Characteristics of Initial Attachment and Biofilm Formation of Pseudomonas aeruginosa on Microplastic Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Analysis of Microplastic Surface Properties
2.3. Biofilm Formation Assay
2.4. Biofilm Quantification
2.5. Effect of QS on Biofilm Formation on the Microplastic Surface
2.6. Effect of QQ on Microplastic Surface Biofilm Formation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Surface Characterization of Microplastics
3.2. Biofilm Formation on Microplastic Surfaces
3.3. Effect of Microplastics at Different Biofilm Developmental Stages
3.4. Effect of QS Signaling on Biofilm Formation on Microplastic Surfaces
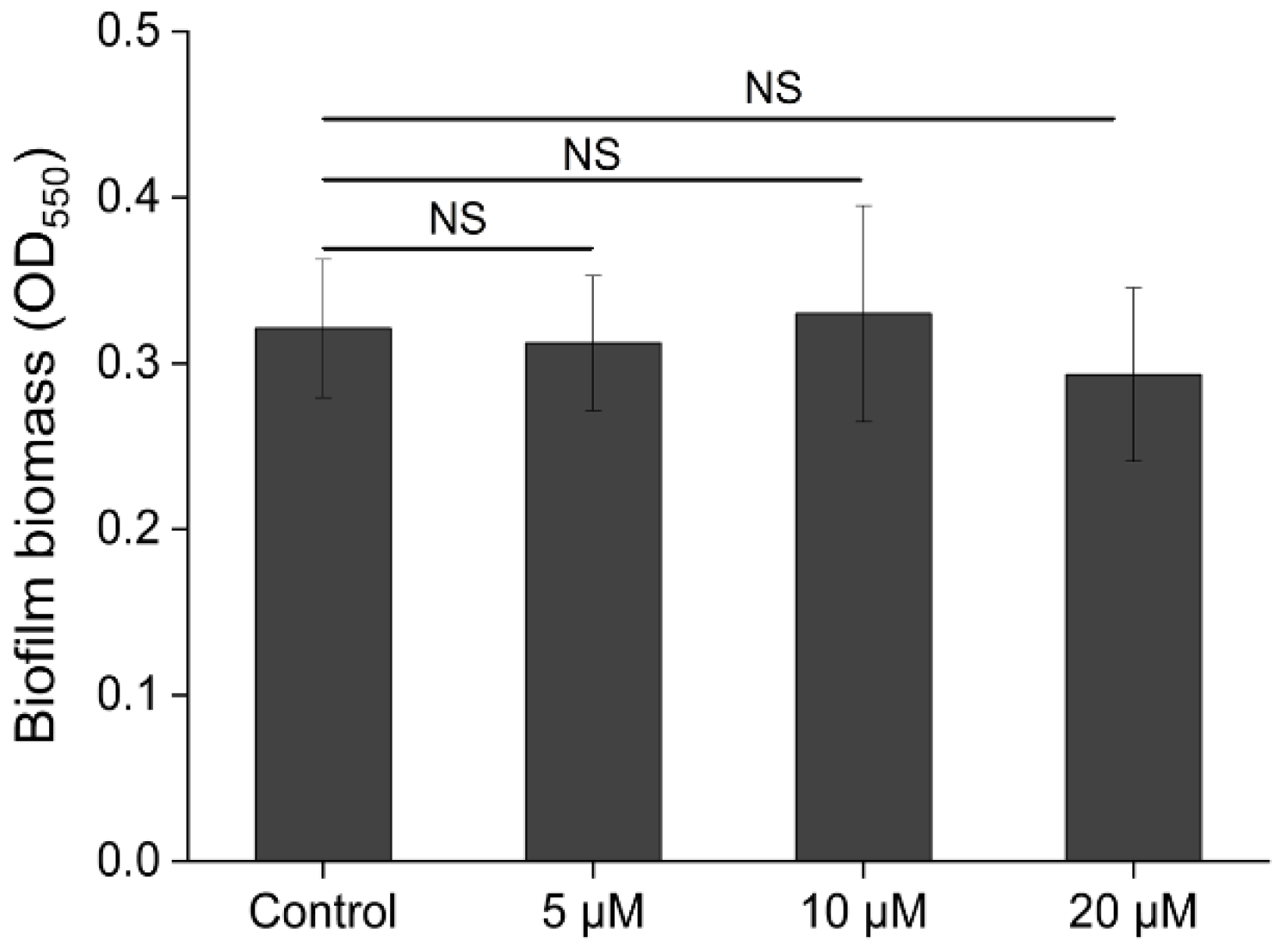
3.4.1. Injection of Exogenous QS Signaling Molecules
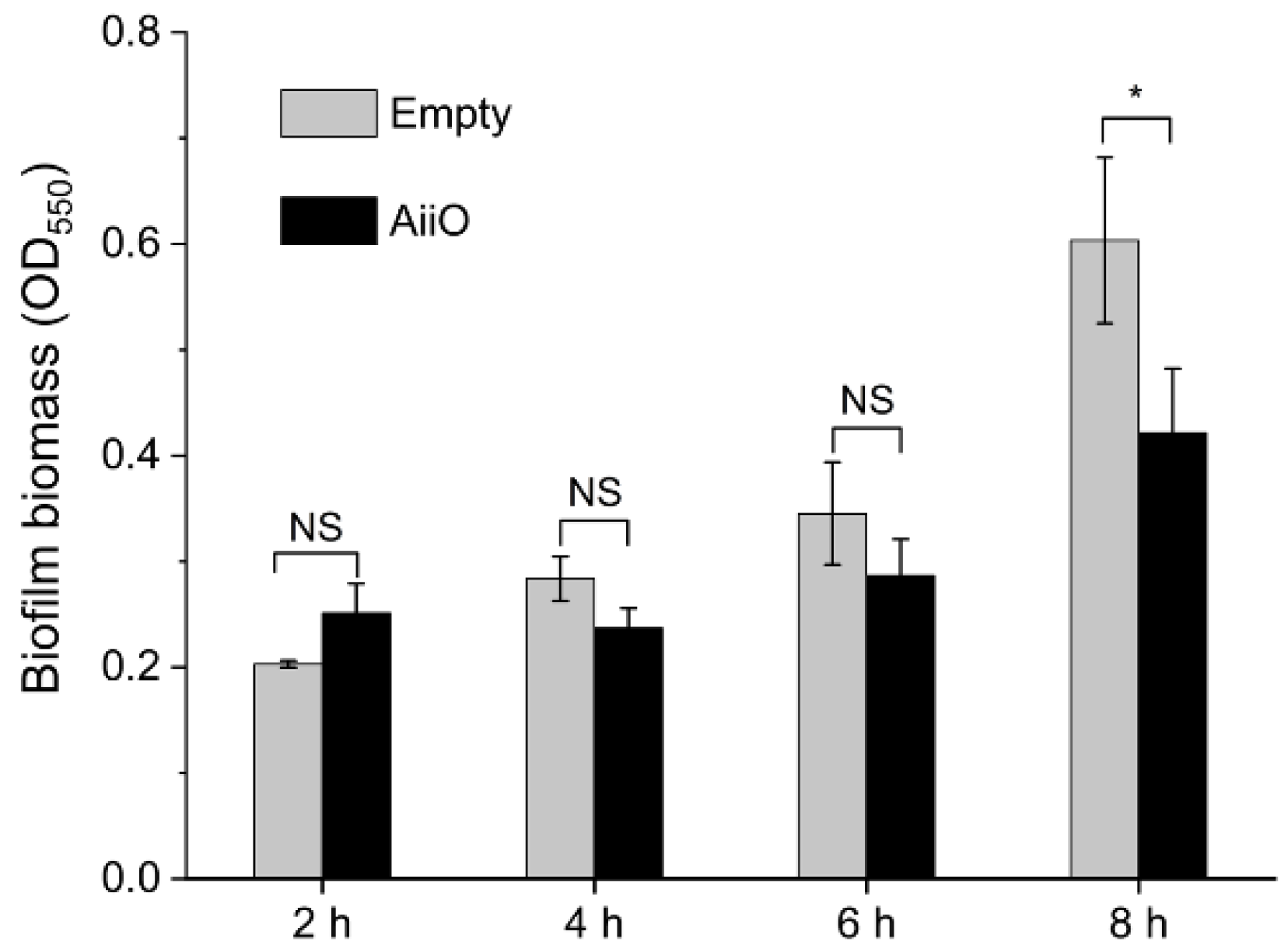
3.4.2. Injection of QQ Enzyme
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, A.A.; Dixon, S.J. Microplastics: An introduction to environmental transport processes. Wiley Interdiscip. Rev. Water 2018, 5, e1268. [Google Scholar] [CrossRef] [Green Version]
