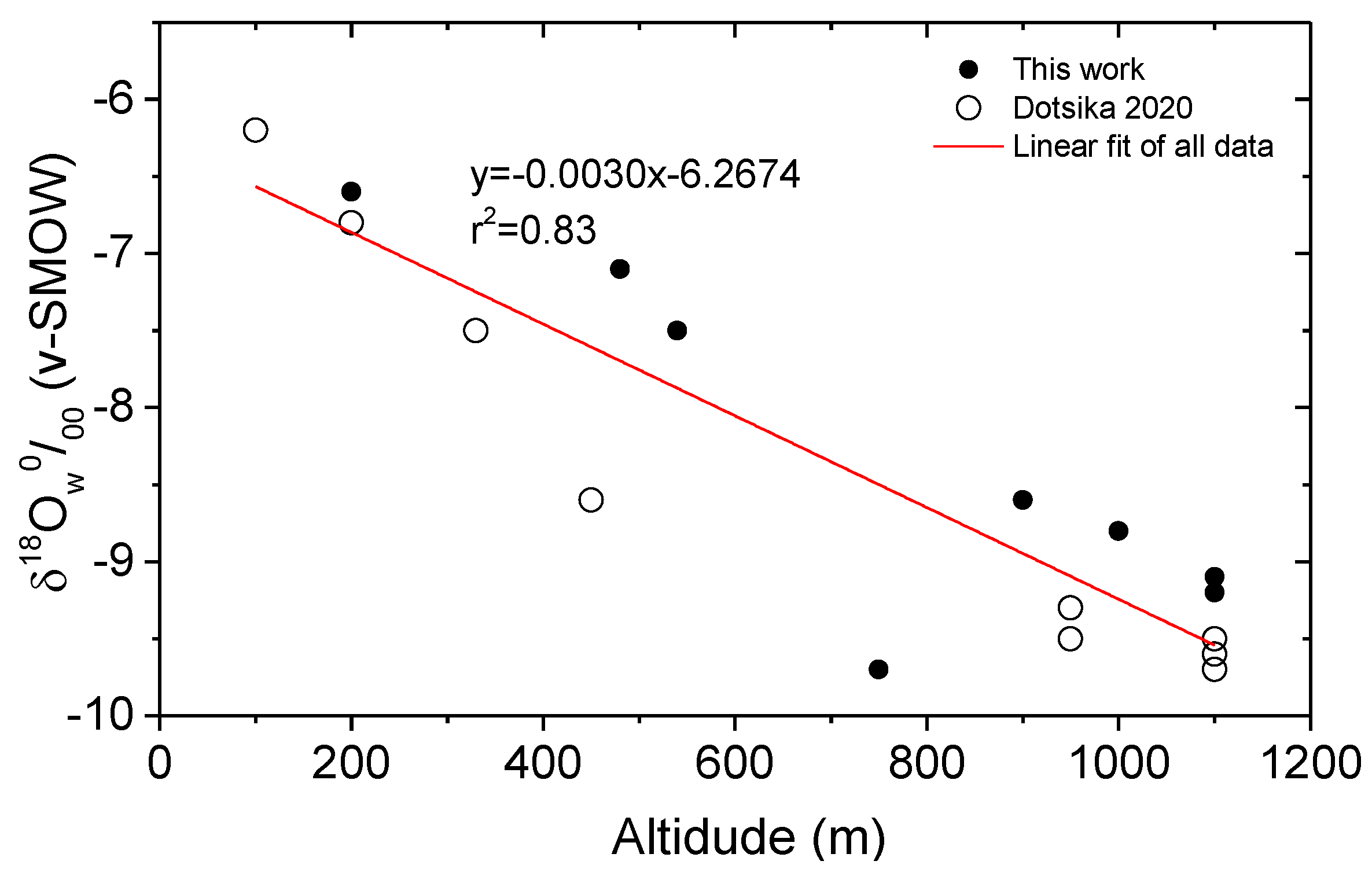
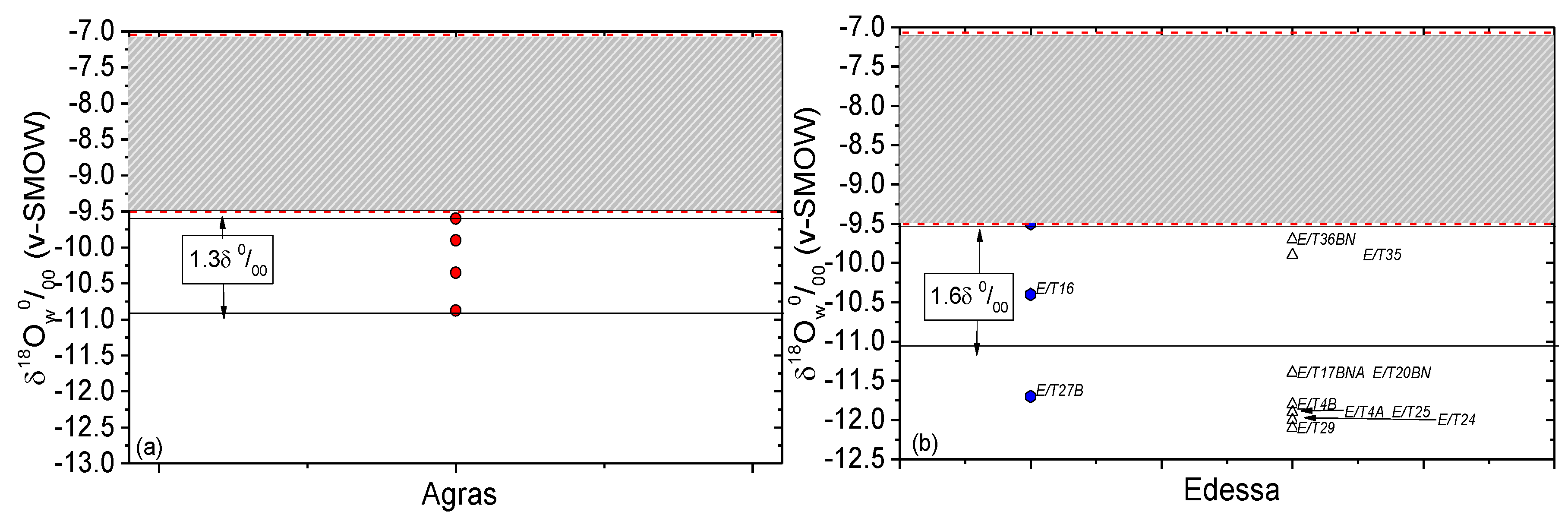
3.3. Climatic Conditions and Mobility
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Katzenberg, M.A. Stable isotope analysis: A tool for studying past diet, demography, and life history. In Biological Anthropology of the Human Skeleton; Katzenberg, M.A., Saunders, S.R., Eds.; Wiley-Liss: Hoboken, NJ, USA, 2008; pp. 413–441. [Google Scholar]
- Dotsika, E.; Diamantopoulos, G.; Lykoudis, S.; Gougoura, S.; Kranioti, E.; Karalis, P.; Michail, D.; Samartzidou, E.; Palaigeorgiou, E. Establishment of a Greek food database for palaeodiet recostruction: Case study of human and fauna remains Neolithic to Late Bronze age from Greece. Geosciences 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Dotsika, E.; Michael, D.E. Using stable isotope technique in order to assess the dietary habits of a Roman population in Greece. J. Archaeol. Sci.-Rep. 2018, 22, 470–481. [Google Scholar] [CrossRef]
- Dotsika, E.; Michael, D.E.; Iliadis, E.; Karalis, P.; Diamantopoulos, G. Isotopic reconstruction of diet in Medieval Thebes (Greece). J. Archaeol. Sci.-Rep. 2018, 22, 482–491. [Google Scholar] [CrossRef]
- Papathanasiou, A.; Richards, M.P.; Fox, S.C. Archaeodiet in the Greek World: Dietary Reconstruction from Stable Isotope Analysis; American School of Classical Studies: Athens, Greece, 2015; Volume 49. [Google Scholar]
- Guede, I.; Zuluaga, M.C.; Ortega, L.A.; Alonso-Olazabal, A.; Murelaga, X.; Camino, I.G.; Iacumin, P. Social structuration in medieval rural society based on stable isotope analysis of dietary habits and mobility patterns: San Juan de Momoitio (Biscay, north Iberian Peninsula). J. Archaeol. Sci. Rep. 2020, 31, 102300. [Google Scholar] [CrossRef]
- Keenleyside, A.; Schwarcz, H.; Stirling, L.; Lazreg, N.B. Stable isotopic evidence for diet in a Roman and Late Roman population from Leptiminus, Tunisia. J. Archaeol. Sci. 2009, 36, 51–63. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. The influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of Diet on the Distribution of Nitrogen Isotopes in Animals. Geochim. Et Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- DeNiro, M.J. Post-mortem preservation and alteration of in vivo bone collagen isotope ratios in relation to paleodietary reconstruction. Nature 1985, 317, 806–809. [Google Scholar] [CrossRef]
- Vika, E.; Theothoropoulou, T. Re-investigating fish consumption in Greek antiquity: Results from d13C and d15N analysis from fish bone collagen. J. Archaeol. Sci. Rep. 2012, 39, 1618–1627. [Google Scholar] [CrossRef]
- Longinelli, A. Oxygen isotopes in mammal bone phosphate: A new tool for paleohydrological and paleoclimatological research? Geochim. Et Cosmochim. Acta 1984, 48, 385–390. [Google Scholar] [CrossRef]
- Michael, D.-E.; Dotsika, E. Using Oxygen Isotopic Values in Order to Infer Palaeoclimatic Differences between Northern and Central-Southern Greece. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Prague, Czech Republic, 11–15 September 2017; p. 042056. [Google Scholar]
- Michael, D.-E.; Dotsika, E. Using Oxygen and Carbon Isotopic Signatures in Order to Infer Climatic and Dietary Information in Roman Edessa, Greece. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Prague, Czech Republic, 11–15 September 2017; p. 042050. [Google Scholar]
- Psomiadis, D.; Dotsika, E.; Albanakis, K.; Ghaleb, B.; Hillaire-Marcel, C. Speleothem record of climatic changes in the northern Aegean region (Greece) from the Bronze Age to the collapse of the Roman Empire. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 489, 272–283. [Google Scholar] [CrossRef]
- Britton, K.; Pederzani, S.; Kindler, L.; Roebroeks, W.; Gaudzinski-Windheuser, S.; Richards, M.P.; Tütken, T. Oxygen isotope analysis of Equus teeth evidences early Eemian and early Weichselian palaeotemperatures at the Middle Palaeolithic site of Neumark-Nord 2, Saxony-Anhalt, Germany. Quat. Sci. Rev. 2019, 226, 106029. [Google Scholar] [CrossRef]
