Applications of Mueller Matrix Polarimetry to Biological and Agricultural Diagnostics: A Review
Abstract
1. Introduction
2. Principles of Mueller Matrix Analysis
- Polarization is modulated continuously or pulse-modulated (periodically, as a rule);
- Polarization sequentially passes a fixed, discrete set of time-constant states.
3. Basic Schemes for Using Mueller Matrix Polarimetry in Optical Diagnostics of Biological and Bioorganic Systems
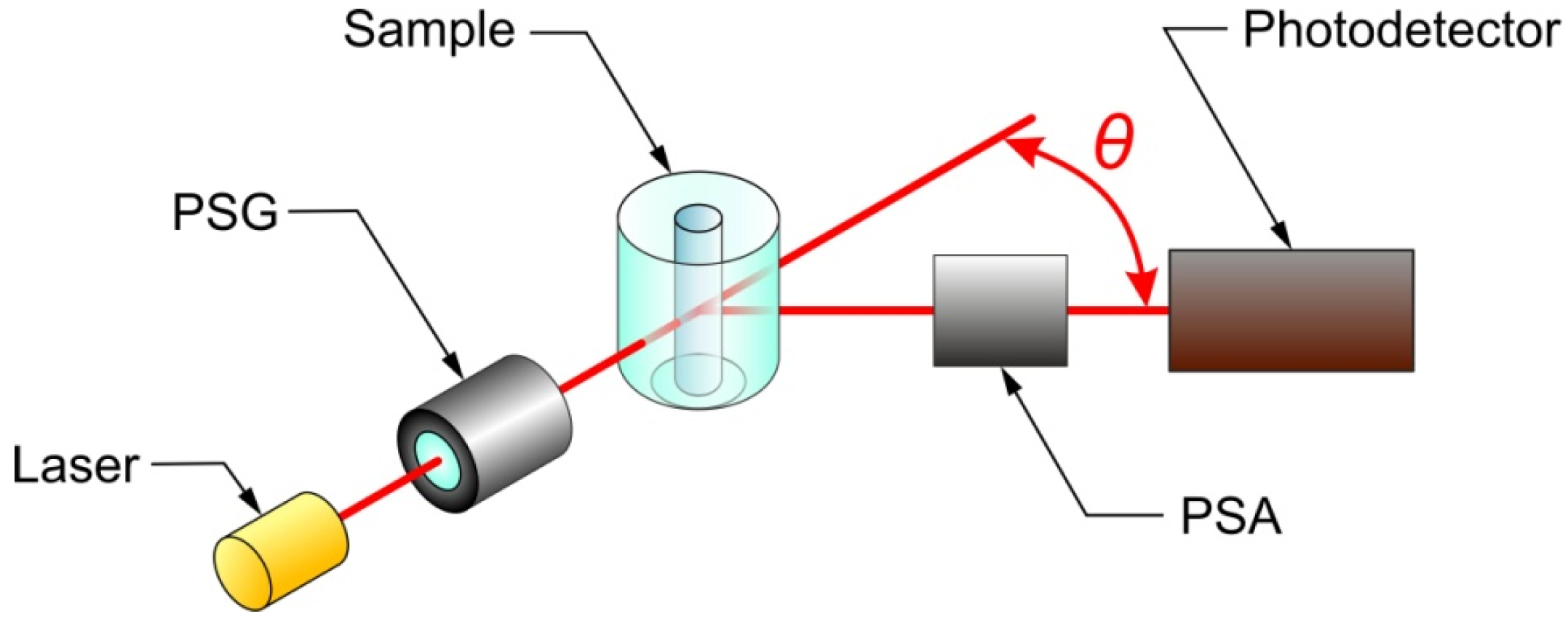
3.1. Mueller Matrix Scatterometry
- Use of multi-wavelength sources. This method is called multi-wavelength scatterometry;
- Use of MM measurements. Such a method is called polarimetric scatterometry or MM scatterometry (MMS).
3.2. Transmission Mueller Matrix Spectropolarimetry
- High sensitivity and accuracy;
- High measurement speed;
- Multicomponent analysis;
- Universality—components of interest can be defined in a variety of objects.
3.3. Mueller Matrix Imaging Polarimetry and Optical Coherence Tomography
- Real-time images of subsurface objects with a resolution close to microscopic;
- Instant, direct visualization of tissue morphology;
- No need for preliminary sample preparation;
- Non-invasiveness;
- Absence of ionizing radiation.
