Medium-Energy Synthesis Gases from Waste as an Energy Source for an Internal Combustion Engine
Abstract
1. Introduction
2. Waste Management in Slovakia
3. Experimental Methods
Analysis of the Pressure in the Cylinder
4. Experimental Results
4.1. Integral Parameters of the Combustion Engine
4.2. Internal Parameters of Combustion Engine
5. Short Discussion
6. Conclusions
- The optimum spark advance angle ranged from 12 °CA BTDC for SG5 gas, as the gas has a high hydrogen content in the mixture (Table 2) that burns the fastest, to 34 °CA BTDC for SG3 that in turn has the highest inert gas content that prevents the development of chemical reactions.
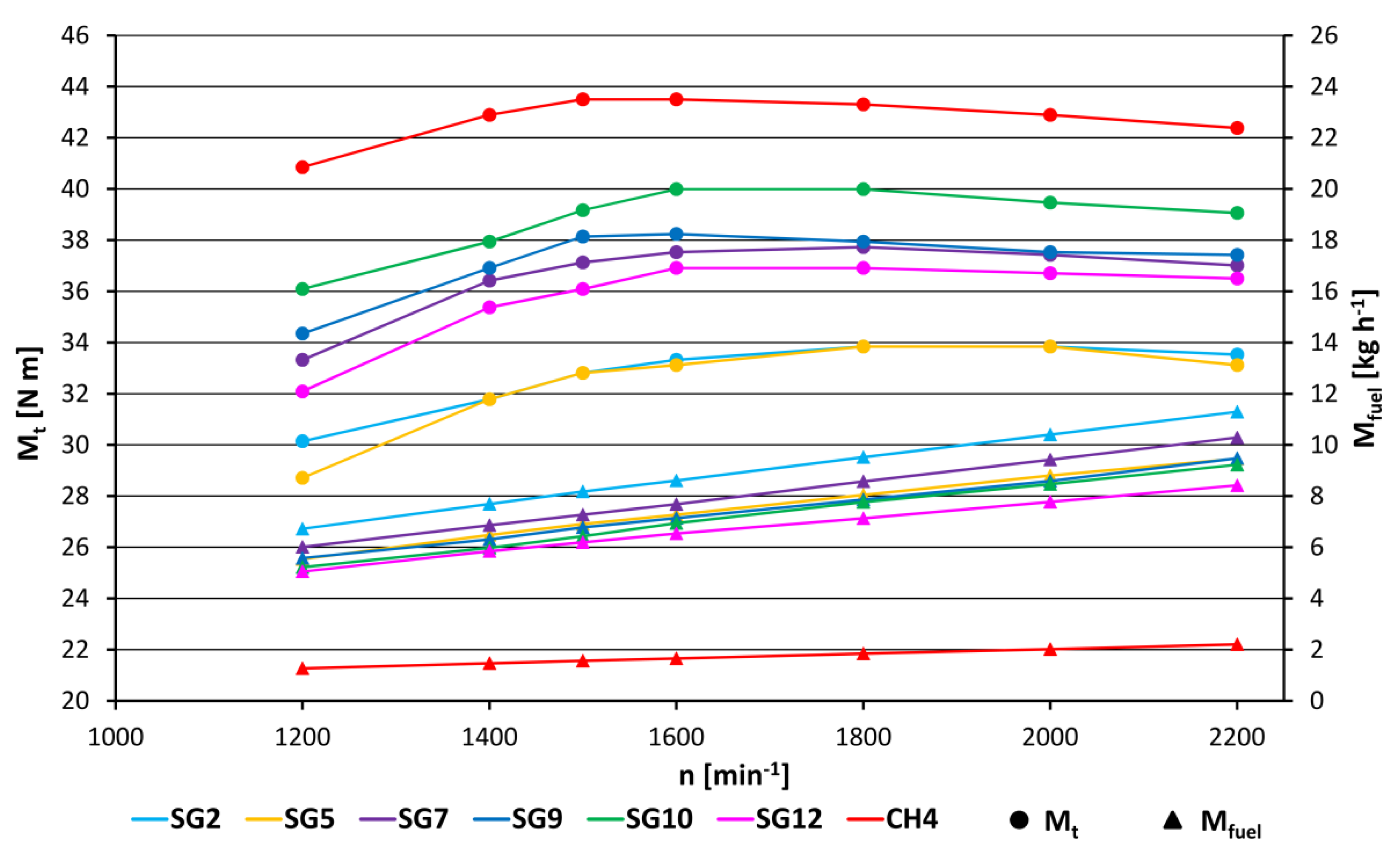
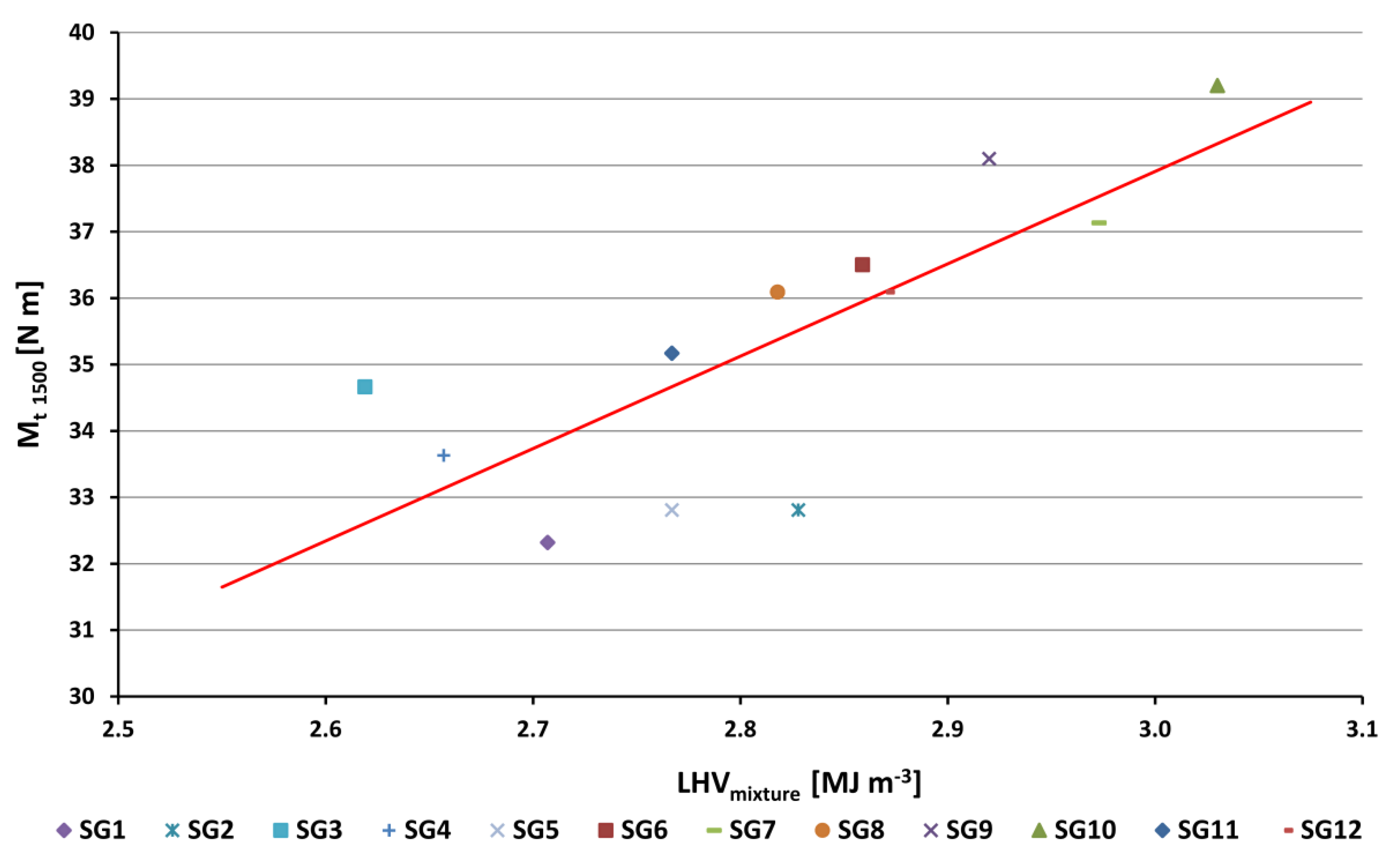
- The torque value for the reference fuel (methane) was 43.2 N m; for the other synthesis gases examined (Figure 3, Figure 4 and Figure 13), this value was lower (from 32.3 N m for SG1 to 39.2 N m for SG10), relative to the fact that the volumetric heating value of stoichiometric mixture was lower for all synthesis gases compared to methane. The linear trend line (red straight line in Figure 13), drawn across the measured values, shows that the deviations of the measured data from the trend line in Figure 13 are for most of the points in the range up to ±5% (except for SG3, SG5 and SG2).