- Clauzel, T.; Richardin, P.; Ricard, J.; Le Béchennec, Y.; Amiot, R.; Fourel, F.; Phouybanhdyt, B.; Vinçon-Laugier, A.; Flandrois, J.-P.; Lécuyer, C. The Gauls experienced the Roman Warm Period: Oxygen isotope study of the Gallic site of Thézy-Glimont, Picardie, France. J. Archaeol. Sci. Rep. 2020, 34, 102595. [Google Scholar] [CrossRef]
- White, C.D.; Spence, M.W.; Stuart-Williams, H.L.Q.; Schwarcz, H.P. Oxygen isotopes and the identification of geographical origins: The Valley of Oaxaca versus the Valley of Mexico. J. Archaeol. Sci. 1998, 25, 643–655. [Google Scholar] [CrossRef]
- Dotsika, E. Correlation between δ 18 Ow and δ 18 Oen for estimating human mobility and paleomobility patterns. Sci. Rep. 2020, 10, 15439. [Google Scholar] [CrossRef]
- Hamre, S.S.; Daux, V. Stable oxygen isotope evidence for mobility in medieval and post-medieval Trondheim, Norway. J. Archaeol. Sci. Rep. 2016, 8, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Maggiano, C.M.; White, C.D.; Stern, R.A.; Peralta, J.S.; Longstaffe, F.J. Focus: Oxygen isotope microanalysis across incremental layers of human bone: Exploring archaeological reconstruction of short term mobility and seasonal climate change. J. Archaeol. Sci. 2019, 111, 105028. [Google Scholar] [CrossRef]
- Stark, R.J.; Emery, M.V.; Schwarcz, H.; Sperduti, A.; Bondioli, L.; Craig, O.E.; Prowse, T. Imperial Roman mobility and migration at Velia (1st to 2nd c. CE) in southern Italy. J. Archaeol. Sci. Rep. 2020, 30, 102217. [Google Scholar] [CrossRef]
- Francisci, G.; Micarelli, I.; Iacumin, P.; Castorina, F.; Di Vincenzo, F.; Di Matteo, M.; Giostra, C.; Manzi, G.; Tafuri, M.A. Strontium and oxygen isotopes as indicators of Longobards mobility in Italy: An investigation at Povegliano Veronese. Sci. Rep. 2020, 10, 11678. [Google Scholar] [CrossRef]
- Symonds, L.; Price, D.T.; Keenleyside, A.; Burton, J. Medieval migrations: Isotope analysis of early Medieval Skeletons on the Isle of Man. Mediev. Archaeol. 2014, 58, 1–20. [Google Scholar] [CrossRef]
- White, C.D.; Spence, M.W.; Longstaffe, F.J.; Law, K.R. Testing the nature of Teotihuacan imperialism at Kaminaljuyu using phosphate oxygen-isotope ratios. J. Anthropol. Res. 2000, 56, 535–558. [Google Scholar] [CrossRef]
- Deines, P. The isotopic composition of reduced organic carbon. In Handbook of Environmental Isotope Geochemistry; Fritz, P., Fontes, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1980; Volume 1, pp. 329–406. [Google Scholar]
- Lee-Thorp, J.A. On Isotopes and Old Bones. Archaeometry 2008, 50, 925–950. [Google Scholar] [CrossRef]
- Smith, B.N.; Epstein, S. Two categories of 13C/12C ratios for higher plants. Plant Physiol. 1971, 47, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Tieszen, L.L.; Boutton, T.W.; Tesdahl, K.G.; Slade, N.A. Fractionation and turnover of stable carbon isotopes in animal tissues: Implications for δ13C analysis of diet. Oecologia 1983, 57, 32–37. [Google Scholar] [CrossRef]
- Ambrose, S.H. Isotopic Analysis of Paleodiets: Methodological and Interpretive Considerations. In Investigations of Ancient Human Tissue: Chemical Analyses in Anthropology; Sandford, M.K., Ed.; Gordon and Breach: Philadelphia, PA, USA, 1993; pp. 59–130. [Google Scholar]
- Chisholm, B.S.; Nelson, D.E.; Schwarcz, H.P. Stable-Carbon Isotope Ratios as a Measure of Marine Versus Terrestrial Protein in Ancient Diets. Science 1982, 216, 1131–1132. [Google Scholar] [CrossRef] [Green Version]
- Schwarcz, H.P.; Melbye, J.; Katzenberg, M.A.; Knyf, M. Stable isotopes in human skeletons of southern Ontario: Reconstructing palaeodiet. J. Archaeol. Sci. 1985, 12, 187–206. [Google Scholar] [CrossRef]