- Haram, L.E.; Carlton, J.T.; Ruiz, G.M.; Maximenko, N.A. A plasticene lexicon. Mar. Pollut. Bull. 2020, 150, 110714. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Zettler, L.A.; Zettler, E.R.; Slikas, B.; Boyd, G.D.; Melvin, D.W.; Morrall, C.E.; Proskurowski, G.; Mincer, T.J. The biogeography of the Plastisphere: Implications for policy. Front. Ecol. Environ. 2015, 13, 541–546. [Google Scholar] [CrossRef]
- Shabbir, S.; Faheem, M.; Ali, N.; Kerr, P.G.; Wang, L.-F.; Kuppusamy, S.; Li, Y. Periphytic biofilm: An innovative approach for biodegradation of microplastics. Sci. Total Environ. 2020, 717, 137064. [Google Scholar] [CrossRef]
- Rosato, A.; Barone, M.; Negroni, A.; Brigidi, P.; Fava, F.; Xu, P.; Candela, M.; Zanaroli, G. Microbial colonization of different microplastic types and biotransformation of sorbed PCBs by a marine anaerobic bacterial community. Sci. Total Environ. 2020, 705, 135790. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Lin, S.; Turner, J.P.; Ke, P.C. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J. Phys. Chem. C 2010, 114, 16556–16561. [Google Scholar] [CrossRef]
- Besseling, E.; Wang, B.; Lürling, M.; Koelmans, A.A. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef]