- The hourly consumption of synthesis gases (Figure 3, Figure 4 and Figure 14) was 3.7 to 5.2 times higher than in operation on methane (1.56 kg/h), because in operation on synthesis gases, a small amount of air is consumed to form a stoichiometric mixture (from 2.23 kg/kg for SG2 up to 3.55 kg/kg for SG11, A/F ratio in Table 2) compared to methane (17.12 kg/kg). The trend in decreasing hourly fuel consumption with increasing values of the mass LHV parameter is virtually linear (red straight line in Figure 14).
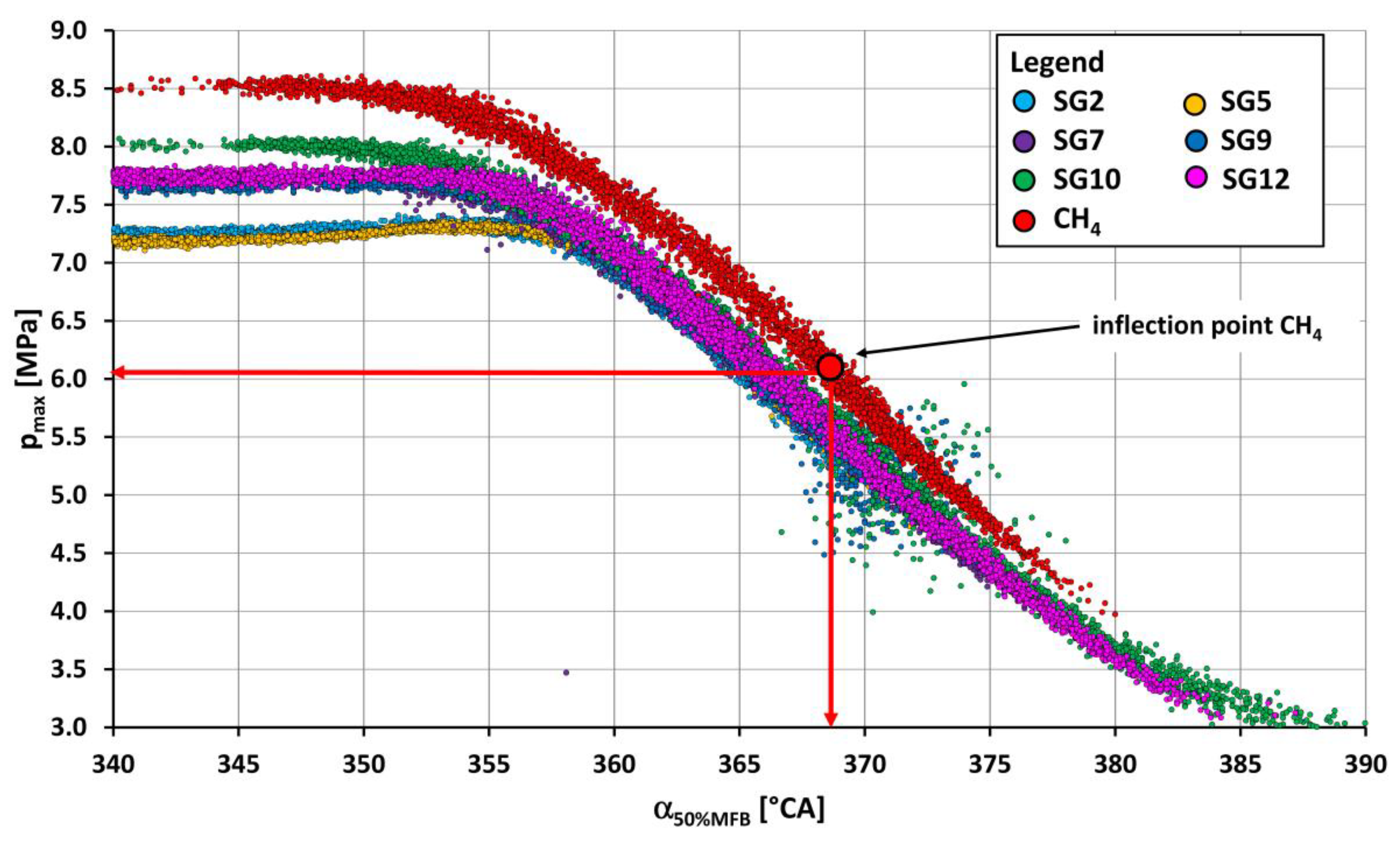
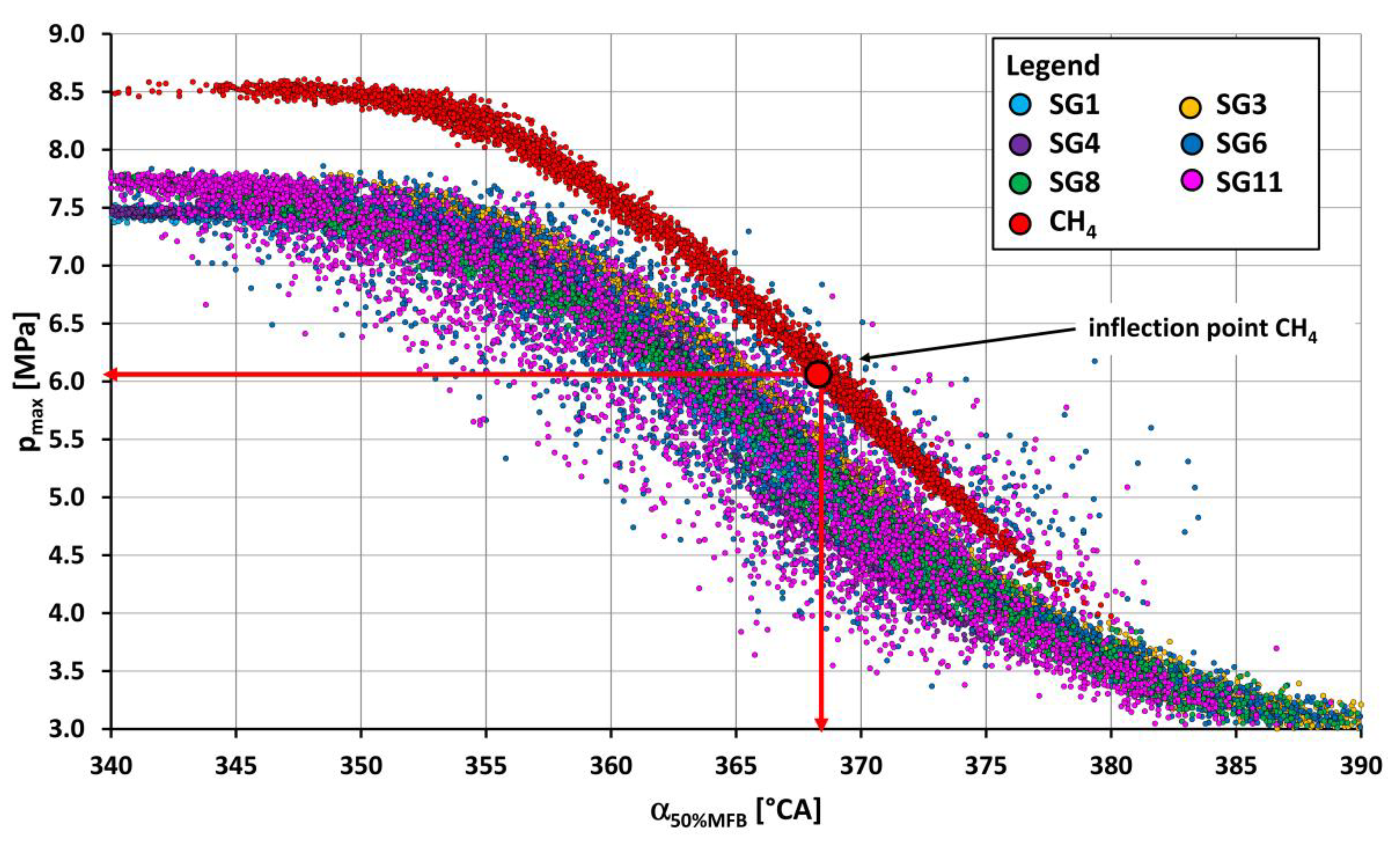
- Out of the synthesis gases, the highest value of maximum pressure (5.98 MPa) was reached by synthesis gas SG5 (Figure 7) and, conversely, the lowest value of maximum pressure (4.77 MPa) at optimum spark advance angle was achieved by burning SG8 synthesis gas (Figure 8). The coefficient of maximum pressure variation had the lowest value (3.2%) when operating on SG2 synthesis gas and, conversely, the highest value (8.9%) was when SG11 gas was burned.
- The lowest value of the maximum of pressure rise rate was achieved when operating on SG11 synthesis gas (0.155 MPa/1 °CA), which contained 45% vol. of internal gases and at the same time approximately the same share of methane (20% vol.) and hydrogen (25% vol.). The synthesis gases, which contained mainly large amounts of hydrogen (35% vol. in SG4 and 50% vol. in SG5), also reached the highest values of the pressure rise rate (0.2518 MPa/1 °CA or 0.2493 MPa/1 °CA) of the engine.
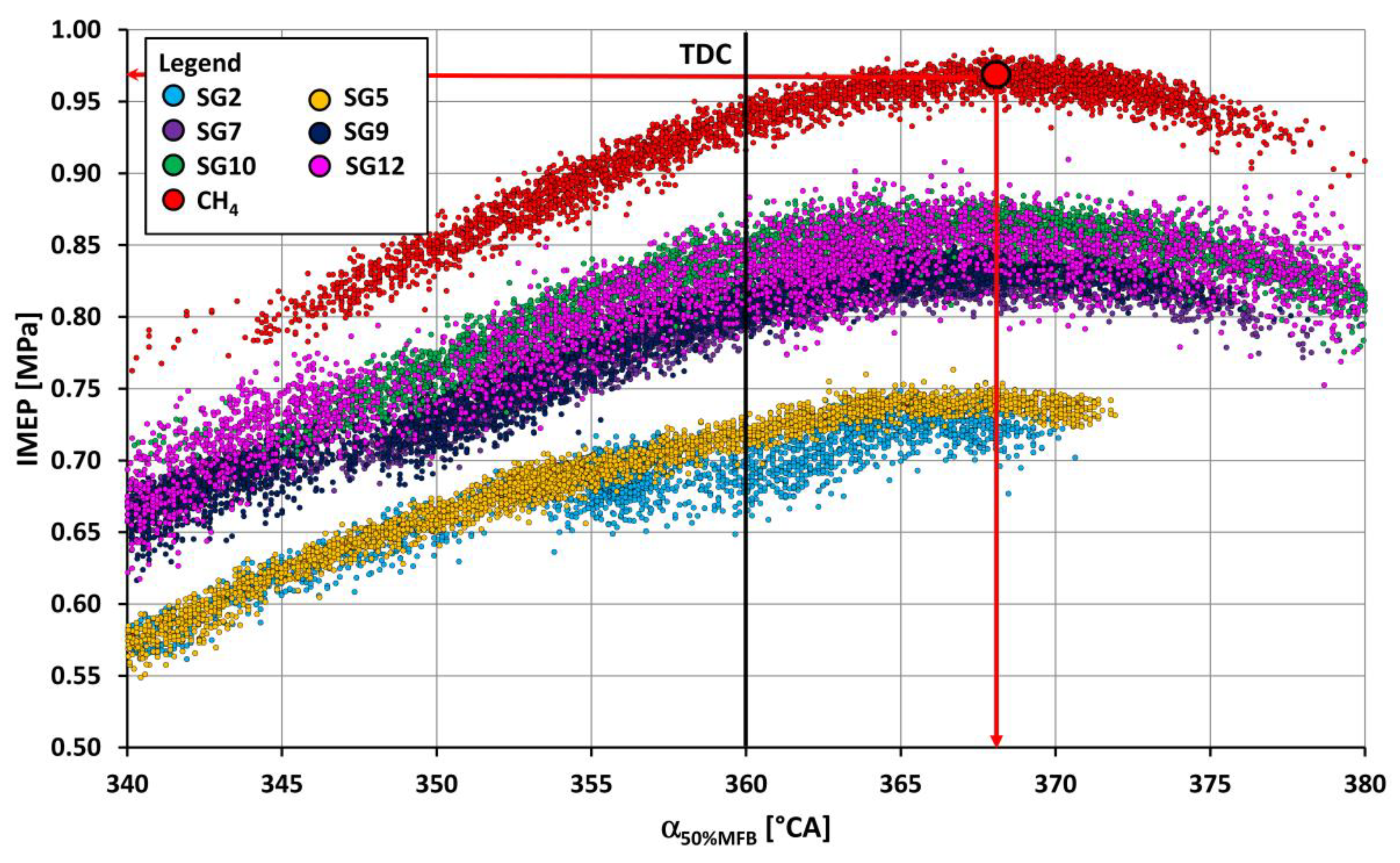
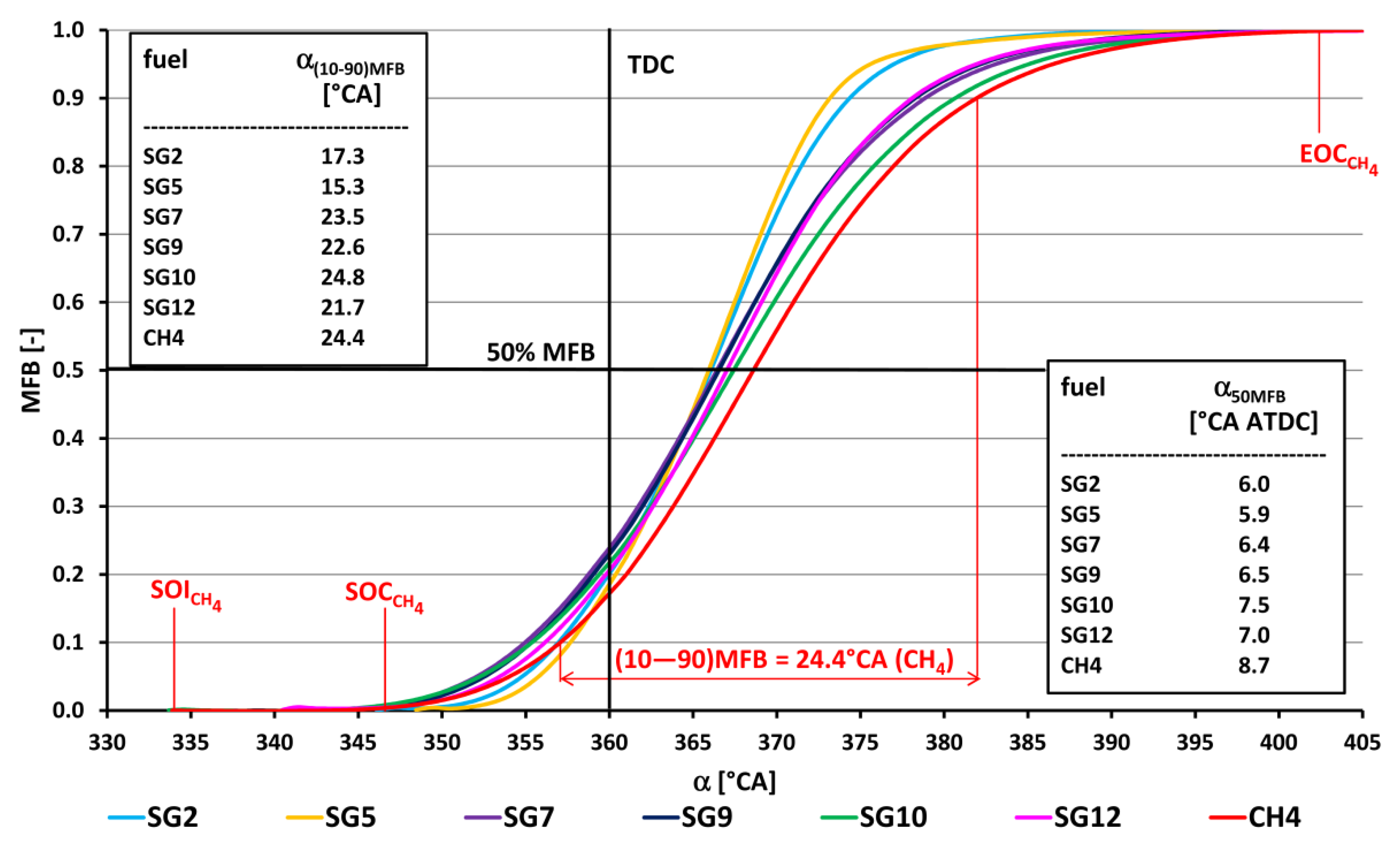
- The course of fuel combustion (Figure 11 and Figure 12) shows that the SG8 synthesis gas has the longest main combustion time (10–90% MFB) and represents a value of 28.9 °CA. The shortest combustion time (15.3 °CA) is for the SG5 synthesis gas combustion, which relates to the highest proportion of