- Richards, M.P. Stable isotope analysis of bone and teeth as a means for reconstructing past human diets in Greece. Hesperia Suppl. 2015, 49, 15–23. [Google Scholar]
- Lee-Thorp, J.A.; Sealy, J.C.; Van Der Merwe, N.J. Stable carbon isotope ratio differences between bone collagen and bone apatite, and their relationship to diet. J. Archaeol. Sci. 1989, 16, 585–599. [Google Scholar] [CrossRef]
- Bocherens, H.; Drucker, D. Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: Case studies from recent and ancient terrestrial ecosystemns. Int. J. Osteoarchaeol. 2003, 13, 46–53. [Google Scholar] [CrossRef]
- Hedges, R.E.M.; Reynard, L.M. Nitrogen isotopes and the trophic level of humans in archaeology. J. Archaeol. Sci. 2007, 34, 1240–1251. [Google Scholar] [CrossRef]
- Schwarcz, H.P.; Schoeninger, M.J. Stable isotope analyses in human nutritional ecology. Am. J. Phys. Anthropol. 1991, 34, 283–321. [Google Scholar] [CrossRef]
- Lubell, D.; Jackes, M.; Schwarcz, H.; Knyf, M.; Meiklejohn, C. The Mesolithic-Neolithic transition in Portugal: Isotopic and dental evidence of diet. J. Archaeol. Sci. 1994, 21, 201–216. [Google Scholar] [CrossRef]
- Schoeninger, M.J.; Deniro, M.J. Nitrogen and Carbon Isotopic Composition of Bone-Collagen from Marine and Terrestrial Animals. Geochim. Et Cosmochim. Acta 1984, 48, 625–639. [Google Scholar] [CrossRef]
- Bourbou, C.; Fuller, B.T.; Garvie-Lok, S.J.; Richards, M.P. Reconstructing the Diets of Greek Byzantine Populations (6th-15th Centuries AD) Using Carbon and Nitrogen Stable Isotope Ratios. Am. J. Phys. Anthropol. 2011, 146, 569–581. [Google Scholar] [CrossRef]
- Lai, L.; Tykot, R.H.; Usai, E.; Beckett, J.F.; Floris, R.; Fonzo, O.; Goddard, E.; Hollander, D.; Manunza, M.R.; Usai, A. Diet in the Sardinian Bronze Age: Models, collagen isotopic data, issues and perspectives. Préhistoires Méditerranéennes 2013, 4, 1–21. [Google Scholar]
- Bryant, J.D.; Froelich, P.N.; Showers, W.J.; Genna, B.J. Biologic and climatic signals in the oxygen isotopic composition of Eocene-Oligocene equid enamel phosphate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 126, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Kovács, J.; Moravcová, M.; Újvári, G.; Pintér, A.G. Reconstructing the paleoenvironment of East Central Europe in the Late Pleistocene using the oxygen and carbon isotopic signal of tooth in large mammal remains. Quat. Int. 2012, 276, 145–154. [Google Scholar] [CrossRef]
- Arppe, L.; Karhu, J. Oxygen isotope values of precipitation and the thermal climate in Europe during the middle to late Weichselian ice age. Quat. Sci. Rev. 2010, 29, 1263–1275. [Google Scholar] [CrossRef]
- Bryant, J.D.; Luz, B.; Froelich, P.N. Oxygen isotopic composition of fossil horse tooth phosphate as a record of continental paleoclimate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1994, 107, 303–316. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pouncett, J.; Jay, M.; Pearson, M.P.; Richards, M.P. Tooth enamel oxygen “isoscapes” show a high degree of human mobility in prehistoric Britain. Sci. Rep. 2016, 6, 34986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luz, B.; Kolodny, Y.; Horowitz, M. Fractionation of oxygen isotopes between mammalian bone-phosphate and environmental drinking water. Geochim. Et Cosmochim. Acta 1984, 48, 1689–1693. [Google Scholar] [CrossRef]
- Daux, V.; Lécuyer, C.; Héran, M.-A.; Amiot, R.; Simon, L.; Fourel, F.; Martineau, F.; Lynnerup, N.; Reychler, H.; Escarguel, G. Oxygen isotope fractionation between human phosphate and water revisited. J. Hum. Evol. 2008, 55, 1138–1147. [Google Scholar] [CrossRef] [Green Version]
- Levinson, A.A.; Luz, B.; Kolodny, Y. Variations in oxygen isotopic compositions of human teeth and urinary stones. Appl. Geochem. 1987, 2, 367–371. [Google Scholar] [CrossRef]
