Thermal Modeling of Ultrasound Diathermy in Tissues with a Circular Inclusion near a Curved Interface
Abstract
:Featured Application
Abstract
1. Introduction
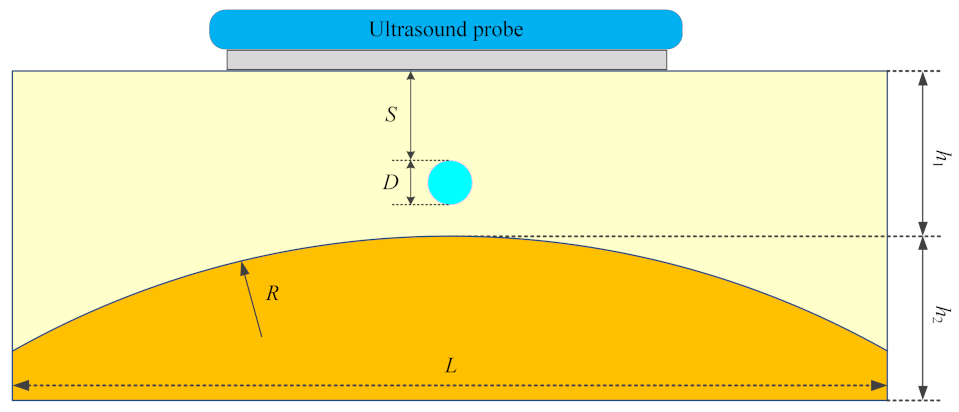
2. Materials and Methods
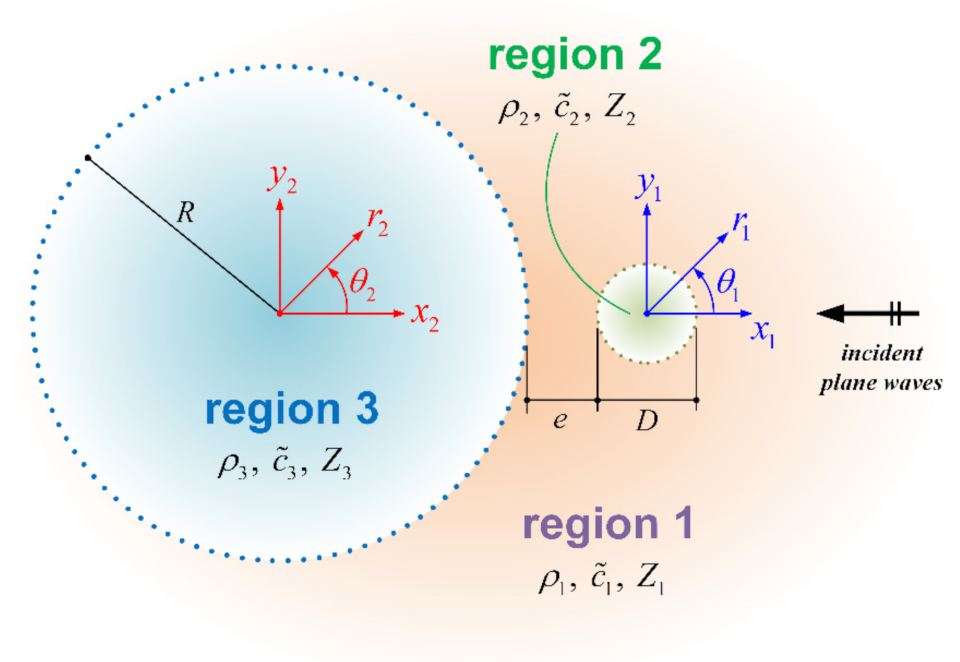
2.1. Pressure Field
2.2. Temperature Field
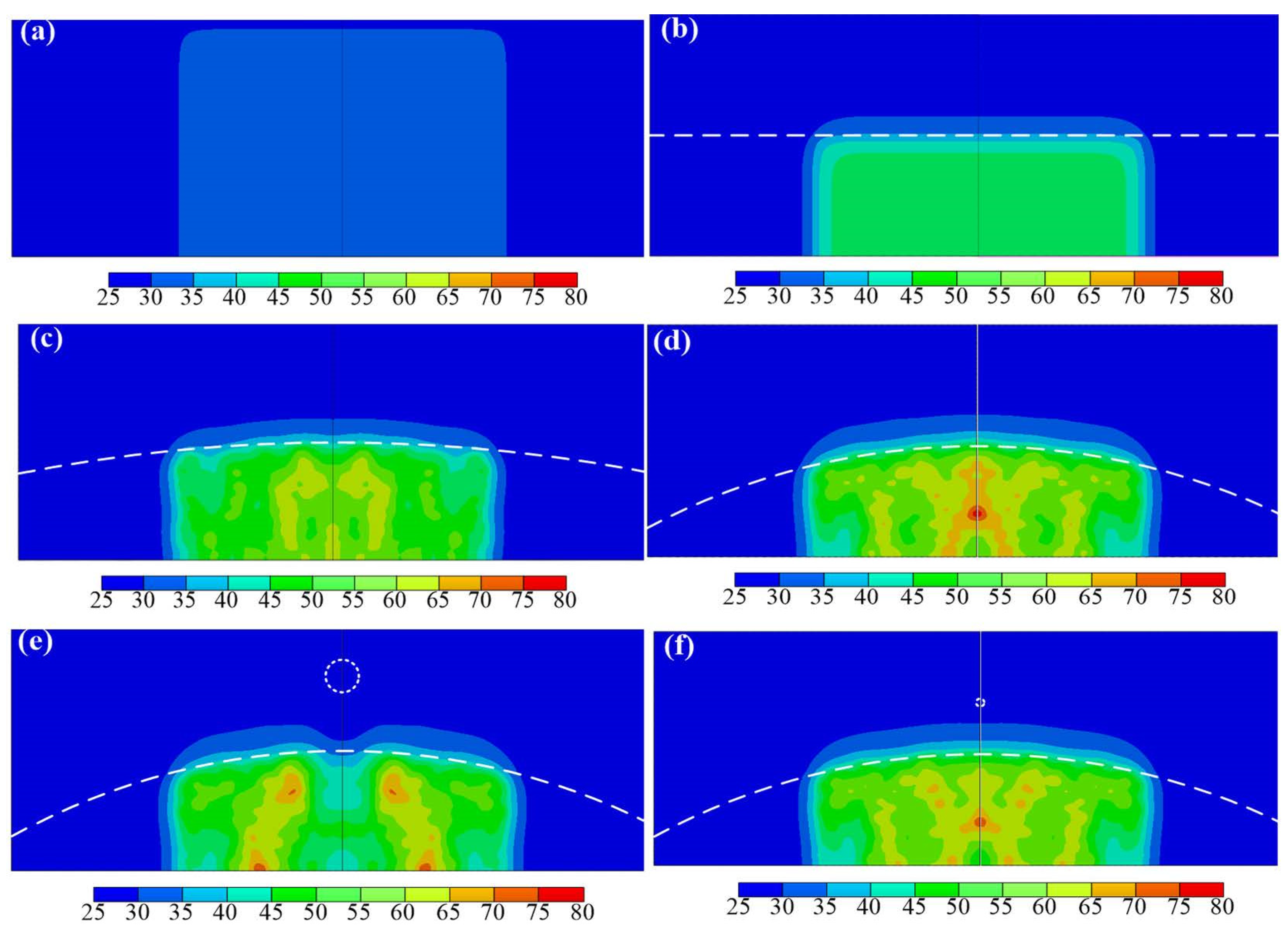
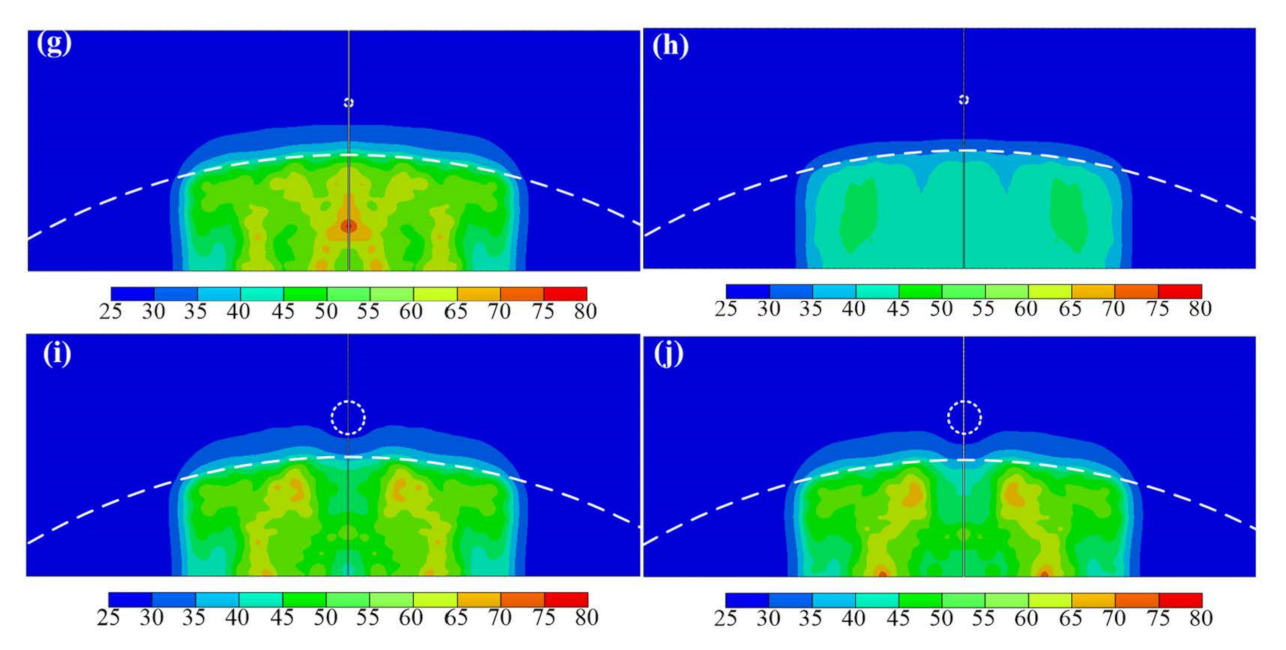
3. Results
4. Discussion
4.1. Effect of a Flat Bone
4.2. Effect of a Convex Bone
4.3. Effect of the Inclusion Size
4.4. Effect of the Inclusion Location
4.5. Effect of the Inclusion Material
4.6. Effect of the Ultrasound Operation Frequency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, B.T.; Merrick, M.A.; Sandrey, M.A.; Cordova, M.L. Three-MHz ultrasound heats deeper into the tissues than originally theorized. J. Athl. Train. 2004, 39, 230–234. [Google Scholar]
- Draper, D.O.; Castel, J.C.; Castel, D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J. Orthop. Sports Phys. Ther. 1995, 22, 142–150. [Google Scholar] [CrossRef]
- Draper, D.O.; Sunderland, S.; Kirkendall, D.T.; Ricard, M. A comparison of temperature rise in human calf muscles following applications of underwater and topical gel ultrasound. J. Orthop. Sports Phys. Ther. 2017, 17, 247–251. [Google Scholar] [CrossRef]