hydrogen in the mixture (50% vol.). The angle at which half of the fuel was burned ranged from 5.9 °CA ATDC for SG5 to 11.3 °CA ATDC for SG8. The combustion onset time (SOI-5% MFB) was the shortest (7.4 °CA) for SG5 synthesis gas with a high hydrogen content and, conversely, the longest (24.8 °CA) for SG3 synthesis gas with the highest inert gas content. The coefficients of variation of the positions during the gradual burning out of the fuel were the lowest for the synthesis gas SG5 and the highest for the synthesis gas SG11.
- If we briefly summarize the performed analyses of the influence of hydrogen in synthesis gases on the combustion process in the internal combustion engine, then we can state the following findings. The hydrogen content in SGs is one of the main causes of their different behaviour during their combustion in the engine. Increasing the proportion of hydrogen in synthesis gases causes an increase in the pressure rise rate values during gas combustion, a decrease in the COV values during the gradual burnout of the fuel, and a shortening of the total combustion period. In other words, synthesis gases with higher hydrogen content lead to a slightly harder engine run, but a more stable combustion process. The effect of hydrogen in the synthesis gases, unless there is abnormal combustion, is positive. The combustion with a higher H2 content is closer to isochoric burning, which contributes to higher thermal and effective engine efficiency and also to lower fuel consumption. At the same time, an important property of hydrogen in the gas mixture [50] during its combustion should be mentioned, namely, that increasing the proportion of hydrogen in the mixture reduces harmful hydrocarbon emissions. A higher content of hydrogen in SGs reduces environmentally neutral CO2 in SGs combustion; vice versa, hydrogen increases the content of nitrogen oxides and water vapour in the exhaust of the internal combustion engine.