- Dotsika, E.; Tsoukala, E.; Zisi, N.; Poutoukis, D.; Psomiadis, D. Palaeo-climatic information from isotopic signatures of fossil teeth in Late Pleistocene from Arkoudospilia Cave (Aridea, N. Greece). In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 2–7 May 2010; p. 12246. [Google Scholar]
- Dotsika, E.; Lykoudis, S.; Poutoukis, D. Spatial distribution of the isotopic composition of precipitation and spring water in Greece. Glob. Planet. Change 2010, 71, 141–149. [Google Scholar] [CrossRef]
- Lykoudis, S.P.; Argiriou, A.A.; Dotsika, E. Spatially interpolated time series of δ18O in Eastern Mediterranean precipitation. Glob. Planet. Change 2010, 71, 150–159. [Google Scholar] [CrossRef]
- Dotsika, E.; Diamantopoulos, G.; Lykoudis, S.; Poutoukis, D.; Kranioti, E. Isotopic composition of spring water in Greece: Spring waters isoscapes. Geosciences 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Kohn, M.J.; Welker, J.M. On the temperature correlation of δ18O in modern precipitation. Earth Planet. Sci. Lett. 2005, 231, 87–96. [Google Scholar] [CrossRef]
- Panagiotopoulou, E.; Papathanasiou, A. Dietary Reconstruction at the Geometric-Period Burial. Site of Ayios Dimitrios. In Archaeodiet in the Greek World. Dietary Reconstruction from Stable Isotope Analysis; Papathanasiou, A., Richards, M.P., Eds.; The American School of Classical Studies at Athens: Princeton, NJ, USA, 2015; pp. 105–117. [Google Scholar]
- Coldstream, J.N. Geometric Greece: 900-700 BC; Psychology Press: Hove, UK, 2003. [Google Scholar]
- Triantaphyllou, S. A Bioarchaeological Approach to Prehistoric Cemetery Populations from Central and Western Greek Macedonia; British Archaeological Reports Limited: Oxford, UK, 2001; Volume 976. [Google Scholar]
- Chrysostomou, A. Agras - Edessa in the Iron Age; Municipality of Edessa: Edessa, Greece, 2021. [Google Scholar]
- Lagia, A. Diet and the Polis: An Isotopic Study of Diet in Athens and Laurion during the Classical, Hellenistic, and Imperial Roman Periods. In Archaeodiet in the Greek World Dietary Reconstruction from Stable Isotope Analysis; Papathanasiou, A., Richards, M.P., Fox, S.C., Eds.; The American School of Classical Studies at Athens: Princeton, NJ, USA, 2015; pp. 119–145. [Google Scholar]
- Panagiotopoulou, E.; van der Plicht, J.; Papathanasiou, A.; Voutsaki, S.; Nikolaou, E.; Tsiouka, F. Isotopic (13C, 15N) investigation of diet and social structure in Early Iron Age Halos, Greece. J. Archaeol. Sci. Rep. 2016, 10, 212–220. [Google Scholar] [CrossRef]
- Fernández-Crespo, T.; Ordoño, J.; Bogaard, A.; Llanos, A.; Schulting, R. A snapshot of subsistence in Iron Age Iberia: The case of La Hoya village. J. Archaeol. Sci. Rep. 2019, 28, 102037. [Google Scholar] [CrossRef]
- Zavodny, E.; Culleton, B.J.; McClure, S.B.; Kennett, D.J.; Balen, J. Minimizing risk on the margins: Insights on Iron Age agriculture from stable isotope analyses in central Croatia. J. Anthropol. Archaeol. 2017, 48, 250–261. [Google Scholar] [CrossRef]
- Mnich, B.; Mueller-Bieniek, A.; Nowak, M.; Wilczyński, J.; Pospuła, S.; Szostek, K. Terrestrial diet in prehistoric human groups from southern Poland based on human, faunal and botanical stable isotope evidence. J. Archaeol. Sci. Rep. 2020, 32, 102382. [Google Scholar] [CrossRef]
- Nicholls, R.A.; Buckberry, J.; Črešnar, M.; Armit, I.; Mason, P.; Koon, H. Interdisciplinary study of human remains from the Early Iron Age cemetery at Zagorje ob Savi (Slovenia). Arheol. Vestn. 2020, 71, 487–498. [Google Scholar]
- Gamarra, B.; Howcroft, R.; McCall, A.; Dani, J.; Hajdú, Z.; Nagy, E.G.; Szabó, L.D.; Domboróczki, L.; Pap, I.; Raczky, P. 5000 years of dietary variations of prehistoric farmers in the Great Hungarian Plain. PLoS ONE 2018, 13, e0197214. [Google Scholar] [CrossRef]
- Longin, R. New method of collagen extraction for radiocarbon dating. Nature 1971, 230, 241–242. [Google Scholar] [CrossRef]