3.4. Mueller Matrix Fluorimetry
3.5. Reflection Mueller Matrix Ellipsometry
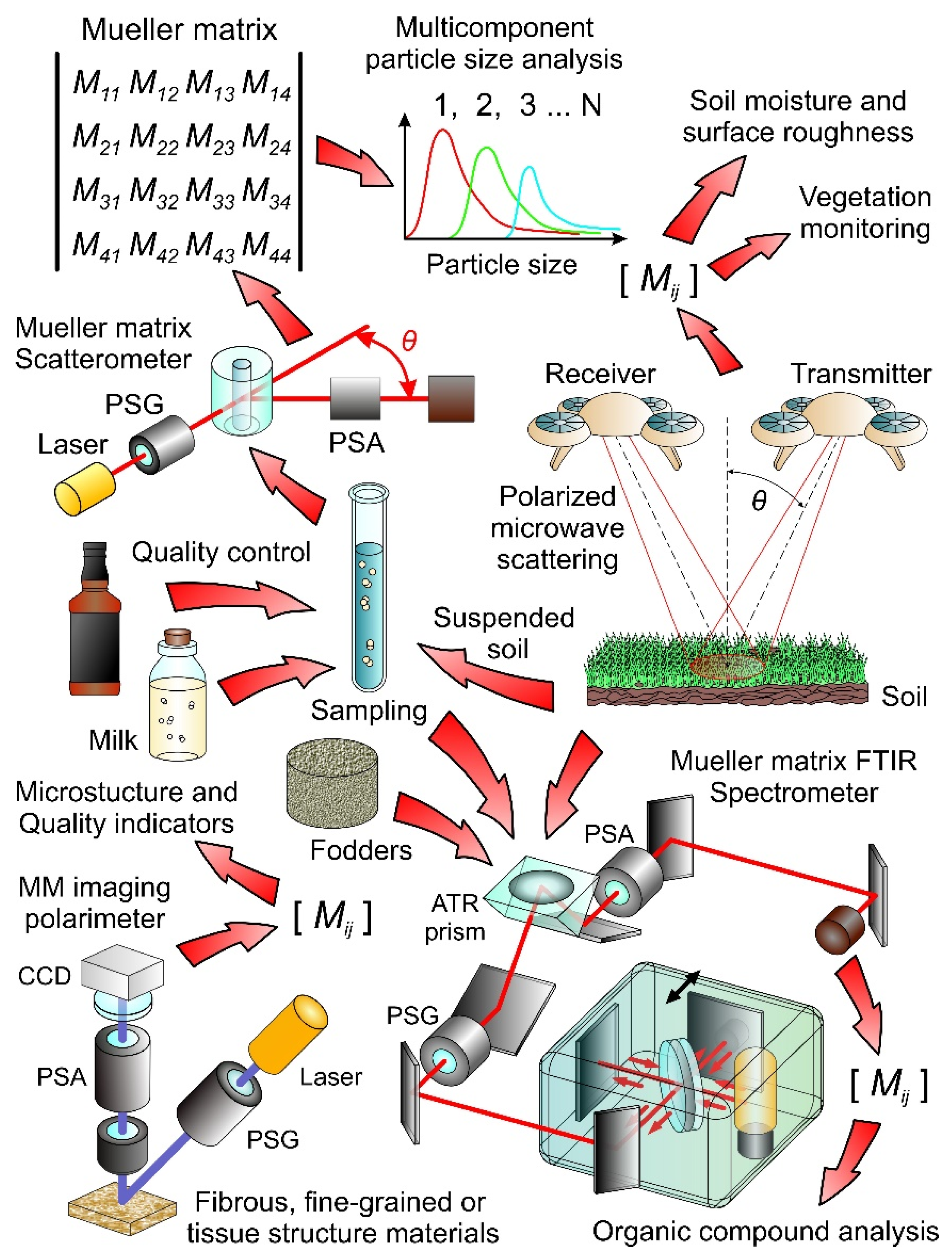
4. Applications of MMP to Biology and Agriculture
- Geometry of the sample—volumetric (MM scatterometry, MM spectrophotometry, MM fluorometry) and surface (MM-OCT and MM ellipsometry);
- Properties of incoming radiation—spectral (spectrophotometry and spectroscopic MM scatterometry, MM fluorometry and MM ellipsometry) and non-spectral (MM-OCT and single-wavelength MM scatterometry, MM fluorometry and MM ellipsometry);
- Received information—visual (MM-OCT and other methods of polarization-sensitive imaging) and structural (all methods that measure the MM with subsequent retrieval of the microphysical parameters of the sample using inverse analysis);
- Operating conditions—stationary and mobile.
4.1. Studying the Structure of Organic Materials
4.2. Microbiological Research
4.3. Biological Tissues
4.4. Milk Composition Analysis and Food Quality Control
4.5. Characterization of Soils and Fodders and Vegetation Monitoring
4.6. Further Development of MMP Diagnostics Using Fiber Optics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuller, G.G. Optical Rheometry of Complex Fluids; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Palberg, T.; Ballauff, M. Optical Methods and Physics of Colloidal Dispersions. In Proceedings of the International Workshop on Optical Methods and the Physics of Colloidal Dispersions held in Memory of Prof. Dr. Klaus Schätzel, Mainz, Germany, 30 September 1996; Steinkopff: Heidelberg, Germany, 1997. [Google Scholar] [CrossRef]
- Xu, R. Particle Characterization: Light Scattering Methods; Kluwer Academic Publishers: New York, NY, USA, 2002. [Google Scholar]
- Mishchenko, M.I.; Travis, L.D.; Lacis, A.A. Scattering, Absorption, and Emission of Light by Small Particles; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Doicu, A.; Wriedt, T.; Eremin, Y.A. Light Scattering by Systems of Particles; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Azzam, R.M. Stokes-vector and Mueller-matrix polarimetry. J. Opt. Soc. Am. A 2016, 33, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Chipman, R.A.; Sornsin, E.A.; Pezzaniti, J.L. Mueller matrix imaging polarimetry: An overview. In Proceedings of the SPIE, International Symposium on Polarization Analysis and Applications to Device Technology, Yokohama, Japan, 12–16 June 1996; Yoshizawa, T., Yokota, H., Eds.; SPIE: Bellingham, WA, USA, 1996; Volume 2873, pp. 5–12. [Google Scholar] [CrossRef]
- Arteaga, O.; Kahr, B. Mueller matrix polarimetry of bianisotropic materials. J. Opt. Soc. Am. B 2019, 36, F72–F83. [Google Scholar] [CrossRef]
- Ghosh, N.; Soni, J.; Wood, M.; Wallenberg, M.; Vitkin, I. Mueller matrix polarimetry for the characterization of complex random medium like biological tissues. Pramana J. Phys. 2010, 75, 1071–1086. [Google Scholar] [CrossRef]
- Tripathi, S.; Toussaint, K.C. Rapid Mueller matrix polarimetry based on parallelized polarization state generation and detection. Opt. Express 2009, 17, 21396–21407. [Google Scholar] [CrossRef]
- Anastasiadou, M.; Hatit, S.B.; Ossikovski, R.; Guyot, S.; De Martino, A. Experimental validation of the reverse polar decomposition of depolarizing Mueller matrices. J. Eur. Opt. Soc. Rapid Publ. 2007, 2, 07018. [Google Scholar] [CrossRef]
- Arwin, H.; Schoeche, S.; Hilfiker, J.; Hartveit, M.; Järrendahl, K.; Juárez-Rivera, O.R.; Mendoza-Galván, A.; Magnusson, R. Optical Chirality Determined from Mueller Matrices. Appl. Sci. 2021, 11, 6742. [Google Scholar] [CrossRef]
- Boulvert, F.; Le Brun, G.; Le Jeune, B.; Cariou, J.; Martin, L. Decomposition algorithm of an experimental Mueller matrix. Opt. Commun. 2009, 282, 692–704. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Huo, Y.; Wang, J.; Zhang, Y. An experimental study on light scattering matrices for Chinese loess dust with different particle size distributions. Atmos. Meas. Tech. 2020, 13, 4097–4109. [Google Scholar] [CrossRef]
- Volten, H.; Jalava, J.P.; Lumme, K.; De Haan, J.F.; Vassen, W.; Hovenier, J.W. Laboratory measurements and T-matrix calculations of the scattering matrix of rutile particles in water. Appl. Opt. 1999, 38, 5232–5240. [Google Scholar] [CrossRef]