- The results presented in this article and summarized in the conclusion give an idea of the integral parameters of the engine (torque and hourly consumption, Figure 1, Figure 2, Figure 3 and Figure 4), but above all, they give an idea of the internal parameters of the engine that relate to the combustion processes of medium-energy synthesis gases (Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12), which we have mainly dealt with in this article. The authors can draw the following conclusions that are similar to those reached by the authors for low-energy synthesis gases [43]:
- If medium-energy synthesis gases are to be used to drive a cogeneration unit, then we assume that the most important criterion for energy recovery from them must be the criterion of low consumption and high efficiency of their use in the internal combustion engine or for cogeneration. This means, that for the gases that have been measured at 1500 L/min, the lowest fuel consumption is in the range approximately up to 6.7 kg/h (Figure 14.). It relates to the gases SG12, 11, 10, 9, and 8, and the calculated values of effective efficiencies for them are as follows: SG10 (31.2%), SG11 (31.1%), SG8 (31.1%), SG9 (30.8%), and SG12 (28.8%). At the same time, the gases SG10 and SG9 also achieved the highest performance parameters (and had the highest volume lower heating value of the mixture - LHVmixture) out of all gases. The gases that achieve low consumption and at the same time a high value of effective efficiency are the gases SG 8, 9, 10, and 11. As for Table 2, these are gases, which in their composition contain from 30% to 45% volume of methane and hydrogen. Therefore, the general conclusion that we can recommend for the production of medium-energy gases is to set up the gasification technology in such a way that the resulting gases contain as much methane and hydrogen as possible.