- Rios, L.M.; Jones, P.R.; Moore, C.; Narayan, U.V. Quantitation of persistent organic pollutants adsorbed on plastic debris from the Northern Pacific Gyre’s “eastern garbage patch”. J. Environ. Monit. 2010, 12, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Reisser, J.; Shaw, J.; Hallegraeff, G.; Proietti, M.; Barnes, D.K.; Thums, M.; Wilcox, C.; Hardesty, B.D.; Pattiaratchi, C. Millimeter-sized marine plastics: A new pelagic habitat for microorganisms and invertebrates. PLoS ONE 2014, 9, e100289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Cappitelli, F.; Sorlini, C. Microorganisms attack synthetic polymers in items representing our cultural heritage. Appl. Environ. Microbiol. 2008, 74, 564–569. [Google Scholar] [CrossRef] [Green Version]
- Ju, W.; An, J.; Jho, E. Adsorption characteristics of Cd and Pb on microplastic films generated in agricultural environment. J. Korean Soc. Environ. Eng. 2021, 43, 32–42. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Rymowicz, W.; Mirończuk, A.M. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 2018, 102, 7669–7678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Alam, A.; Rani, M.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med. Microbiol. 2017, 307, 481–489. [Google Scholar] [CrossRef]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal efficiency of micro-and nanoplastics (180 nm–125 μm) during drinking water treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Siddique, M.S.; Graham, N.J.; Yu, W. Towards microplastics contribution for membrane biofouling and disinfection by-products precursors: The effect on microbes. J. Hazard. Mater. 2022, 426, 127797. [Google Scholar] [CrossRef] [PubMed]
- Arias-Andres, M.; Rojas-Jimenez, K.; Grossart, H.-P. Collateral effects of microplastic pollution on aquatic microorganisms: An ecological perspective. TrAC Trends Anal. Chem. 2019, 112, 234–240. [Google Scholar] [CrossRef]
- Evans, L.R.; Linker, A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J. Bacteriol. 1973, 116, 915–924. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.-S.; Tan, C.H.; Low, J.H.; Rzechowicz, M.; Siddiqui, M.F.; Winters, H.; Kjelleberg, S.; Fane, A.G.; Rice, S.A. Quorum quenching bacteria can be used to inhibit the biofouling of reverse osmosis membranes. Water Res. 2017, 112, 29–37. [Google Scholar] [CrossRef]