- Bocherens, H.; Drucker, D.; Fizet, M.; Mariotti, A.; Lange-Badre, B.; Vandermeersch, B.; Borel, J.P.; Bellon, G. Isotopic biogeochemistry (13C, 15N) of fossil vertebrate collagen: Application to the study of a past food web including Neandertal man. J. Hum. Evol. 1991, 20, 481–492. [Google Scholar] [CrossRef]
- van Klinken, G.J. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archeol. Sci. 1999, 26, 687–695. [Google Scholar] [CrossRef]
- Bocherens, H.; Koch, P.L.; Mariotti, A.; Geraads, D.; Jaeger, J.-J. Isotopic biogeochemistry (13C, 18O) of mammalian enamel from African Pleistocene hominid sites. Palaios 1996, 306–318. [Google Scholar] [CrossRef] [Green Version]
- Dotsika, E.; Zisi, N.; Tsoukala, E.; Poutoukis, D.; Lykoudis, S.; Giannakopoulos, A. Palaeoclimatic information from isotopic signatures of Late Pleistocene Ursus ingressus bone and teeth apatite (Loutra Arideas Cave, Macedonia, Greece). Quat. Int. 2011, 245, 291–301. [Google Scholar] [CrossRef]
- O’Connell, T.C.; Hedges, R.E.; Healey, M.; Simpson, A. Isotopic comparison of hair, nail and bone: Modern analyses. J. Archaeol. Sci. 2001, 28, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Suess, H.E. The radioactivity of the atmosphere and hydrosphere. Annu. Rev. Nucl. Sci. 1958, 8, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.J.; Bard, E.; Bayliss, A.; Beck, J.W.; Blackwell, P.G.; Ramsey, C.B.; Buck, C.E.; Cheng, H.; Edwards, R.L.; Friedrich, M. IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon 2013, 55, 1869–1887. [Google Scholar] [CrossRef] [Green Version]
- Higham, T.; Hogg, A. Evidence for late Polynesian colonization of New Zealand: University of Waikato radiocarbon measurements. Radiocarbon 1997, 39, 149–192. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.L.; Gregg, J.W. Source partitioning using stable isotopes: Coping with too many sources. Oecologia 2003, 136, 261–269. [Google Scholar] [CrossRef]
- Fernandes, R.; Millard, A.R.; Brabec, M.; Nadeau, M.-J.; Grootes, P. Food Reconstruction Using Isotopic Transferred Signals (FRUITS): A Bayesian Model for Diet Reconstruction. PLoS ONE 2014, 9, e87436. [Google Scholar] [CrossRef] [Green Version]
- Van der Merwe, N.J. Carbon Isotopes, Photosynthesis, and Archaeology: Different pathways of photosynthesis cause characteristic changes in carbon isotope ratios that make possible the study of prehistoric human diets. Am. Sci. 1982, 70, 596–606. [Google Scholar]
- Garvie-Lok, S.J. Loaves and Fishes: A Stable Isotope Reconstruction of Diet in Medieval Greece; University of Calgary: Calgary, Canada, 2001. [Google Scholar]
- Harrison, R.G.; Katzenberg, M.A. Paleodiet studies using stable carbon isotopes from bone apatite and collagen: Examples from Southern Ontario and San Nicolas Island, California. J. Anthropol. Archaeol. 2003, 22, 227–244. [Google Scholar] [CrossRef]
- Ambrose, S.H.; Norr, L. Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate. In Prehistoric Human Bone: Archaeology at the Molecular Level; Lambert, J., Grupe, G., Eds.; Springer: New York, NY, USA, 1993; pp. 1–37. [Google Scholar]
- Panagiotopoulou, E.; Papathanasiou, A. Dietary Reconstruction at the Geometric-Period Burial Site of Ayios Dimitrios. Hesperia Suppl. 2015, 49, 105–117. [Google Scholar]
- Lagia, A. Diet and the polis: An isotopic study of diet in Athens and Laurion during the classical, Hellenistic, and imperial Roman periods. Hesperia Suppl. 2015, 49, 119–145. [Google Scholar]
- Triantaphyllou, S. Stable isotope analysis of skeletal assemblages from prehistoric northern Greece. Hesperia Suppl. 2015, 49, 57–75. [Google Scholar]
- Valamoti, S.M. Food across Borders: A Consideration of the Neolithic and Bronze Age Archaeobotanical Evidence from Northern Greece; Aegeum: 2007; Volume 30. Available online: http://ikee.lib.auth.gr/record/215301/?ln=en (accessed on 30 October 2021).