- In order to prevent abnormal combustion in the form of knocking of the engine or backfire of the mixture in the intake manifold, it is necessary to take into account the fact that in the case of methane-free gases (SG2 and SG5, Table 2), if the hydrogen content exceeds 25% by volume, then the volume of inert gases must not fall below 25% vol. [43]. During experiments with SGs, which contain methane, we did not measure any signs of abnormal combustion.
- When changing the composition of synthesis gas, similar to low-energy synthesis gases [43], it is necessary to optimise the engine in terms of the change in the compression ratio, spark advance angle (SOI) for each gas, ignition system (spark energy value, spark plug heat value), geometry of the piping system in terms of achieving maximum filling of the cylinders (valve timing, use of the wave effect), shape of the combustion chamber, or shape of the holes (flow coefficients), optimum turbulence of the charge, use of supercharging, etc. The results of this SG analysis are directly applicable in practice. They provide several suggestions on how to set up waste gasification technologies to obtain optimal performance and the best economic parameters from cogeneration units.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A/F | Air to fuel ratio [kg /kg] |
| ATDC | After top-dead centre |
| BTDC | Before top-dead centre |
| CHP | Combined Heat and Power |
| CH4 | Methane |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| COV | Coefficient of variation |
| COVα10%MFB | Coefficient of variation at the point of 10% mass fraction burned |
| COVα50%MFB | Coefficient of variation at the point of 50% mass fraction burned |
| COVα90%MFB | Coefficient of variation at the point of 90% mass fraction burned |
| D10 | Disposal—Incineration on land |
| dpc | Pressure change due to combustion [Pa] |
| dpp | Pressure change due to compression [Pa] |
| dQ | Heat delivered to the system |
| dU | Change of internal energy of matter in the system |
| dW | Work produced by system |
| EGR | Exhaust gas recirculation |
| EOC | End of combustion [°CA ATDC] |
| EU | European Union |
| FT | Fischer-Tropsch |
| hi.dmi | i-th component of enthalpy of mass flow across system boundaries |
| ICE | Internal combustion engine |
| IMEP | Indicated mean effective pressure [MPa] |
| LHV | Fuel lower heating value [MJ/kg], [MJ/m3], |
| LHVmixture | Lower heating value of mixture (volumetric) [MJ/m3] |
| M | Molar mass [kg/kmol] |
| Mfuel | Hourly fuel consumption [kg/h] |
| Mfuel1500 | Hourly fuel consumption at 1500 L/min [kg/h] |
| Mt | Brake torque [N m] |
| MAF | Mass air flow |
| MFB | Mass fraction burned |
| MSW | Municipal solid waste |
| N2 | Nitrogen |
| NTP | Normal temperature and pressure (293.15 K, 101,325 Pa) |
| O2 | Oxygen |
| P | in-cylinder pressure [MPa] |
| PM | Particulate matter |
| PPC | Partially premixed combustion |
| R1 | Recovery—Use principally as a fuel or other means to generate energy |
| SG | Syngas |
| SOI | Start of ignition [°CA BTDC] |
| SOC | Start of combustion [°CA BTDC] |
| TDC | Top-dead centre |
| WtE | Waste-to-Energy |
| α | Crankshaft angle [°CA] |
| α50%MFB | Crank angle at which 50% of fuel mass is burned |
| ρNTP fuel | Density of fuel [kg/m3] |
| ρNTP mixture | Density of stoichiometric mixture [kg/m3] |
References
- Dace, E.; Blumberga, D.; Kuplais, G.; Bozko, L.; Khabdullina, Z.; Khabdullin, A. Optimization of landfill Gas Use in Municipal Solid Waste Landfills in Latvia. Energy Procedia 2015, 72, 293–299. [Google Scholar] [CrossRef][Green Version]
- Swarbrick, G.E.; Stuetz, M.R. Handbook for the Design, Construction, Operation, Monitoring and Maintenance of a Passive Landfill Gas Drainage and Biofiltration System; Department of Environment, Climate Change and Water: New South Wales, Australia, 2010; pp. 4–7.
- Ali, J.; Rasheed, T.; Afreen, M.; Anwar, M.T.; Nawaz, Z.; Anwar, H.; Rizwan, K. Modalities for conversion of waste to energy—Challenges and perspectives. Sci. Total Environ. 2020, 727, 138610. [Google Scholar] [CrossRef]
- Alzate-Arias, S.; Jaramillo-Duque, Á.; Villada, F.; Restrepo-Cuestas, B. Assessment of government incentives for energy from waste in Colombia. Sustainability 2018, 10, 1294. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov/HAC/landfill/PDFs/Landfill_2001_ch2mod.pdf (accessed on 30 September 2021).
- Markulik, S.; Šolc, M.; Petrík, J.; Balážiková, M.; Blaško, P.; Kliment, J.; Bezák, M. Application of FTA Analysis for Calculation of the Probability of the Failure of the Pressure Leaching Process. Appl. Sci. 2021, 11, 6731. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard. Mater. 2021, 403, 123970. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, C.; Baškovič, U.Ž.; Renzi, M.; Seljak, T.; Oprešnik, S.R.; Baratieri, M.; Katrašnik, T. Complementing syngas with natural gas in spark ignition engines for power production: Effects on emissions and combustion. Energies 2021, 14, 3688. [Google Scholar] [CrossRef]
- Pukalskas, S.; Kriaučiūnas, D.; Rimkus, A.; Przybyła, G.; Droździel, P.; Barta, D. Effect of hydrogen addition on the energetic and ecologic parameters of an SI engine fueled by biogas. Appl. Sci. 2021, 11, 742. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Saini, A.K.; Saini, V.; Pareek, B.; Gaidukovs, S.; Thakur, V.K. Recovery processes of sustainable energy using different biomass and wastes. Renew. Sustain. Energy Rev. 2021, 150, 111483. [Google Scholar] [CrossRef]
- Dong, J.; Chi, Y.; Tang, Y.; Ni, M.; Nzihou, A.; Weiss-Hortala, E.; Huang, Q. Effect of Operating Parameters and Moisture Content on Municipal Solid Waste Pyrolysis and Gasification. Energy Fuels 2016, 30, 3994–4001. [Google Scholar] [CrossRef]
- Mojaver, M.; Hasanzadeh, R.; Azdast, T.; Park, C.B. Comparative study on air gasification of plastic waste and conventional biomass based on coupling of AHP/TOPSIS multi-criteria decision analysis. Chemosphere 2022, 286, 131867. [Google Scholar] [CrossRef]
- Gu, Q.; Wu, W.; Jin, B.; Zhou, Z. Analyses for synthesis gas from municipal solid waste gasification under medium temperatures. Processes 2020, 8, 10084. [Google Scholar] [CrossRef]
- Ramos, A.; Teixeira, C.A.; Rouboa, A. Environmental assessment of municipal solid waste by two-stage plasma gasification. Energies 2019, 12, 10137. [Google Scholar] [CrossRef]
- Villarini, M.; Marcantonio, V.; Colantoni, A.; Bocci, E. Sensitivity analysis of different parameters on the performance of a CHP internal combustion engine system fed by a biomass waste gasifier. Energies 2019, 12, 40688. [Google Scholar] [CrossRef]