- McLean, R.J.; Whiteley, M.; Stickler, D.J.; Fuqua, W.C. Evidence of autoinducer activity in naturally occurring biofilms. FEMS Microbiol. Lett. 1997, 154, 259–263. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J.-m. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Krishnan, S.; Weinman, C.J.; Ober, C.K. Advances in polymers for anti-biofouling surfaces. J. Mater. Chem. 2008, 18, 3405–3413. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Meier, K.; Klöckner, W.; Bonhage, B.; Antonov, E.; Regestein, L.; Büchs, J. Correlation for the maximum oxygen transfer capacity in shake flasks for a wide range of operating conditions and for different culture media. Biochem. Eng. J. 2016, 109, 228–235. [Google Scholar] [CrossRef]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wu, D.; Xia, J.; Shi, H.; Kim, H. Influence of physicochemical surface properties on the adhesion of bacteria onto four types of plastics. Sci. Total Environ. 2019, 671, 1101–1107. [Google Scholar] [CrossRef]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Anburajan, P.; Kim, Y.; Rice, S.A.; Oh, H.-S. Bacterial signaling and signal responses as key factors in water and wastewater treatment. J. Water Process Eng. 2021, 44, 102434. [Google Scholar] [CrossRef]
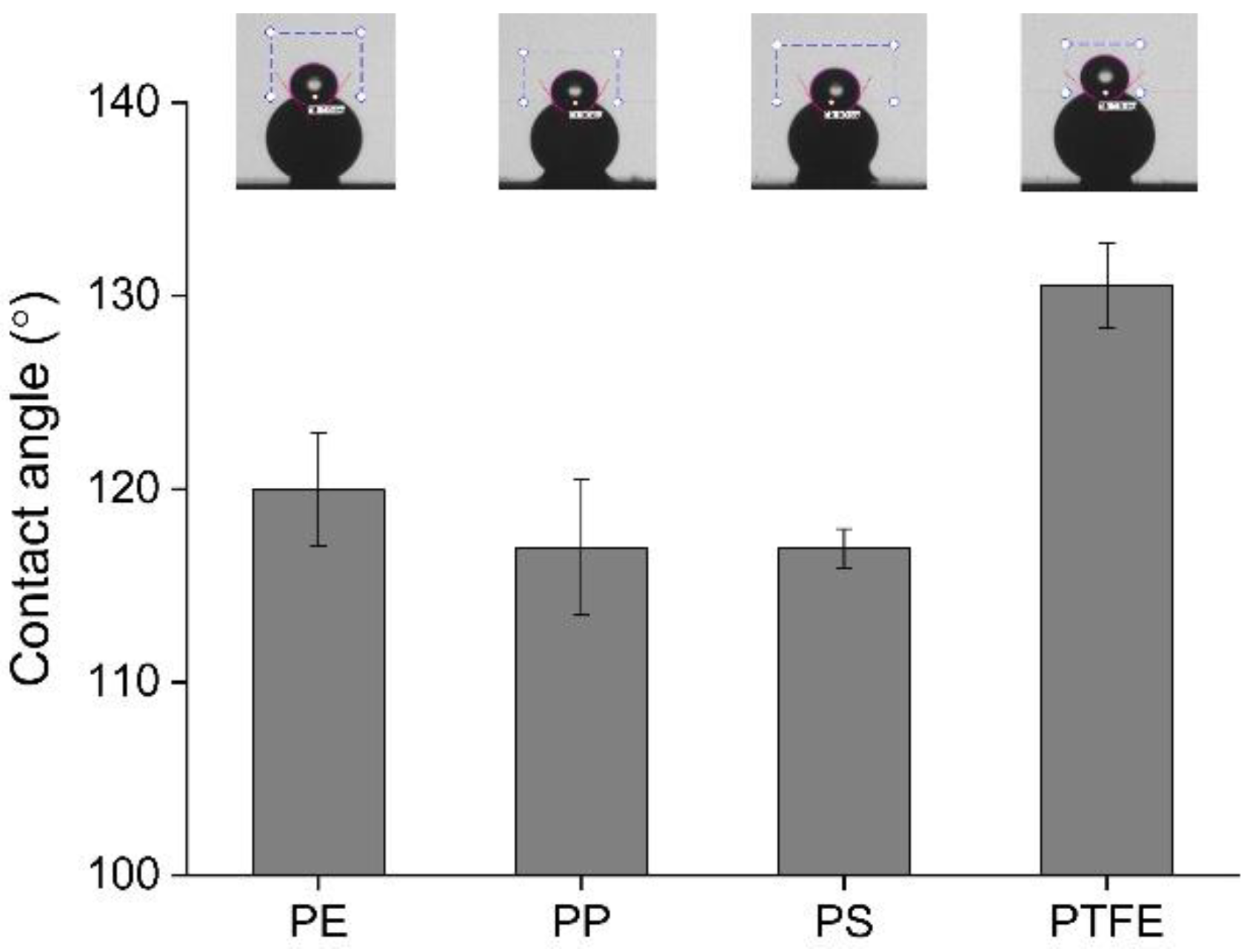
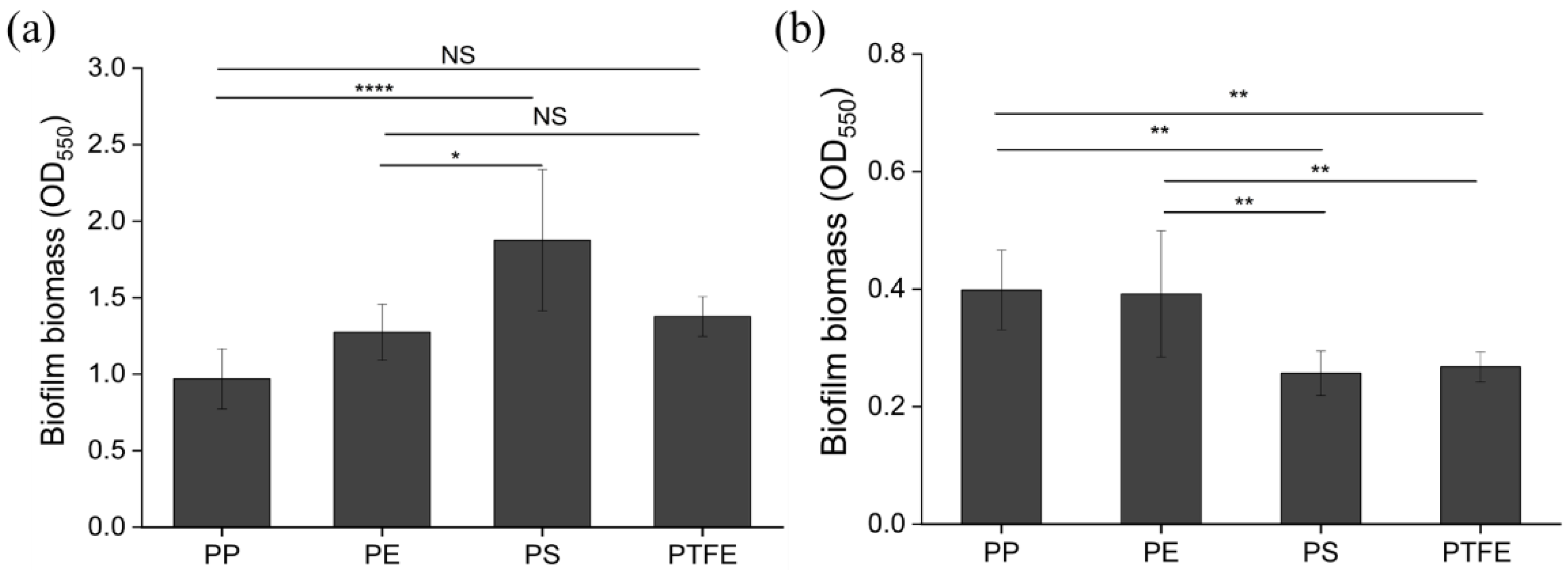
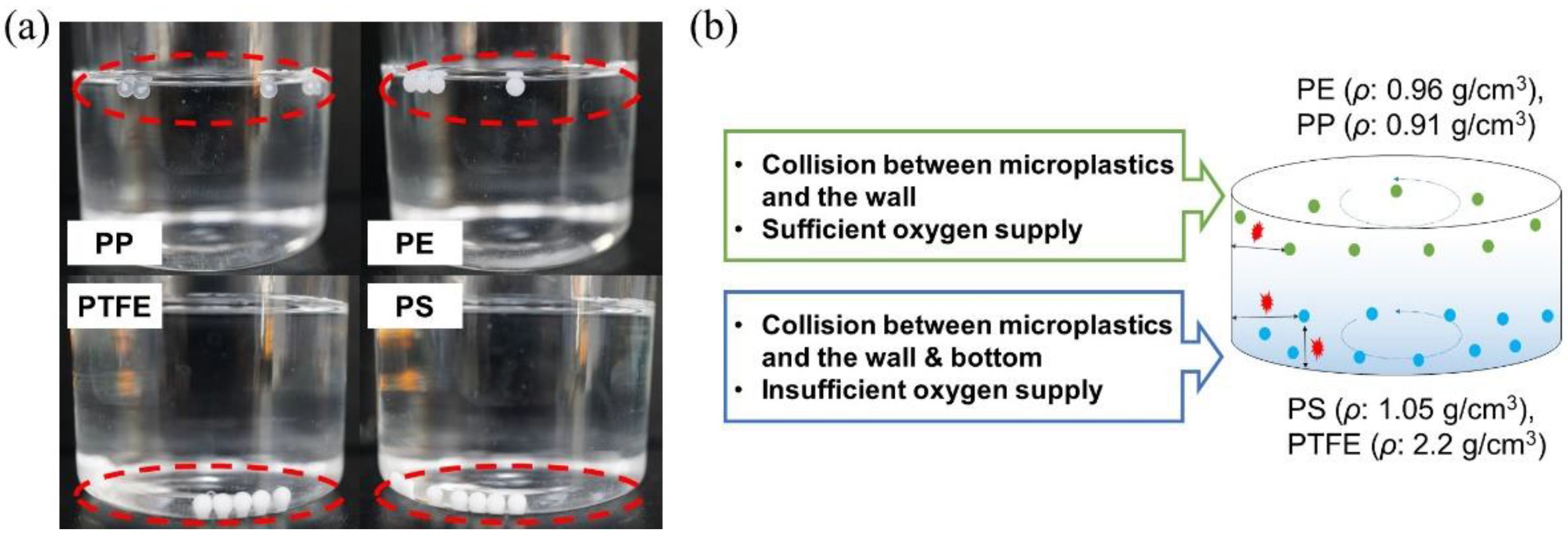