- Chenery, C.A.; Pashley, V.; Lamb, A.L.; Sloane, H.J.; Evans, J.A. The oxygen isotope relationship between the phosphate and structural carbonate fractions of human bioapatite. Rapid Commun. Mass Spectrom. 2012, 26, 309–319. [Google Scholar] [CrossRef]
- d’Angela, D.; Longinelli, A. Oxygen isotopes in living mammal’s bone phosphate: Further results. Chem. Geol. Isot. Geosci. Sect. 1990, 86, 75–82. [Google Scholar] [CrossRef]
- Sponheimer, M.; Lee-Thorp, J.A. Oxygen isotopes in enamel carbonate and their ecological significance. J. Archaeol. Sci. 1999, 26, 723–728. [Google Scholar] [CrossRef] [Green Version]
- Finné, M.; Holmgren, K.; Sundqvist, H.S.; Weiberg, E.; Lindblom, M. Climate in the eastern Mediterranean, and adjacent regions, during the past 6000 years–A review. J. Archaeol. Sci. 2011, 38, 3153–3173. [Google Scholar] [CrossRef]
- Finné, M.; Bar-Matthews, M.; Holmgren, K.; Sundqvist, H.S.; Liakopoulos, I.; Zhang, Q. Speleothem evidence for late Holocene climate variability and floods in Southern Greece. Quat. Res. 2014, 81, 213–227. [Google Scholar] [CrossRef]
- Theodorakopoulou, K.; Pavlopoulos, K.; Athanassas, C.; Zacharias, N.; Bassiakos, Y. Sedimentological response to Holocene climate events in the Istron area, Gulf of Mirabello, NE Crete. Quat. Int. 2012, 266, 62–73. [Google Scholar] [CrossRef]
- Denton, G.H.; Karlén, W. Holocene climatic variations—their pattern and possible cause. Quat. Res. 1973, 3, 155–205. [Google Scholar] [CrossRef]
- Mayewski, P.A.; Rohling, E.E.; Stager, J.C.; Karlén, W.; Maasch, K.A.; Meeker, L.D.; Meyerson, E.A.; Gasse, F.; van Kreveld, S.; Holmgren, K. Holocene climate variability. Quat. Res. 2004, 62, 243–255. [Google Scholar] [CrossRef]
- Davis, B.A.; Brewer, S.; Stevenson, A.C.; Guiot, J. The temperature of Europe during the Holocene reconstructed from pollen data. Quat. Sci. Rev. 2003, 22, 1701–1716. [Google Scholar] [CrossRef]
- Rosén, P.; Segerström, U.; Eriksson, L.; Renberg, I.; Birks, H.J.B. Holocene climatic change reconstructed from diatoms, chironomids, pollen and near-infrared spectroscopy at an alpine lake (Sjuodjijaure) in northern Sweden. Holocene 2001, 11, 551–562. [Google Scholar] [CrossRef] [Green Version]
- Karlén, W. Scandinavian glacial and climatic fluctuations during the Holocene. Quat. Sci. Rev. 1988, 7, 199–209. [Google Scholar] [CrossRef]


| Sample’s Code | Sex | Age | δ13Ccol (‰) (v-PDB) | δ13Cap (‰) (v-PDB) | δ15Ncol (‰) (v-AIR) | C:N | Δ13Cap-col | Date (BC) | Date Error (Years) |