- Puškár, M.; Kopas, M.; Kádárová, J. Ecological analysis related to creation of gaseous emissions within transport focused on fulfilment of the future emission standards. Transp. Res. Part D: Transp. Environ. 2017, 57, 413–421. [Google Scholar] [CrossRef]
- Pieta, I.S.; Epling, W.S.; Kazmierczuk, A.; Lisowski, P.; Nowakowski, R.; Serwicka, E.M. Waste into fuel—catalyst and process development for MSW valorisation. Catalysts 2018, 8, 30113. [Google Scholar] [CrossRef]
- Puškár, M.; Kopas, M.; Puškár, D.; Lumnitzer, J.; Faltinová, E. Method for reduction of the NOX emissions in marine auxiliary diesel engine using the fuel mixtures containing biodiesel using HCCI combustion. Mar. Pollut. Bull. 2018, 127, 752–760. [Google Scholar] [CrossRef]
- Sinay, J.; Puškár, M.; Kopas, M. Reduction of the NOx emissions in vehicle diesel engine in order to fulfill future rules concerning emissions released into air. Sci. Total Environ. 2018, 624, 1421–1428. [Google Scholar] [CrossRef]
- Puškár, M.; Jahnátek, A.; Kuric, I.; Kádárová, J.; Kopas, M.; Šoltésová, M. Complex analysis focused on influence of biodiesel and its mixture on regulated and unregulated emissions of motor vehicles with the aim to protect air quality and environment. Air Qual. Atmos. Health 2019, 12, 855–864. [Google Scholar] [CrossRef]
- Nasreen, S.; Nafees, M.; Qureshi, L.A.; Asad, M.S.; Sadiq, A.; Ali, S.D. Review of Catalytic Transesterification Methods for Biodiesel Production. In Biofuels—State of Development. Biofuels: State Dev. 2018, 93–119. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Mahmoudi, M.; Doustdar, O.; Jahangiri, H.; Tsolakis, A.; Gu, S.; LechWyszynski, M. A review of Fischer Tropsch synthesis process, mechanism, surface chemistry and catalyst formulation. Biofuels Eng. 2018, 2, 11–31. [Google Scholar] [CrossRef]
- Asadullah, M. Barriers of commercial power generation using biomass gasification gas: A review. Renew. Sustain. Energy Rev. 2014, 29, 201–215. [Google Scholar] [CrossRef]
- Asadullah, M. Biomass gasification gas cleaning for downstream applications: A comparative critical review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Thapa, S.; Bhoi, P.R.; Kumar, A.; Huhnke, R.L. Effects of syngas cooling and biomass filter medium on tar removal. Energies 2017, 10, 30349. [Google Scholar] [CrossRef]
- De Filippis, P.; Scarsella, M.; de Caprariis, B.; Uccellari, R. Biomass Gasification Plant and Syngas Clean-up System. Energy Procedia 2015, 75, 240–245. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Duan, J.; Liu, F.; Sun, B. Backfire control and power enhancement of a hydrogen internal combustion engine. Int. J. Hydrogen Energy 2014, 39, 4581–4589. [Google Scholar] [CrossRef]
- Verhelst, S.; Maesschalck, P.; Rombaut, N.; Sierens, R. Increasing the power output of hydrogen internal combustion engines by means of supercharging and exhaust gas recirculation. Int. J. Hydrogen Energy 2009, 34, 4406–4412. [Google Scholar] [CrossRef]
- White, C.M.; Steeper, R.R.; Lutz, A.E. The hydrogen-fueled internal combustion engine: A technical review. Int. J. Hydrogen Energy 2006, 31, 1292–1305. [Google Scholar] [CrossRef]
- García-Cascallana, J.; Carrillo-Peña, D.; Morán, A.; Smith, R.; Gómez, X. Energy Balance of Turbocharged Engines Operating in a WWTP with Thermal Hydrolysis. Co-Digestion Provides the Full Plant Energy Demand. Appl. Sci. 2021, 11, 11103. [Google Scholar] [CrossRef]
- Kriaučiūnas, D.; Pukalskas, S.; Rimkus, A.; Barta, D. Analysis of the influence of CO2 concentration on a spark ignition engine fueled with biogas. Appl. Sci. 2021, 11, 46379. [Google Scholar] [CrossRef]
- Shamun, S.; Belgiorno, G.; di Blasio, G. Engine Parameters Assessment for Alcohols Fuels Application in Compression Ignition Engines. In Energy, Environment, and Sustainability; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 125–139. [Google Scholar] [CrossRef]
- Dimitrakopoulos, N.; Belgiorno, G.; Tuner, M.; Blasio G di Beatrice, C. PPC Operation with Low RON Gasoline Fuel. A Study on Load Range on a Euro 6 Light Duty Diesel Engine. In Proceedings of the Ninth International Conference on Modeling and Diagnostics for Advanced Engine Systems, Japan Society of Mechanical Engineers, Okayama, Japan, 26–28 July 2017. [Google Scholar]
- Beatrice, C.; Belgiorno, G.; Di Blasio, G.; Mancaruso, E.; Sequino, L.; Vaglieco, B.M. Analysis of a Prototype High-Pressure “Hollow Cone Spray” Diesel Injector Performance in Optical and Metal Research Engines; SAE Technical Paper 2017-24-0073; SAE: Warrendale, PA, USA, 2017. [Google Scholar] [CrossRef]
- Ministry of Environment of the Slovak Republic. Available online: https://www.enviroportal.sk/uploads/report/9341.pdf (accessed on 5 October 2021).
- Ministry of Environment of the Slovak Republic. Available online: https://www.minzp.sk/files/iep/01_2017_tri-vyzvy_zivotneho_prostredia.pdf (accessed on 5 October 2021).
- Institute of Circular Economy. Available online: https://www.incien.sk/preco-sa-oplati-zbavit-sa-takmer-vsetkych-starosti-odpadov/ (accessed on 7 October 2021).
- Institute of Circular Economy. Available online: https://www.incien.sk/wp-content/uploads/2020/11/analyza-odpad-hosp-2020-final.pdf (accessed on 7 October 2021).
- Levaggi, L.; Levaggi, R.; Marchiori, C.; Trecroci, C. Waste-to-energy in the EU: The effects of plant ownership, waste mobility, and decentralization on environmental outcomes and welfare. Sustainability 2020, 12, 5743. [Google Scholar] [CrossRef]
- EUROSTAT Municipal Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Municipal_waste_statistics&oldid=343958#Municipal_waste_treatment (accessed on 9 December 2021).
- Scarlat, N.; Fahl, F.; Dallemand, J.F. Status and Opportunities for Energy Recovery from Municipal Solid Waste in Europe. Waste Biomass Valorization 2019, 10, 2425–2444. [Google Scholar] [CrossRef]
- Polóni, M.; Chríbik, A. Low-Energy Synthesis Gases from Waste as Energy Source for Internal Combustion Engine. SAE Int. J. Engines 2020, 13, 633–648. [Google Scholar] [CrossRef]
- Pavlenko, I.; Saga, M.; Kuric, I.; Kotliar, A.; Basova, Y.; Trojanowska, J.; Ivanov, V. Parameter Identification of Cutting Forces in Crankshaft Grinding Using Artificial Neural Networks. Materials 2020, 13, 5357. [Google Scholar] [CrossRef]
- General Instructions Digital Mass Flow. Available online: https://www.bronkhorst.com/getmedia/50bed9ce-0445-4eba-9d37-f113ec53cb34/917022-Manual-general-instructions-digital-laboratory-style-and-IN-FLOW.pdf (accessed on 12 December 2021).
