Synthesis of Gold-PVP Nanostructured Composites by Microplasma: A Test to Study Their Inhibiting Tendency of Avian Influenza Virus Activity
Abstract
:1. Introduction
2. Materials and Method
2.1. Synthesis of Gold Nanostructured Particles and Gold-PVP Nanostructured Composites
2.2. Investigations of Antiviral Activity
Hemagglutination Test (HA)
3. Results and Discussions
3.1. X-rays Diffraction (XRD) Analyses
3.2. SEM Analysis
3.3. Dynamic Light Scattering (DLS) Analysis
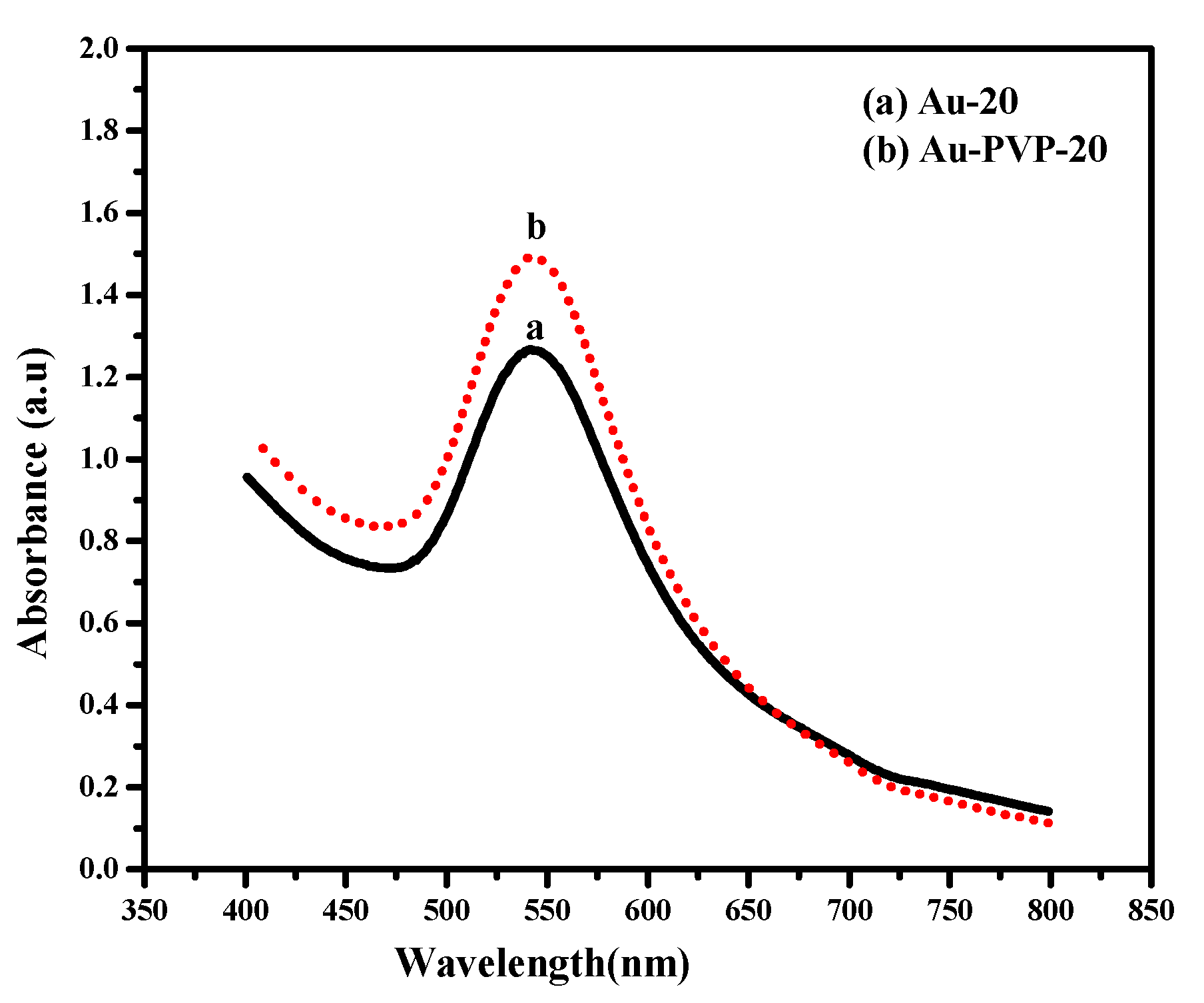
3.4. UV-VIS Spectroscopic Analysis
3.5. Antiviral Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wieczorek, K.; Szutkowska, B.; Kierzek, E. Anti-influenza strategies based on nanoparticle applications. Pathogens 2020, 9, 1020. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.H. Summary of avian influenza activity in Europe, Asia, and Africa, 2006–2009. Avian Dis. 2010, 54, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Peacock, T.T.P.; James, J.; Sealy, J.E.; Iqbal, M. A global perspective on H9N2 avian influenza virus. Viruses 2019, 11, 620. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.-H.; Song, H. Antiviral potential of nanoparticles—Can nanoparticles fight against coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dou, J.; Teng, Z.; Yu, J.; Wang, T.; Lu, N.; Wang, H.; Zhou, C. Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Arch. Virol. 2014, 159, 3269–3278. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Zheng, Y.; Duan, W.; Li, X.; Yin, J.; Shigdar, S.; O’Connor, M.L.; Marappan, M.; Zhao, X.; Miao, Y. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 4103. [Google Scholar] [CrossRef] [Green Version]
- Xiang, D.-X.; Chen, Q.; Pang, L.; Zheng, C.-L. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J. Virol. Methods 2011, 178, 137–142. [Google Scholar] [CrossRef]
- Draz, M.S.; Shafiee, H. Applications of gold nanoparticles in virus detection. Theranostics 2018, 8, 1985. [Google Scholar] [CrossRef]
- Kim, J.; Yeom, M.; Lee, T.; Kim, H.-O.; Na, W.; Kang, A.; Lim, J.-W.; Park, G.; Park, C.; Song, D. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnology 2020, 18, 54. [Google Scholar] [CrossRef] [Green Version]
- Papp, I.; Sieben, C.; Ludwig, K.; Roskamp, M.; Böttcher, C.; Schlecht, S.; Herrmann, A.; Haag, R. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small 2010, 6, 2900–2906. [Google Scholar] [CrossRef]
- Gupta, I.; Duran, N.; Rai, M. Nano-silver toxicity: Emerging concerns and consequences in human health. In Nano-Antimicrobials; Springer: Berlin/Heidelberg, Germany, 2012; pp. 525–548. [Google Scholar]
- Kumar, H.; Venkatesh, N.; Bhowmik, H.; Kuila, A. Metallic nanoparticle: A review. Biomed. J. Sci. Tech. Res. 2018, 4, 3765–3775. [Google Scholar]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Basak, P.; Das, P.; Biswas, S.; Biswas, N.C.; Mahapatra, G.K.D. Green synthesis and characterization of gelatin-PVA silver nanocomposite films for improved antimicrobial activity. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Suzhou, China, 22–24 June 2018; p. 012019. [Google Scholar]