- Diaspro, A.; Radicchi, G.; Nicolini, C. Polarized light scattering: A biophysical method for studying bacterial cells. IEEE Trans. Biomed. Eng. 1995, 42, 1038–1043. [Google Scholar] [CrossRef]
- Mengüç, M.; Manickavasagam, S. Characterization of size and structure of agglomerates and inhomogeneous particles via polarized light. Int. J. Eng. Sci. 1998, 36, 1569–1593. [Google Scholar] [CrossRef]
- Kolokolova, L.; Kimura, H.; Ziegler, K.; Mann, I. Light-scattering properties of random-oriented aggregates: Do they represent the properties of an ensemble of aggregates? J. Quant. Spectrosc. Radiat. Transf. 2006, 100, 199–206. [Google Scholar] [CrossRef]
- He, C.; He, H.; Chang, J.; Chen, B.; Ma, H.; Booth, M.J. Polarisation optics for biomedical and clinical applications: A review. Light Sci. Appl. 2021, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Vitkin, I.A. Tissue polarimetry: Concepts, challenges, applications, and outlook. J. Biomed. Opt. 2011, 16, 110801. [Google Scholar] [CrossRef] [PubMed]
- Ramella-Roman, J.C.; Saytashev, I.; Piccini, M. A review of polarization-based imaging technologies for clinical and preclinical applications. J. Opt. 2020, 22, 123001. [Google Scholar] [CrossRef]
- He, H.; Liao, R.; Zeng, N.; Li, P.; Chen, Z.; Liu, X.; Ma, H. Mueller matrix polarimetry—An emerging new tool for characterizing the microstructural feature of complex biological specimen. J. Lightwave Technol. 2019, 37, 2534–2548. [Google Scholar] [CrossRef]
- Tuchin, V.V. Polarized light interaction with tissues. J. Biomed. Opt. 2016, 21, 071114. [Google Scholar] [CrossRef]
- Ahmad, I.; Khaliq, A.; Iqbal, M.; Khan, S. Mueller matrix polarimetry for characterization of skin tissue samples: A review. Photodiagnosis Photodyn. Ther. 2020, 30, 101708. [Google Scholar] [CrossRef]
- Smith, M.H.; Burke, P.D.; Lompado, A.; Tanner, E.A.; Hillman, L.W. Mueller matrix imaging polarimetry in dermatology. In Proceedings of the SPIE 3911, Biomedical Diagnostic, Guidance, and Surgical-Assist Systems II, San Jose, CA, USA, 3 May 2000. [Google Scholar] [CrossRef]
- Anwar, S.; Firdous, S. Optical diagnosis of dengue virus infected human blood using Mueller matrix polarimetry. Opt. Spectrosc. 2016, 121, 322–325. [Google Scholar] [CrossRef]
- Le Gratiet, A.; Mohebi, A.; Callegar, F.; Bianchini, P.; Diaspro, A. Review on Complete Mueller Matrix Optical Scanning Microscopy Imaging. Appl. Sci. 2021, 11, 1632. [Google Scholar] [CrossRef]
- Ghosh, N.; Wood, M.F.; Li, S.H.; Weisel, R.D.; Wilson, B.C.; Li, R.K.; Vitkin, I.A. Mueller matrix decomposition for polarized light assessment of biological tissues. J. Biophotonics 2009, 2, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Badieyan, S.; Ameri, A.; Razzaghi, M.R.; Rafii-Tabar, H.; Sasanpour, P. Mueller matrix imaging of prostate bulk tissues; Polarization parameters as a discriminating benchmark. Photodiagnosis Photodyn. Ther. 2019, 26, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; He, H.; Zeng, N.; Sun, M.; Guo, Y.; Wu, J.; Liu, S.; Ma, H. Mueller matrix polarimetry for differentiating characteristic features of cancerous tissues. J. Biomed. Opt. 2014, 19, 076013. [Google Scholar] [CrossRef] [PubMed]
- Badieyan, S.; Dilmaghani-Marand, A.; Hajipour, M.J.; Ameri, A.; Razzaghi, M.R.; Rafii-Tabar, H.; Mahmoudi, M.; Sasanpour, P. Detection and Discrimination of Bacterial Colonies with Mueller Matrix Imaging. Sci. Rep. 2018, 8, 10815. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, T.; He, H.; Liu, S.; Dong, Y.; Wu, J.; Ma, H. Comparative study of the imaging contrasts of Mueller matrix derived parameters between transmission and backscattering polarimetry. Biomed. Opt. Express 2018, 9, 4413–4428. [Google Scholar] [CrossRef]
- Jiao, S.; Yu, W.; Stoica, G.; Wang, L.V. Multiple-channel Mueller-matrix optical coherence tomography in biological tissue. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society, Houston, TX, USA, 23–26 October 2002; Volume 3, pp. 2321–2322. [Google Scholar] [CrossRef]
- Chue-Sang, J.; Bai, Y.; Stoff, S.; Straton, D.; Ramaswamy, S.; Ramella-Roman, J.C. Use of combined polarization-sensitive optical coherence tomography and Mueller matrix imaging for the polarimetric characterization of excised biological tissue. J. Biomed. Opt. 2016, 21, 71109. [Google Scholar] [CrossRef]