|---|---|---|---|---|---|---|---|---|---|
| T4b | female | 36–50 | −17.8 | −13.2 | 8 | 3.2 | 4.3 | - | |
| T6b | male | 25–35 | −20.5 | −14.9 | 8.3 | 3.3 | 5.6 | 942–899 | 50 |
| T7 | unknown | subadult | −15.6 | - | 8.9 | 3.5 | - | - | |
| T12b | unknown | subadult | −16.6 | - | 11.1 | 2.9 | - | - | |
| Τ13 | unknown | subadult | −18 | - | 9.4 | 3.1 | - | 881–844 | 50 |
| Τ14 | indeterminate | subadult | −16 | - | 10 | 3 | - | - | |
| T24 KT | female | 17–25 | −16 | - | 6 | 3.1 | - | - | |
| T24 BN | female | 36–50 | −16.5 | - | 7.8 | 3.2 | - | - | |
| T33Ba | unknown | adult | −17.4 | −12.3 | 8.1 | 3 | 5.1 | - | |
| T50b | female | 36–50 | −16 | −12.2 | 10.9 | 3.2 | 3.8 | 980–924 | 50 |
| Samples Excluding Outliers | T6 Outlier | T24KT Outlier | ||||
|---|---|---|---|---|---|---|
| Source | IsoSource ‰ | Fruits ‰ | IsoSource ‰ | Fruits ‰ | IsoSource ‰ | Fruits ‰ |
| C3 | 19.6 | 20.8 | 59.4 | 59.4 | - | 28.6 |
| Terrestrial Animals | 15.1 | 24.6 | 4.3 | 3.6 | - | 27.8 |
| Freshwater | 43.2 | 22.8 | 30.8 | 32 | - | 3.7 |
| Marine | 18.6 | 17.9 | 4.9 | 3.7 | - | 4 |
| C4 | 3.6 | 13.7 | 0.7 | 0.9 | - | 35 |
| Errors | ||||||
| Samples Excluding the Outliers | T6 Outlier | T24KT Outlier | ||||
| Source | IsoSource ‰ | Fruits ‰ | IsoSource ‰ | Fruits ‰ | IsoSource ‰ | Fruits ‰ |
| C3 | 9 | 12 | 3 | 2 | - | 13 |
| Terrestrial Animals | 10 | 17 | 3 | 3 | - | 19 |
| Freshwater | 14 | 16 | 5 | 3 | - | 2 |
| Marine | 13 | 12 | 4 | 0.1 | - | 2 |
| C4 | 2 | 9 | 0.8 | 0.7 | - | 4 |
| Site | Reference | n | Type of Value | δ13Ccol (‰) (v-PDB) | δ15Ncol (‰) (v-AIR) |
|---|---|---|---|---|---|
| Treis Elies | [58] | 6 | mean | −16.9 | 8.3 |
| s.d. | 1.6 | 0.7 | |||
| Karitsa | [58] | 2 | mean | −16.7 | 9.6 |
| s.d. | 0.3 | 0.5 | |||
| Kladeri | [58] | 5 | mean | −17.1 | 9.3 |
| s.d. | 0.6 | 0.7 | |||
| Makriyalos | [5] | 14 | mean | −18.9 | 7.1 |
| s.d. | 0.5 | 0.6 | |||
| Ayios Dimitrios | [82] | 36 | mean | −19.7 | 8.3 |
| s.d. | 1.2 | 1.9 | |||
| Athens | [83] | 12 | mean | −19.2 | 10.6 |
| s.d. | 0.2 | 0.8 | |||
| Halos, Greece | [61] | 20 | mean | −19.2 | 8.2 |
| s.d. | 0.7 | 0.7 | |||
| Spain | [62] | 19 | mean | −18.7 | 10.2 |
| s.d. | 0.4 | 0.9 | |||
| Croatia | [63] | 36 | mean | −17.0 | 9.3 |
| s.d. | 1.1 | 1.0 | |||
| Slovenia | [65] | 3 | mean | −14.8 | 8.8 |
| s.d. | 0.4 | 0.2 | |||
| Poland | [64] | 26 | mean | −20.1 | 9.2 |
| s.d. | 0.8 | 0.6 | |||
| Hungary | [66] | 52 | mean | −16.6 | 10.7 |
| s.d. | 2.0 | 0.3 |