- Dhakal, T.R.; Mishra, S.R.; Glenn, Z.; Rai, B.K. Synergistic effect of PVP and PEG on the behavior of silver nanoparticle-polymer composites. J. Nanosci. Nanotechnol. 2012, 12, 6389–6396. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, V.; Varunkumar, K.; Ravikumar, V.; Rajaram, R. Target delivery of doxorubicin tethered with PVP stabilized gold nanoparticles for effective treatment of lung cancer. Sci. Rep. 2018, 8, 3815. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Shamim, A.; Ahmad, Z.; Mahmood, T.; Nawaz, F.; Asghar, F.; Naheed, S.; Ajmal, M. Synthesis of metallic nanoparticles by physical, chemical and biological methods and their characterization. Cell 2009, 92, 317-5051024. [Google Scholar]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Thong, Y.L.; Chin, O.H.; Ong, B.H.; Huang, N.M. Synthesis of silver nanoparticles prepared in aqueous solutions using helium dc microplasma jet. Jpn. J. Appl. Phys. 2015, 55, 01AE19. [Google Scholar] [CrossRef]
- Huang, X.; Zhong, X.; Lu, Y.; Li, Y.; Rider, A.; Furman, S.; Ostrikov, K. Plasmonic Ag nanoparticles via environment-benign atmospheric microplasma electrochemistry. Nanotechnology 2013, 24, 095604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Mariotti, D.; Sankaran, R.M. Microplasmas for nanomaterials synthesis. J. Phys. D Appl. Phys. 2010, 43, 323001. [Google Scholar] [CrossRef]
- Bisht, A.; Roshan Deen, G.; Ilyas, U. Synthesis of nanoparticles using atmospheric microplasma discharge. In Proceedings of the International Conference on the Frontiers of Plasma Physics and Technology, Singapore, 18–22 April 2011. [Google Scholar]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Teodorescu, M.; Bercea, M. Poly (vinylpyrrolidone)—A versatile polymer for biomedical and beyond medical applications. Polym.-Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Larez, J.; Castell, R.; Rojas, C. Colloids and composite materials Au/PVP and Ag/PVP generated by laser ablation in polymeric liquid environment. Rev. Mex. de Física 2016, 62, 188–192. [Google Scholar]
- Das, T.; Kolli, V.; Karmakar, S.; Sarkar, N. Functionalisation of polyvinylpyrrolidone on gold nanoparticles enhances its anti-amyloidogenic propensity towards hen egg white lysozyme. Biomedicines 2017, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Kim, H.; Song, J.; Oh, H.; Song, H.; Jang, J. Synthesis of silver nanoparticles decorated polypyrrole nanotubes for antimicrobial application. Macromol. Res. 2012, 20, 1096–1101. [Google Scholar] [CrossRef]
- Kim, F.; Connor, S.; Song, H.; Kuykendall, T.; Yang, P. Platonic gold nanocrystals. Angew. Chem. Int. Ed. 2004, 43, 3673–3677. [Google Scholar] [CrossRef] [PubMed]
- Pillai, Z.S.; Kamat, P.V. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J. Phys. Chem. B 2004, 108, 945–951. [Google Scholar] [CrossRef]
- Hoppe, C.E.; Lazzari, M.; Pardinas-Blanco, I.; López-Quintela, M.A. One-step synthesis of gold and silver hydrosols using poly (N-vinyl-2-pyrrolidone) as a reducing agent. Langmuir 2006, 22, 7027–7034. [Google Scholar] [CrossRef] [PubMed]
- Baygazieva, E.; Yesmurzayeva, N.; Tatykhanova, G.; Mun, G.; Khutoryanskiy, V.; Kudaibergenov, S. Polymer protected gold nanoparticles: Synthesis, characterization and application in catalysis. Int. J. Biol. Chem. 2014, 7, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Cortez-Lemus, N.A.; Licea-Claverie, A.; Paraguay-Delgado, F.; Alonso-Nuñez, G. Gold nanoparticles size design and control by poly (N,N′-diethylaminoethyl methacrylate). J. Nanomater. 2015, 16, 270. [Google Scholar] [CrossRef] [Green Version]