- Crofcheck, C.; Wade, J.; Swamy, J.N.; Aslan, M.M.; Mengüç, M.P. Effect of Fat and Casein Particles in Milk on the Scattering of Elliptically Polarized Light. Trans. ASAE 2005, 48, 1147–1155. [Google Scholar] [CrossRef]
- Shkirin, A.V.; Ignatenko, D.N.; Chirikov, S.N.; Bunkin, N.F.; Astashev, M.E.; Gudkov, S.V. Analysis of Fat and Protein Content in Milk Using Laser Polarimetric Scatterometry. Agriculture 2021, 11, 1028. [Google Scholar] [CrossRef]
- Lamelas, F.; Swaminathan, S. Optical absorption, scattering, and multiple scattering: Experimental measurements using food coloring, India ink, and milk. Am. J. Phys. 2020, 88, 137–140. [Google Scholar] [CrossRef]
- Lehmann, P.; Osten, W.; Albertazzi Gonçalves, A.; Jain, P.; Sarma, S.E. Light scattering and transmission measurement using digital imaging for online analysis of constituents in milk. In Proceedings of the Optical Measurement Systems for Industrial Inspection, Munich, Germany, 21 June 2015. [Google Scholar]
- Veenstra, C.; Every, D.E.; Petersen, W.; van Goudoever, J.B.; Steenbergen, W.; Bosschaart, N. Dependency of the optical scattering properties of human milk on casein content and common sample preparation methods. J. Biomed. Opt. 2020, 25, 045001. [Google Scholar] [CrossRef]
- Firdous, S. Polarization Sensitive Optical Imaging and Characterization of Soybean Using Stokes-Mueller Matrix Model. In Soybean—Genetics and Novel Techniques for Yield Enhancement; Krezhova, D., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Derman, D.; Şenel, E.C.; Opar, E.; Ferhanoğlu, O.; Polat, Ö. Optical characterization of olive and sun flower oils via Mueller matrix polarimetry in combination with principal component analysis. J. Food Meas. Charact. 2021, 15, 2309–2317. [Google Scholar] [CrossRef]
- Peyvasteh, M.; Popov, A.; Bykov, A.; Pierangelo, A.; Novikova, T.; Meglinski, I. Evolution of raw meat polarization-based properties by means of Mueller matrix imaging. J. Biophotonics 2021, 14, e202000376. [Google Scholar] [CrossRef] [PubMed]
- Patty, C.H.L.; Luo, D.A.; Snik, F.; Ariese, F.; Buma, W.J.; ten Kate, I.L.; van Spanning, R.J.M.; Sparks, W.B.; Germer, T.A.; Garab, G.; et al. Imaging linear and circular polarization features in leaves with complete Mueller matrix polarimetry. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1350–1363. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Glinushkin, A.P.; Shkirin, A.V.; Ignatenko, D.N.; Chirikov, S.N.; Savchenko, I.V.; Meshalkin, V.P.; Samarin, G.N.; Maleki, A.; Kalinitchenko, V.P. Identification of Organic Matter Dispersions Based on Light Scattering Matrices Focusing on Soil Organic Matter Management. ACS Omega 2020, 5, 33214–33224. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Sarabandi, K.; Ulaby, F.T. Semi-empirical model of the ensemble-averaged differential Mueller matrix for microwave backscattering from bare soil surfaces. IEEE Trans. Geosci. Remote Sens. 2002, 40, 1348–1355. [Google Scholar] [CrossRef]
- Zallat, J.; Stoll, M.P. Polarized bidirectional scattering by bare soils. J. Opt. A Pure Appl. Opt. 2000, 2, 169. [Google Scholar] [CrossRef]
- Seitz, R.; Brings, R.; Geiger, R. Protein adsorption on solid–liquid interfaces monitored by laser-ellipsometry. Appl. Surf. Sci. 2005, 252, 154–157. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, C. Polarized synchronous light scattering characterization of the interaction of proteins with sodium dodecyl sulfonate. Chin. Sci. Bull. 2007, 52, 456–460. [Google Scholar] [CrossRef]
- Goldstein, D.H.; Chipman, R.A. Error analysis of a Mueller matrix polarimeter. J. Opt. Soc. Am. A 1990, 7, 693–700. [Google Scholar] [CrossRef]
- Jagtap, J.; Chandel, S.; Das, N.; Soni, J.; Chatterjee, S.; Pradhan, A.; Ghosh, N. Quantitative Mueller matrix fluorescence spectroscopy for precancer detection. Opt. Lett. 2014, 39, 243–246. [Google Scholar] [CrossRef]