- Puškár, M.; Bigoš, P.; Kelemen, M.; Markulik, Š.; Puškárová, P. Method for accurate measurement of output ignition curves for combustion engines. Measurement 2013, 46, 1379–1384. [Google Scholar] [CrossRef]
- Merker, G.P.; Schwarz, C.; Teichmann, R. Combustion Engines Development: Mixture Formation, Combustion, Emissions and Simulation; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-02951-6. [Google Scholar] [CrossRef]
- Sága, M.; Bulej, V.; Čuboňova, N.; Kuric, I.; Virgala, I.; Eberth, M. Case study: Performance analysis and development of robotized screwing application with integrated vision sensing system for automotive industry. Int. J. Adv. Robot. Syst. 2020, 17, 172988142092399. [Google Scholar] [CrossRef]
- Tazerout, M.; Le Corre, O.; Ramesh, A. A New Method to Determine the Start and End of Combustion in an Internal Combustion Engine Using Entropy Changes. Int. J. Thermodyn. 2000, 3, 49–55. [Google Scholar]
- Chríbik, A.; Polóni, M.; Lach, J. Combustion engine powered by a mixture of natural gas and hydrogen. MECCA J. Middle Eur. Constr. Des. Cars 2012, 10, 31–36. [Google Scholar] [CrossRef]
- Chríbik, A.; Polóni, M.; Minárik, M.; Mitrovič, R.; Miškovič, Z. The effect of inert gas in the mixture with natural gas on the parameters of the combustion engine. In Computational and Experimental Approaches in Materials Science and Engineering; Springer: Cham, Switzerland, 2020; pp. 410–426. ISBN 978-3-030-30852-0. [Google Scholar]
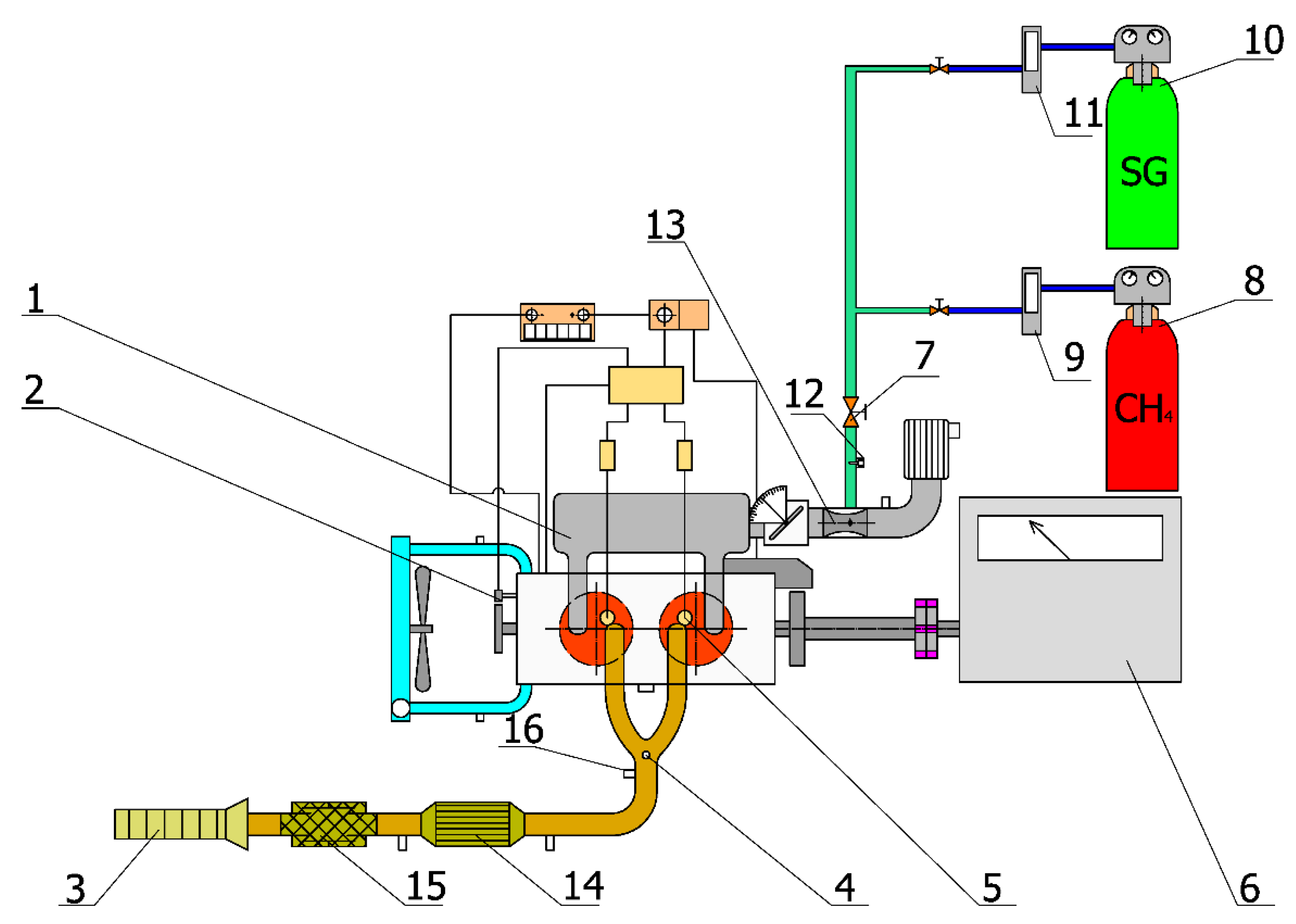






| City | MSW Quantity [tons] | Landfilling | Recycling | WtE | Other |
|---|---|---|---|---|---|
| Bratislava | 213,047.580 | 9% | 33% | 56% | 2% |
| Košice | 97,403.323 | 1% | 36% | 63% | 0% |
| Prešov | 41,586.348 | 60% | 40% | 0% | 0% |
| Žilina | 46,497.679 | 53% | 43% | 0% | 4% |
| Nitra | 47,382.150 | 57% | 43% | 0% | 0% |
| B. Bystrica | 45.070 | 46% | 54% | 0% | 0% |
| Trnava | 39,717.635 | 44% | 56% | 0% | 0% |
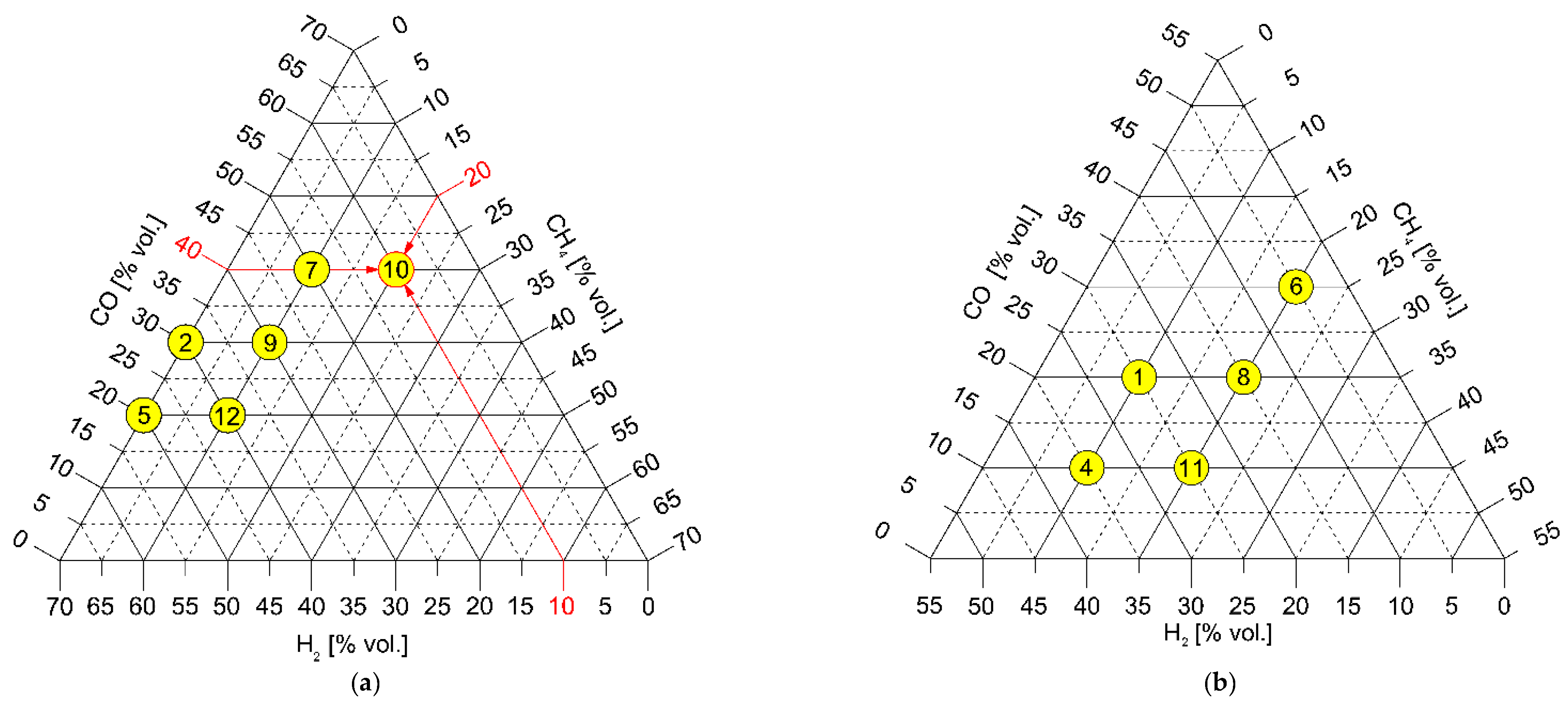
| Name | Unit | CH4 | SG1 | SG2 | SG3 | SG4 | SG5 | SG6 | SG7 | SG8 | SG9 | SG10 | SG11 | SG12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | [% vol.] | 100 | 10 | 0 | 20 | 10 | 0 | 20 | 10 | 20 | 10 | 20 | 20 | 10 |
| H2 | [% vol.] | 0 | 25 | 40 | 10 | 35 | 50 | 5 | 20 | 15 | 30 | 10 | 25 | 40 |
| CO | [% vol.] | 0 | 20 | 30 | 10 | 10 | 20 | 30 | 40 | 20 | 30 | 40 | 10 | 20 |
| CO2 | [% vol.] | 0 | 20 | 25 | 10 | 20 | 25 | 20 | 25 | 20 | 25 | 25 | 20 | 25 |
| N2 | [% vol.] | 0 | 25 | 5 | 50 | 25 | 5 | 25 | 5 | 25 | 5 | 5 | 25 | 5 |
| LHV | [MJ/kg] | 50.012 | 8.385 | 8.403 | 8.650 | 9.231 | 9.334 | 9.361 | 9.433 | 10.164 | 10.330 | 11.031 | 11.171 | 11.442 |