| PE | PP | PS | PTFE | |
|---|---|---|---|---|
| Diameter (mm) | 3.2 | 3.2 | 2.96 | 3.2 |
| Density (g/cm3) | 0.96 | 0.91 | 1.05 | 2.2 |
| PE | PP | PS | PTFE | |
|---|---|---|---|---|
| Rq * (nm) | 116.42 | 55.09 | 119.28 | 71.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayush, P.T.; Ko, J.-H.; Oh, H.-S. Characteristics of Initial Attachment and Biofilm Formation of Pseudomonas aeruginosa on Microplastic Surfaces. Appl. Sci. 2022, 12, 5245. https://doi.org/10.3390/app12105245
Ayush PT, Ko J-H, Oh H-S. Characteristics of Initial Attachment and Biofilm Formation of Pseudomonas aeruginosa on Microplastic Surfaces. Applied Sciences. 2022; 12(10):5245. https://doi.org/10.3390/app12105245
Chicago/Turabian StyleAyush, Purevdash Tsend, Je-Hyeon Ko, and Hyun-Suk Oh. 2022. "Characteristics of Initial Attachment and Biofilm Formation of Pseudomonas aeruginosa on Microplastic Surfaces" Applied Sciences 12, no. 10: 5245. https://doi.org/10.3390/app12105245
APA StyleAyush, P. T., Ko, J.-H., & Oh, H.-S. (2022). Characteristics of Initial Attachment and Biofilm Formation of Pseudomonas aeruginosa on Microplastic Surfaces. Applied Sciences, 12(10), 5245. https://doi.org/10.3390/app12105245