- Soni, J.; Purwar, H.; Lakhotia, H.; Chandel, S.; Banerjee, C.; Kumar, U.; Ghosh, N. Quantitative fluorescence and elastic scattering tissue polarimetry using an Eigenvalue calibrated spectroscopic Mueller matrix system. Opt. Express 2013, 21, 15475–15489. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Li, W.; Qi, J.; Liu, T.; He, H.; Dong, Y.; Liu, S.; Wu, J.; Elson, D.S.; Ma, H. Quantitative Analysis of 4 × 4 Mueller Matrix Transformation Parameters for Biomedical Imaging. Photonics 2019, 6, 34. [Google Scholar] [CrossRef]
- He, H.; Zeng, N.; Li, D.; Liao, R.; Ma, H. Quantitative Mueller matrix polarimetry techniques for biological tissues. J. Innov. Opt. Health Sci. 2012, 5, 1250017. [Google Scholar] [CrossRef]
- Borovkova, M.; Trifonyuk, L.; Ushenko, V.; Dubolazov, O.; Vanchulyak, O.; Bodnar, G.; Ushenko, Y.; Olar, O.; Ushenko, O.; Sakhnovskiy, M.; et al. Mueller-matrix-based polarization imaging and quantitative assessment of optically anisotropic polycrystalline networks. PLoS ONE 2019, 14, e0214494. [Google Scholar] [CrossRef]
- Ding, Z.; Liang, C.P.; Tang, Q.; Chen, Y. Quantitative single-mode fiber based PS-OCT with single input polarization state using Mueller matrix. Biomed. Opt. Express 2015, 6, 1828–1843. [Google Scholar] [CrossRef]
- Meng, R.; Chen, Z.; Wang, X.; Liu, Y.; He, H.; Ma, H. Comparison of different calibration methods for Mueller matrix microscopy of cells. Appl. Opt. 2021, 60, 1380–1386. [Google Scholar] [CrossRef]
- AxoScan™ Mueller Matrix Polarimeter. Available online: https://www.axometrics.com/products/polarimeters-ellipsometers/axoscan (accessed on 5 May 2021).
- RC2 Ellipsometer. Available online: https://www.jawoollam.com/products/rc2-ellipsometer (accessed on 22 July 2010).
- Aiello, A.; Woerdman, J. Linear Algebra for Mueller Calculus. arXiv 2004, arXiv:Math-ph/0412061. Available online: https://arxiv.org/abs/math-ph/0412061 (accessed on 17 December 2004).
- Gil, J.J. Review on Mueller matrix algebra for the analysis of polarimetric measurements. J. Appl. Rem. Sens. 2014, 8, 081599. [Google Scholar] [CrossRef]
- Ding, H.; Lu, J.Q.; Brock, R.S.; McConnell, T.J.; Ojeda, J.F.; Jacobs, K.M.; Hu, X.H. Angle-resolved Mueller matrix study of light scattering by B-cells at three wavelengths of 442, 633, and 850 nm. J. Biomed. Opt. 2007, 12, 034032. [Google Scholar] [CrossRef]
- Van de Merwe, W.; Li, Z.Z.; Bronk, B.V.; Czégé, J. Polarized light scattering for rapid observation of bacterial size changes. Biophys. J. 1997, 73, 500–506. [Google Scholar] [CrossRef][Green Version]
- Le Gratiet, A.; Marongiu, R.; Diaspro, A. Circular Intensity Differential Scattering for Label-Free Chromatin Characterization: A Review for Optical Microscopy. Polymers 2020, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.L.; Aptowicz, K.; Arnold, J.; Cheng, S.; Kalume, A.; Piedra, P.; Wang, C.; Santarpia, J.; Videen, G. Review of elastic light scattering from single aerosol particles and application in bioaerosol detection. J. Quant. Spectrosc. Radiat. Transf. 2022, 279, 108067. [Google Scholar] [CrossRef]
- Neuman, M. Angle Resolved Light Scattering in Turbid Media: Analysis and Applications. Ph.D. Thesis, Mid Sweden University, Härnösand, Sweden, 2011. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:miun:diva-13154 (accessed on 25 January 2011).
- Fanjul-Velez, F.; Samperio-Garcia, D.; Pereda-Cubian, D.; Arce-Diego, J.L. Mueller matrix group theory Formalism for tissue imaging polarimetry contrast increase. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007. [Google Scholar] [CrossRef]
- Ramella-Roman, J.C.; Prahl, S.A.; Jacques, S.L. Three Monte Carlo programs of polarized light transport into scattering media: Part I. Opt. Express 2005, 13, 4420–4438. [Google Scholar] [CrossRef] [PubMed]
- Ramella-Roman, J.C.; Prahl, S.A.; Jacques, S.L. Three Monte Carlo programs of polarized light transport into scattering media: Part II. Opt. Express 2005, 13, 10392–10405. [Google Scholar] [CrossRef]
- Wang, X.; Yao, G.; Wang, L.V. Monte Carlo model and single-scattering approximation of the propagation of polarized light in turbid media containing glucose. Appl. Opt. 2002, 41, 792–801. [Google Scholar] [CrossRef]
- Wang, L.F. Monte Carlo Simulation Model for Electromagnetic Scattering from Vegetation and Inversion of Vegetation Parameters. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2007. Available online: http://hdl.handle.net/1721.1/38923 (accessed on 28 September 2007).