- Oliveira, D.P.; Areias, L.M.A.; Pereira, W.C.A.; von Krüger, M.A. Therapeutic ultrasound heat propagation in a cylindrical four-layer phantom. In Proceedings of the XXIV Brazilian Congress on Biomedical Engineering (CBEB 2014), Armação de Buzios, Brazil, 21–25 October 2014; Volume 1037, p. 41. [Google Scholar]
- Speed, C.A. Therapeutic ultrasound in soft tissue lesions. Rheumatology 2001, 40, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- Swanson, A.B.; Maupin, B.K.; Gajjar, N.V.; de Groot Swanson, G. Flexible implant arthroplasty in the proximal interphalangeal joint of the hand. J. Hand Surg. Am. 1985, 10, 796–805. [Google Scholar] [CrossRef]
- Cofield, R.H.; Edgerton, B.C. Total shoulder arthroplasty: Complications and revision surgery. Instr. Course Lect. 1990, 39, 449–462. [Google Scholar]
- Ghidella, S.D.; Segalman, K.A.; Murphey, M.S. Long-term results of surgical management of proximal interphalangeal joint contracture. J. Hand Surg. Am. 2002, 27, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Planas, J. Prophylactic use of external ultrasound for breast implant capsular contracture. Aesthet. Surg. J. 2002, 22, 205–207. [Google Scholar] [CrossRef]
- Cameron, M.H. Physical Agents in Rehabilitation: From Research to Practice, 4th ed.; Elsevier/Saunders: St. Louis, MO, USA, 2012; pp. 185–214. [Google Scholar]
- Gersten, J.W. Effect of metallic objects on temperature rises produced in tissue by ultrasound. Am. J. Phys. Med. Rehabil. 1958, 37, 75–82. [Google Scholar] [CrossRef]