| LHV | [MJ/m3] | 33.358 | 8.197 | 7.550 | 8.852 | 8.027 | 7.378 | 10.708 | 10.045 | 10.529 | 9.883 | 12.389 | 10.364 | 9.711 |
| A/F ratio | [kg/kg] | 17.12 | 2.49 | 2.23 | 2.79 | 2.79 | 2.53 | 2.88 | 2.68 | 3.17 | 2.99 | 3.30 | 3.55 | 3.37 |
| M | [kg/kmol] | 16.04 | 23.52 | 21.61 | 24.62 | 20.92 | 19.01 | 27.52 | 25.61 | 24.92 | 23.01 | 27.02 | 22.32 | 20.42 |
| ρNTP fuel | [kg/m3] | 0.667 | 0.978 | 0.899 | 1.024 | 0.870 | 0.790 | 1.144 | 1.065 | 1.036 | 0.957 | 1.123 | 0.928 | 0.849 |
| ρNTP mixture | [kg/m3] | 1.153 | 1.130 | 1.090 | 1.151 | 1.094 | 1.049 | 1.188 | 1.163 | 1.159 | 1.131 | 1.185 | 1.131 | 1.099 |
| Fuel in mix. | [% vol.] | 9.51 | 33.0 | 37.5 | 29.6 | 33.1 | 37.5 | 26.7 | 29.6 | 26.8 | 29.6 | 24.5 | 26.7 | 29.6 |
| LHVmixture | [MJ/m3] | 3.172 | 2.707 | 2.828 | 2.619 | 2.657 | 2.767 | 2.859 | 2.973 | 2.818 | 2.920 | 3.030 | 2.767 | 2.871 |
 | Principle of the work | Spark ignition |
| Number of cylinders and arrangement | 2 in a row | |
| Crankshaft angle [°] | 360 | |
| Bore/Stroke [mm] | 75/77.6 | |
| Swept volume [cm3] | 686 | |
| Compression ratio [–] | 12.5:1 | |
| Valve timing, drive | OHC, timing belt | |
| Preparation of the mixture | External in a mixer with electronic control of mixture richness to stoichiometric mixture | |
| Cooling | Liquid with forced circulation, two-circuit thermostatically controlled, radiator blown by a fan driven by an electric motor | |
| Regulation | Electronically controlled throttle | |
| Ignition system | Brisk10DS—the highest thermal value. Ignition coil Bosch, energy 65 mJ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chríbik, A.; Polóni, M.; Magdolen, Ľ.; Minárik, M. Medium-Energy Synthesis Gases from Waste as an Energy Source for an Internal Combustion Engine. Appl. Sci. 2022, 12, 98. https://doi.org/10.3390/app12010098
Chríbik A, Polóni M, Magdolen Ľ, Minárik M. Medium-Energy Synthesis Gases from Waste as an Energy Source for an Internal Combustion Engine. Applied Sciences. 2022; 12(1):98. https://doi.org/10.3390/app12010098
Chicago/Turabian StyleChríbik, Andrej, Marián Polóni, Ľuboš Magdolen, and Matej Minárik. 2022. "Medium-Energy Synthesis Gases from Waste as an Energy Source for an Internal Combustion Engine" Applied Sciences 12, no. 1: 98. https://doi.org/10.3390/app12010098
APA StyleChríbik, A., Polóni, M., Magdolen, Ľ., & Minárik, M. (2022). Medium-Energy Synthesis Gases from Waste as an Energy Source for an Internal Combustion Engine. Applied Sciences, 12(1), 98. https://doi.org/10.3390/app12010098