- Tuchin, V.V.; Zhu, D.; Genina, E.A. (Eds.) Handbook of Tissue Optical Clearing: New Prospects in Optical Imaging; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Lu, S.; Chipman, R. Interpretation of Mueller matrices based on polar decomposition. J. Opt. Soc. Am. A 1996, 13, 1106–1113. [Google Scholar] [CrossRef]
- Shindo, Y.; Oda, Y.; Oshima, A.; Maeda, S. New type of CD spectropolarimeter with LD option. Rev. Sci. Instrum. 1993, 64, 1161–1168. [Google Scholar] [CrossRef]
- Garcia-Caurel, E.; De Martino, A.; Drévillon, B. Spectroscopic Mueller polarimeter based on liquid crystal devices. Thin Solid Film 2004, 455–456, 120–123. [Google Scholar] [CrossRef]
- Dubreuil, M.; Rivet, S.; Le Jeune, B.; Cariou, J. Snapshot Mueller matrix polarimeter by wavelength polarization coding. Opt. Express 2007, 15, 13660–13668. [Google Scholar] [CrossRef]
- Protsenko, E.D.; Tymper, S.I.; Shkirin, A.V. Automated laser IR spectropolarimeter for surface Mueller matrix measurements. Instrum. Exp. Tech. 2011, 51, 268–274. [Google Scholar] [CrossRef]
- Bueno, J.M. Polarimetry using liquid-crystal variable retarders: Theory and calibration. J. Opt. A Pure Appl. Opt. 2000, 2, 216–222. [Google Scholar] [CrossRef]
- Suárez-Bermejo, J.C.; de Sande, J.C.G.; Santarsiero, M.; Piquero, G. Mueller matrix polarimetry using full Poincaré beams. Opt. Lasers Eng. 2019, 122, 134–141. [Google Scholar] [CrossRef]
- Arteaga, O.; Freudenthal, J.; Wang, B.; Kahr, B. Mueller matrix polarimetry with four photoelastic modulators: Theory and calibration. Appl. Opt. 2012, 51, 6805–6817. [Google Scholar] [CrossRef] [PubMed]
- Bunkin, N.F.; Shkirin, A.V.; Kozlov, V.A.; Starosvetskiy, A.V. Laser Scattering in Water and Aqueous Solutions of Salts. In Proceedings of the SPIE—Society of Photo-Optical Instrumentation Engineers, Laser Applications in Life Sciences, Oulu, Finland, 24 November 2010; SPIE: Bellingham, WA, USA, 2010. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Shkirin, A.V.; Ninham, B.W.; Chirikov, S.N.; Chaikov, L.L.; Penkov, N.V.; Kozlov, V.A.; Gudkov, S.V. Shaking-Induced Aggregation and Flotation in Immunoglobulin Dispersions: Differences between Water and Water–Ethanol Mixtures. ACS Omega 2020, 5, 14689–14701. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Twardowski, M.; Hedley, J.D.; McFarland, M.; Nayak, A.R.; Moore, T. Optical backscattering and linear polarization properties of the colony forming cyanobacterium Microcystis. Opt. Express 2020, 28, 37149–37166. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Shi, Y.; Gu, H.; Jiang, H.; Liu, S. Metrology of Nanostructures by Tomographic Mueller-Matrix Scatterometry. Appl. Sci. 2018, 8, 2583. [Google Scholar] [CrossRef]
- Goldstein, D.H. Mueller matrix dual-rotating retarder polarimeter. Appl. Opt. 1992, 31, 6676–6683. [Google Scholar] [CrossRef]
- Dong, H.; Gong, Y.; Paulose, V.; Hong, M. Polarization state and Mueller matrix measurements in terahertz-time domain spectroscopy. Opt. Commun. 2009, 282, 3671–3675. [Google Scholar] [CrossRef]
- Kuroda, R.; Harada, T.; Shindo, Y. A solid-state dedicated circular dichroism spectrophotometer: Development and application. Rev. Sci. Instrum. 2001, 72, 3802–3810. [Google Scholar] [CrossRef]
- Pezzaniti, J.L.; Chipman, R.A. Mueller matrix imaging polarimetry. Opt. Eng. 1995, 34, 1558–1568. [Google Scholar] [CrossRef]
- Mujat, M.; Ferguson, R.D.; Iftimia, N. Mueller matrix microscopy. In Proceedings of the SPIE 8873, Polarization Science and Remote Sensing VI, San Diego, CA, USA, 27 September 2013. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Raele, M.P.; Izatt, J.A.; Amaral, M.M.; Dias Vieira, J.N.; Tuchin, V.V.; Zanardi de Freitas, A. Polarization sensitive and Mueller matrix OCT measurements and data analysis. In Proceedings of the SPIE—Society of Photo-Optical Instrumentation Engineers, Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XV, San Francisco, CA, USA, 24–26 January 2011; Fujimoto, J.G., Izatt, J.A., Tuchin, V.V., Eds.; SPIE: Bellingham, WA, USA, 2011. [Google Scholar] [CrossRef]
- Jiao, S.; Yu, W.; Stoica, G.; Wang, L. Optical-fiber-based Mueller optical coherence tomography. Opt. Lett. 2003, 28, 1206–1208. [Google Scholar] [CrossRef] [PubMed]
- Lundblad, J.R.; Laurance, M.; Goodman, R.H. Fluorescence polarization analysis of protein-DNA and protein-protein interactions. Mol. Endocrinol. 1996, 10, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Maji, K.; Saha, S.; Dey, R.; Ghosh, N.; Haldar, D. Mueller Matrix Fluorescence Spectroscopy for Probing Self-Assembled Peptide-Based Hybrid Supramolecular Structure and Orientation. J. Phys. Chem. C 2017, 121, 19519–19529. [Google Scholar] [CrossRef]
- Azzam, R.M.A.; Bashara, N.M. Ellipsometry and Polarized Light; North-Holland: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Laskarakis, A.; Logothetidis, S.; Pavlopoulou, E.; Gioti, M. Mueller matrix spectroscopic ellipsometry: Formulation and application. Thin Solid Films 2004, 455, 43–49. [Google Scholar] [CrossRef]
- Mendoza-Galván, A.; Muñoz-Pineda, E.; Ribeiro, S.J.L.; Santos, M.V.; Järrendahl, K.; Arwin, H. Mueller matrix spectroscopic ellipsometry study of chiral nanocrystalline cellulose films. J. Opt. 2018, 20, 024001. [Google Scholar] [CrossRef]