- Brunner, G.D.; Lehmann, J.F.; McMillan, J.A.; Lane, K.E.; Bell, J.W. Can ultrasound be used in the presence of surgical metal implants: An experimental approach. Phys. Ther. 1958, 38, 823–824. [Google Scholar] [CrossRef]
- Lehmann, J.F.; Brunner, G.D.; McMillan, J.A. Influence of surgical metal implants on the temperature distribution in thigh specimens exposed to ultrasound. Arch. Phys. Med. Rehabil. 1958, 39, 692–695. [Google Scholar]
- Lehmann, J.F.; Lane, K.E.; Bell, J.W.; Brunner, G.D. Influence of surgical metal implants on the distribution of the intensity in the ultrasonic field. Arch. Phys. Med. Rehabil. 1958, 39, 756–760. [Google Scholar]
- Sun, M.K.; Shieh, J.; Chen, C.S.; Chiang, H.G.; Huang, C.W.; Chen, W.S. Effects of an implant on temperature distribution in tissue during ultrasound diathermy. Ultrason. Sonochem. 2016, 32, 44–53. [Google Scholar] [CrossRef]
- Andrades, A.O.; Mazzanti, A.; Beckmann, D.V.; Aiello, G.R.; Chaves, O.; Santos, R.P. Heating produced by therapeutic ultrasound in the presence of a metal plate in the femur of canine cadavers. Arq. Bras. Med. Vet. Zootec. 2014, 66, 1343–1350. [Google Scholar] [CrossRef]
- Huang, C.W. Simplified theoretical model for temperature evaluation in tissue–implant–bone systems during ultrasound diathermy. Appl. Sci. 2020, 10, 1306. [Google Scholar] [CrossRef] [Green Version]