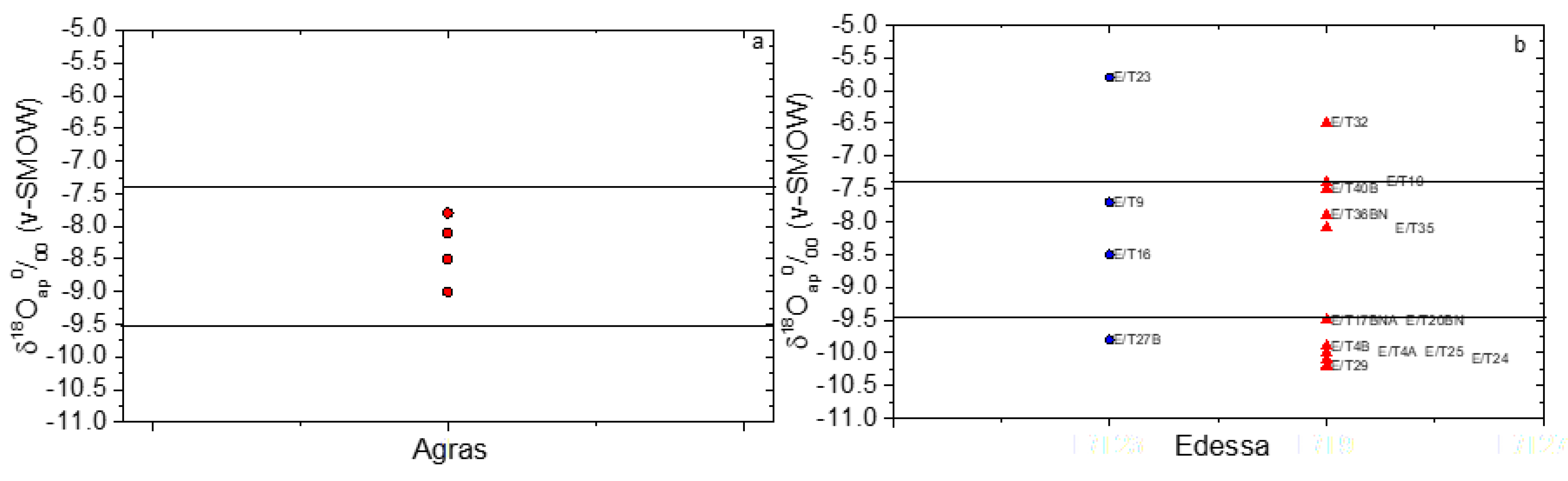
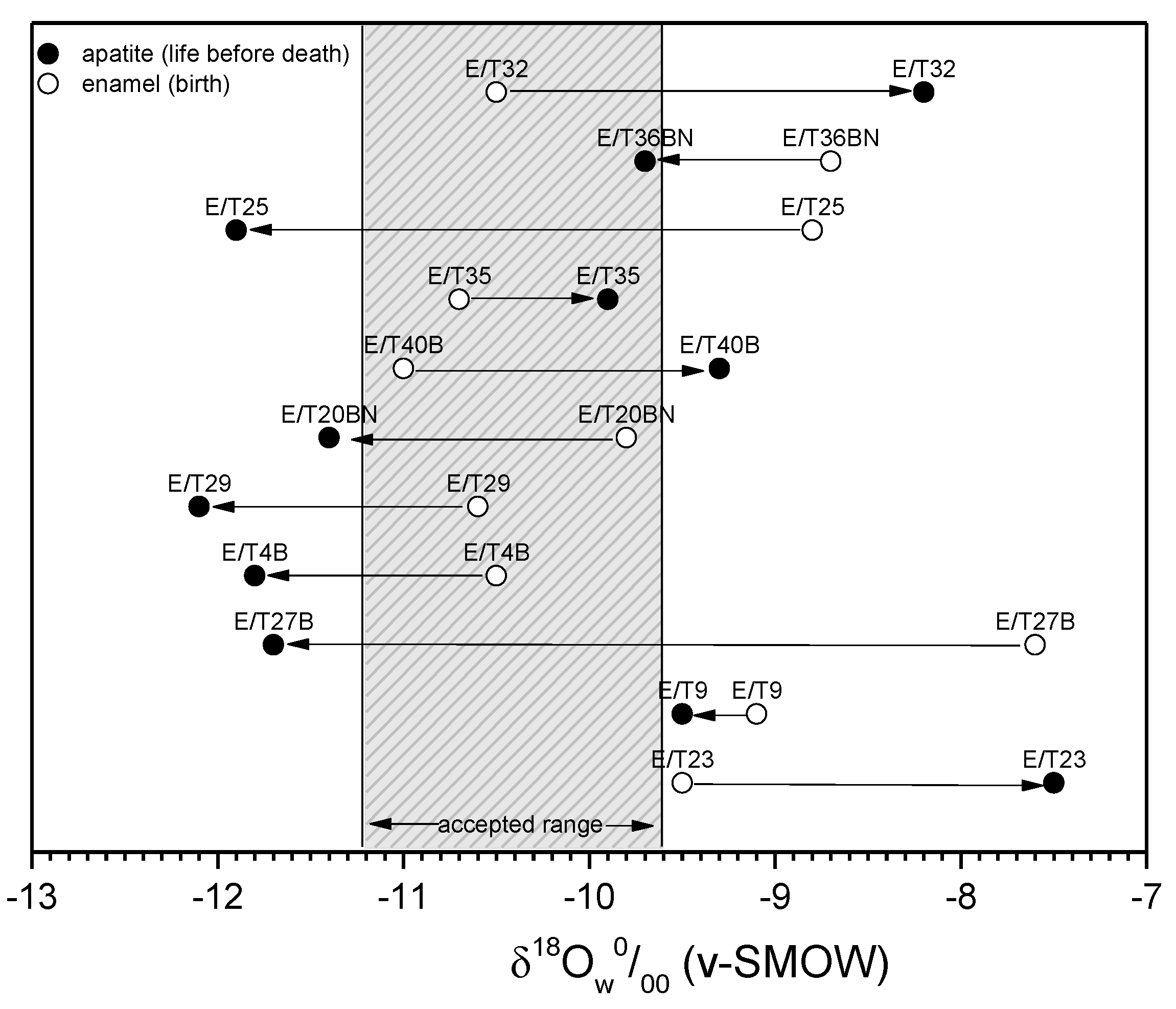
| Sample’s Code | Sex | δ18Oap (‰) (v-PDB) Measured | δ18Oap (‰) (v-SMOW) Calculated 1 | δ18Ow(ap) (‰) (v-SMOW) Calculated 2 | δ18Ow(en) (‰) (v-SMOW) 3 | abs(Δen-ap) (‰) |
|---|---|---|---|---|---|---|
| EIA Agras | ||||||
| T4b | female | −9 | 21.6 | −10.9 | - | |
| T6b | male | −8.5 | 22.1 | −10.4 | - | |
| T33Ba | unknown | −7.8 | 22.9 | −9.6 | - | |
| T50b | female | −8.1 | 22.6 | −9.9 | - | |
| Roman Edessa | ||||||
| E/T23 | male | −5.8 | 24.9 | −7.5 | −9.5 | 2.0 |
| E/T9 | male | −7.7 | 23.0 | −9.5 | −9.1 | 0.4 |
| E/T27B | male | −9.8 | 20.8 | −11.7 | −7.6 | 4.1 |
| E/T16 | male | −8.5 | 22.1 | −10.4 | - | - |
| E/T4B | female | −9.9 | 20.7 | −11.8 | −10.5 | 1.3 |
| E/T29 | female | −10.2 | 20.4 | −12.1 | −10.6 | 1.5 |
| E/T20BN | female | −9.5 | 21.1 | −11.4 | −9.8 | 1.6 |
| E/T40B | female | −7.5 | 23.2 | −9.3 | −11.0 | 1.7 |
| E/T35 | female | −8.1 | 22.6 | −9.9 | −10.7 | 0.8 |
| E/T25 | female | −10 | 20.6 | −11.9 | −8.8 | 3.1 |
| E/T36BN | female | −7.9 | 22.8 | −9.7 | −8.7 | 1.0 |
| E/T32 | female | −6.5 | 24.2 | −8.2 | −10.5 | 2.3 |
| E/T24 | female | −10.1 | 20.5 | −12.0 | - | - |
| E/T10 | female | −7.4 | 23.3 | −9.2 | - | - |
| E/T17BNA | female | −9.5 | 21.1 | −11.4 | - | - |
| E/T4A | female | −10 | 20.6 | −11.9 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsika, E.; Tassi, M.; Karalis, P.; Chrysostomou, A.; Michael, D.E.; Poutouki, A.E.; Theodorakopoulou, K.; Diamantopoulos, G. Stable Isotope and Radiocarbon Analysis for Diet, Climate and Mobility Reconstruction in Agras (Early Iron Age) and Edessa (Roman Age), Northern Greece. Appl. Sci. 2022, 12, 498. https://doi.org/10.3390/app12010498
Dotsika E, Tassi M, Karalis P, Chrysostomou A, Michael DE, Poutouki AE, Theodorakopoulou K, Diamantopoulos G. Stable Isotope and Radiocarbon Analysis for Diet, Climate and Mobility Reconstruction in Agras (Early Iron Age) and Edessa (Roman Age), Northern Greece. Applied Sciences. 2022; 12(1):498. https://doi.org/10.3390/app12010498
Chicago/Turabian StyleDotsika, Elissavet, Maria Tassi, Petros Karalis, Anastasia Chrysostomou, Dimitra Ermioni Michael, Anastasia Elektra Poutouki, Katerina Theodorakopoulou, and Georgios Diamantopoulos. 2022. "Stable Isotope and Radiocarbon Analysis for Diet, Climate and Mobility Reconstruction in Agras (Early Iron Age) and Edessa (Roman Age), Northern Greece" Applied Sciences 12, no. 1: 498. https://doi.org/10.3390/app12010498