- Stabo-Eeg, F.; Kildemo, M.; Nerbo, I.; Lindgren, M. Well-conditioned multiple laser Mueller matrix ellipsometer. Opt. Eng. 2008, 47, 073604. [Google Scholar] [CrossRef]
- Deibler, L.L.; Smith, M.H. Measurement of the complex refractive index of isotropic materials with Mueller matrix polarimetry. Appl. Opt. 2001, 40, 3659–3667. [Google Scholar] [CrossRef]
- Furchner, A.; Walder, C.; Zellmeier, M.; Rappich, J.; Hinrichs, K. Broadband infrared Mueller-matrix ellipsometry for studies of structured surfaces and thin films. Appl. Opt. 2018, 57, 7895–7904. [Google Scholar] [CrossRef]
- Furchner, A.; Kratz, C.; Ogieglo, W.; Pinnau, I.; Rappich, J.; Hinrichs, K. Ultrasensitive broadband infrared 4 × 4 Mueller-matrix ellipsometry for studies of depolarizing and anisotropic thin films. J. Vac. Sci. Technol. B 2020, 38, 014003. [Google Scholar] [CrossRef]
- Den Boer, J.H.W.G. Spectroscopic Infrared Ellipsometry: Components, Calibration, and Application; Technische Universiteit: Eindhoven, The Netherlands, 1995. [Google Scholar] [CrossRef]
- Chen, C.; An, I.; Ferreira, G.M.; Podraza, N.J.; Zapien, J.A.; Collins, R.W. Multichannel Mueller matrix ellipsometer based on the dual rotating compensator principle. Thin Solid Films 2004, 455–456, 14–23. [Google Scholar] [CrossRef]
- Lee, J.; Koh, J.; Collins, R. Dual rotating-compensator multichannel ellipsometer: Instrument development for high-speed Mueller matrix spectroscopy of surfaces and thin films. Rev. Sci. Instrum. 2001, 72, 1742–1754. [Google Scholar] [CrossRef]
- Collins, R.; Koh, J. Dual rotating-compensator multichannel ellipsometer: Instrument design for real-time Mueller matrix spectroscopy of surfaces and films. J. Opt. Soc. Am. A 1999, 16, 1997–2006. [Google Scholar] [CrossRef]
- Chen, C.; Horn, M.; Pursel, S.; Ross, C.; Collins, R. The ultimate in real-time ellipsometry: Multichannel Mueller matrix spectroscopy. Appl. Surf. Sci. 2006, 253, 38–46. [Google Scholar] [CrossRef]
- Compain, E.; Drevillon, B. Complete high-frequency measurement of Mueller matrices based on a new coupled-phase modulator. Rev. Sci. Instrum. 1997, 68, 2671–2680. [Google Scholar] [CrossRef]
- Han, C.Y.; Chao, Y.F.; Tsai, H.M. Photoelastic Modulated Imaging Ellipsometry. In Ellipsometry—Principles and Techniques for Materials Characterization; Wahaia, F., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Hilfiker, J.; Herzinger, C.; Wagner, T.; Marino, A.; Delgais, G.; Abbate, G. Mueller-matrix characterization of liquid crystals. Thin Solid Films 2004, 455, 591–595. [Google Scholar] [CrossRef]
- Kupinski, M.; Li, L. Evaluating the Utility of Mueller Matrix Imaging for Diffuse Material Classification. J. Imaging Sci. Technol. 2020, 64, 60409. [Google Scholar] [CrossRef]
- Juárez-Rivera, O.R.; Mauricio-Sánchez, R.A.; Järrendahl, K.; Arwin, H.; Mendoza-Galván, A. Shear-Coated Linear Birefringent and Chiral Cellulose Nanocrystal Films Prepared from Non-Sonicated Suspensions with Different Storage Time. Nanomaterials 2021, 11, 2239. [Google Scholar] [CrossRef]
- Mendoza-Galván, A.; Li, Y.; Yang, X.; Magnusson, R.; Järrendahl, K.; Berglund, L.; Arwin, H. Transmission Mueller-matrix characterization of transparent ramie films. J. Vac. Sci. Technol. B 2020, 38, 014008. [Google Scholar] [CrossRef]
- Fricke, D.; Becker, A.; Jutte, L.; Bode, M.; de Cassan, D.; Wollweber, M.; Glasmacher, B.; Roth, B. Mueller Matrix Measurement of Electrospun Fiber Scaffolds for Tissue Engineering. Polymers 2019, 11, 2062. [Google Scholar] [CrossRef]
- Guo, X.; Wood, M.; Vitkin, A.I. Angular measurements of light scattered by turbid chiral media using linear Stokes polarimeter. J. Biomed. Opt. 2006, 11, 041105. [Google Scholar] [CrossRef]
- Svensen, Ø.; Stamnes, J.; Kildemo, M.; Aas, L.; Erga, S.; Frette, Ø. Mueller matrix measurements of algae with different shape and size distributions. Appl. Opt. 2011, 50, 5149–5157. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, M.; Babilotte, P.; Martin, L.; Sevrain, D.; Rivet, S.; Le Grand, Y.; Le Brun, G.; Turlin, B.; Le Jeune, B. Mueller matrix polarimetry for improved liver fibrosis diagnosis. Opt. Lett. 2012, 37, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.Q. Theoretical Modeling for Polarimetric Scattering and Information Retrieval of SAR Remote Sensing. In Advances in Geoscience and Remote Sensing; IntechOpen: London, UK, 2009. [Google Scholar] [CrossRef]
- Wu, X.; Calabia, A.; Xu, J.; Bai, W.; Guo, P. Forest canopy scattering properties with signal of opportunity reflectometry: Theoretical simulations. Geosci. Lett. 2021, 8, 25. [Google Scholar] [CrossRef]
- Swamy, J.N.; Crofcheck, C.; Mengüç, M.P. Time dependent scattering properties of slow decaying liquid foams. Colloids Surf. A Physicochem. Eng. Asp. 2009, 338, 80–86. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Molecular Probes Handbook. Fluorescence Polarization (FP). Available online: https://www.thermofisher.com/ru/ru/home/references/molecular-probes-the-handbook/technical-notes-and-product-highlights/fluorescence-polarization-fp.html (accessed on 18 July 2017).