- de Araújo Loures, E.; Costa, F.B.; Júnior, A.F.M.; Rocha, G.M.; Loures, D.N. Fatigue fracture of femoral stem in total hip arthroplasty after use of ultrasound: A case report. Int. J. Case Rep. Images 2018, 9, 100923Z01EL2018. [Google Scholar]
- Moros, E.G.; Straube, W.L.; Myerson, R.J.; Fan, X. The impact of ultrasonic parameters on chest wall hyperthermia. Int. J. Hyperth. 2000, 16, 523–538. [Google Scholar] [CrossRef]
- Moros, E.G.; Novak, P.; Straube, W.L.; Kolluri, P.; Yablonskiy, D.A.; Myerson, R.J. Thermal contribution of compact bone to intervening tissue-like media exposed to planar ultrasound. Phys. Med. Biol. 2004, 49, 869–886. [Google Scholar] [CrossRef]
- Omena, T.P.; Fontes-Pereira, A.J.; Costa, R.M.; Simões, R.J.; von Krüger, M.A.; Pereira, W.C.A. Why we should care about soft tissue interfaces when applying ultrasonic diathermy: An experimental and computer simulation study. J. Ther. Ultrasound. 2017, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Varadan, V.V.; Ma, Y.; Varadan, V.K.; Lakhtakia, A. Scattering of waves by spheres and cylinders. In Field Representations and Introduction to Scattering; Elsevier Science: New York, NY, USA, 1991; pp. 211–324. [Google Scholar]
- Hahn, D.W.; Özisik, M.N. Heat Conduction, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- SIMULIA-Abaqus 2020 User’s Manual; Dassault Syst′emes Simulia, Inc.: Providence, RI, USA, 2020.