- Chami, M. Importance of the polarization in the retrieval of oceanic constituents from the remote sensing reflectance. J. Geophys. Res. 2007, 112, C05026. [Google Scholar] [CrossRef]
- Ahmad, I.; Gribble, A.; Murtza, I.; Ikram, M.; Pop, M.; Vitkin, A. Polarization image segmentation of radiofrequency ablated porcine myocardial tissue. PLoS ONE 2017, 12, e0175173. [Google Scholar] [CrossRef]
- Dong, H.; Shum, P.; Gong, Y.D.; Yan, M.; Zhou, J.Q.; Wu, C.Q. Virtual Generalized Mueller Matrix Method for Measurement of Complex Polarization-Mode Dispersion Vector in Optical Fibers. IEEE Photonics Technol. Lett. 2007, 19, 27–29. [Google Scholar] [CrossRef][Green Version]
- Dong, H.; Shum, P.; Yan, M.; Zhou, J.Q.; Ning, G.X.; Gong, Y.D.; Wu, C.Q. Measurement of Mueller matrix for an optical fiber system with birefringence and polarization-dependent loss or gain. Opt. Commun. 2007, 274, 116–123. [Google Scholar] [CrossRef]
- Qi, J.; Elson, D.S. Mueller polarimetric imaging for surgical and diagnostic applications: A review. J. Biophotonics 2017, 10, 950–982. [Google Scholar] [CrossRef]






| Sample Type | Methods | Sample Properties | References |
|---|---|---|---|
| Organic materials and molecular systems | MM ellipsometry and Transmission MMP | Complex refractive index, film thickness, | [93,97] |
| Anisotropy, orientation of polymer molecules and fibers | [8,94,95,107,108,109,110,111] | ||
| MM scatterometry, Transmission MMP and MM fluorometry | Structure and size of molecular aggregates | [64,81] | |
| Chirality, molecular structure and orientation | [8,12,91,92,112] | ||
| MM IR spectroscopy | Spectral properties, composition | [97,98,99,100] | |
| Cell and microbiological systems | MM scatterometry | Size and shape of cells and microorganisms, | [16,61,62,82,113] |
| structural changes in DNA | [16,63,64] | ||
| MM imaging polarimetry | Distinguishing colonies | [31] | |
| Tissues | MM-OCT and MM imaging polarimetry, MM fluorometry | Optical polarization properties, | [20,32,33,34] |
| microstructural characteristics, | [27,54] | ||
| cell properties, | [56,88] | ||
| pathology detection, | [24,25,28,29,30,50,114] | ||
| microbial contamination | [26] | ||
| Milk | MM scatterometry | Size distribution, | [35,36] |
| fat and protein content | |||
| Vegetable oils | Transmission MMP | Optical polarization properties, | [40,41] |
| authenticity | |||
| Meat | MM imaging polarimetry | Freshness assessment | [42] |
| Plants | MM spectopolarimetry Microwave MMP | Chlorophyll organization | [43] |
| Vegetation | [70,115,116] | ||
| Soils | MM scatterometry | Particle size and optical properties | [46] |
| Microwave MMP | Moisture content, surface roughness | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatenko, D.N.; Shkirin, A.V.; Lobachevsky, Y.P.; Gudkov, S.V. Applications of Mueller Matrix Polarimetry to Biological and Agricultural Diagnostics: A Review. Appl. Sci. 2022, 12, 5258. https://doi.org/10.3390/app12105258
Ignatenko DN, Shkirin AV, Lobachevsky YP, Gudkov SV. Applications of Mueller Matrix Polarimetry to Biological and Agricultural Diagnostics: A Review. Applied Sciences. 2022; 12(10):5258. https://doi.org/10.3390/app12105258
Chicago/Turabian StyleIgnatenko, Dmitry N., Alexey V. Shkirin, Yakov P. Lobachevsky, and Sergey V. Gudkov. 2022. "Applications of Mueller Matrix Polarimetry to Biological and Agricultural Diagnostics: A Review" Applied Sciences 12, no. 10: 5258. https://doi.org/10.3390/app12105258
APA StyleIgnatenko, D. N., Shkirin, A. V., Lobachevsky, Y. P., & Gudkov, S. V. (2022). Applications of Mueller Matrix Polarimetry to Biological and Agricultural Diagnostics: A Review. Applied Sciences, 12(10), 5258. https://doi.org/10.3390/app12105258