- Gradshteyn, I.S.; Ryzhik, I.M. Table of Integrals, Series and Products, 7th ed.; Elsevier-Academic Press: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Jacobsen, F.; Juhl, P.M. Fundamentals of General Linear Acoustics; John Wiley & Sons: London, UK, 2013; pp. 171–186. [Google Scholar]
- Watson, G.N. A Treatise on the Theory of Bessel Functions, 2nd ed.; Cambridge University Press: Cambridge, UK, 1966. [Google Scholar]
- Lebedev, N.N. Special Functions and Their Applications; Selected Russian Publications in the Mathematical Sciences; Silverman, R.A., Ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1965. [Google Scholar]
- Nyborg, W.L. Heat generation by ultrasound in a relaxing medium. J Acoust. Soc. Am. 1981, 70, 310–312. [Google Scholar] [CrossRef]
- Meaney, P.M.; Clarke, R.L.; Haar, G.R.T.; Rivens, I.H. A 3-D finite-element model for computation of temperature profiles and regions of thermal damage during focused ultrasound surgery exposures. Ultrasound Med. Biol. 1998, 24, 1489–1499. [Google Scholar] [CrossRef]


| Material Property | Tissue | Stainless Steel | HDPE | Bone |
|---|---|---|---|---|
| Density (kg/m3) | 1190 | 7710 | 960 | 1912 |
| Specific heat (J/kg °C) | 3431 | 502 | 2300 | 1313 |
| Speed of sound (m/s) | 1512 | 5790 | 2460 | 4400 |
| Thermal conductivity (W/m °C) | 0.6 | 16.27 | 0.442 | 0.32 |
| Attenuation coefficient (db/m) | 54 | 124 | 66 | 900 |
| Model | S (mm) | D (mm) | M | R (mm) | h1 (mm) | h2 (mm) | f (MHz) |
|---|---|---|---|---|---|---|---|
| Case a | 0 | 0 | - | infinity | 30 | 0 | 1 |
| Case b | 0 | 0 | - | infinity | 15 | 15 | 1 |
| Case c | 0 | 0 | - | 200 | 15 | 15 | 1 |
| Case d | 0 | 0 | - | 80 | 15 | 15 | 1 |
| Case e | 4 | 4 | Steel | 80 | 15 | 15 | 1 |
| Case f | 8 | 1 | HDPE | 80 | 15 | 15 | 1 |
| Case g | 8 | 1 | Steel | 80 | 15 | 15 | 1 |
| Case h | 8 | 1 | Steel | 80 | 15 | 15 | 3 |
| Case i | 8 | 4 | HDPE | 80 | 15 | 15 | 1 |
| Case j | 8 | 4 | Steel | 80 | 15 | 15 | 1 |
| Model | Tissue | Inclusion | Bone |
|---|---|---|---|
| Case a | 33.96 | ̶ | ̶ |
| Case b | 35.62 | ̶ | 47.90 |
| Case c | 41.55 | ̶ | 64.86 |
| Case d | 42.34 | ̶ | 77.04 |
| Case e | 41.43 | 27.77 | 73.64 |
| Case f | 40.67 | 28.73 | 73.66 |
| Case g | 41.77 | 28.53 | 75.54 |
| Case h | 34.32 | 27.02 | 47.38 |
| Case i | 42.22 | 29.35 | 67.11 |
| Case j | 41.17 | 28.32 | 71.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-H.; Huang, C.-W. Thermal Modeling of Ultrasound Diathermy in Tissues with a Circular Inclusion near a Curved Interface. Appl. Sci. 2022, 12, 5166. https://doi.org/10.3390/app12105166
Chang K-H, Huang C-W. Thermal Modeling of Ultrasound Diathermy in Tissues with a Circular Inclusion near a Curved Interface. Applied Sciences. 2022; 12(10):5166. https://doi.org/10.3390/app12105166
Chicago/Turabian StyleChang, Kao-Hao, and Chang-Wei Huang. 2022. "Thermal Modeling of Ultrasound Diathermy in Tissues with a Circular Inclusion near a Curved Interface" Applied Sciences 12, no. 10: 5166. https://doi.org/10.3390/app12105166
APA StyleChang, K.-H., & Huang, C.-W. (2022). Thermal Modeling of Ultrasound Diathermy in Tissues with a Circular Inclusion near a Curved Interface. Applied Sciences, 12(10), 5166. https://doi.org/10.3390/app12105166