- Dkhilalli, F.; Megdiche Borchani, S.; Rasheed, M.; Barille, R.; Shihab, S.; Guidara, K.; Megdiche, M. Characterizations and morphology of sodium tungstate particles. R. Soc. Open Sci. 2018, 5, 172214. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, M.; Barillé, R. Comparison the optical properties for Bi2O3 and NiO ultrathin films deposited on different substrates by DC sputtering technique for transparent electronics. J. Alloys Compd. 2017, 728, 1186–1198. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Y.; Sun, G.; Zhang, G.; Liu, H.; Du, J.; Yang, S.; Bai, J.; Yang, Q. Fabrication of Au/PVP nanofiber composites by electrospinning. J. Appl. Polym. Sci. 2007, 105, 3618–3622. [Google Scholar] [CrossRef]
- Alexander, D.; Chettle, N. Procedures for the haemagglutination and the haemagglutination inhibition tests for avian infectious bronchitis virus. Avian Pathol. 1977, 6, 9–17. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Babaei, A.; Mousavi, S.M.; Ghasemi, M.; Pirbonyeh, N.; Soleimani, M.; Moattari, A. Gold nanoparticles show potential in vitro antiviral and anticancer activity. Life Sci. 2021, 284, 119652. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.; Ataei-Pirkooh, A.; Mm Sadeghi, G.; Bokharaei-Salim, F.; Sahrapour, P.; Kiani, S.J.; Moghoofei, M.; Farahmand, M.; Javanmard, D.; Monavari, S.H. Polyethylene glycol-coated zinc oxide nanoparticle: An efficient nanoweapon to fight against herpes simplex virus type 1. Nanomedicine 2018, 13, 2675–2690. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.; Hongo, S.; Kaverin, N.; Kochs, G.; Lamb, R.; Matrosovich, M.; Perez, D.; Palese, P.; Presti, R.; Rimstad, E. Family orthomyxoviridae. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2012; pp. 749–761. [Google Scholar]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Boopathi, S.; Senthilkumar, S.; Phani, K.L. Facile and one pot synthesis of gold nanoparticles using tetraphenylborate and polyvinylpyrrolidone for selective colorimetric detection of mercury ions in aqueous medium. J. Anal. Methods Chem. 2012, 2012, 348965. [Google Scholar] [CrossRef] [PubMed]
- Sametband, M.; Shukla, S.; Meningher, T.; Hirsh, S.; Mendelson, E.; Sarid, R.; Gedanken, A.; Mandelboim, M. Effective multi-strain inhibition of influenza virus by anionic gold nanoparticles. MedChemComm 2011, 2, 421–423. [Google Scholar] [CrossRef]
- Paradowska, E.; Studzińska, M.; Jabłońska, A.; Lozovski, V.; Rusinchuk, N.; Mukha, I.; Vitiuk, N.; Leśnikowski, Z.J. Antiviral effect of nonfunctionalized gold nanoparticles against herpes simplex virus type-1 (HSV-1) and possible contribution of near-field interaction mechanism. Molecules 2021, 26, 5960. [Google Scholar] [CrossRef] [PubMed]
- Bastian, A.R.; Nangarlia, A.; Bailey, L.D.; Holmes, A.; Sundaram, R.V.K.; Ang, C.; Moreira, D.R.; Freedman, K.; Duffy, C.; Contarino, M. Mechanism of multivalent nanoparticle encounter with HIV-1 for potency enhancement of peptide triazole virus inactivation. J. Biol. Chem. 2015, 290, 529–543. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubair, M.; Rafique, M.S.; Khalid, A.; Yaqub, T.; Alomar, S.Y.; Gohar, H. Synthesis of Gold-PVP Nanostructured Composites by Microplasma: A Test to Study Their Inhibiting Tendency of Avian Influenza Virus Activity. Appl. Sci. 2022, 12, 5352. https://doi.org/10.3390/app12115352
Zubair M, Rafique MS, Khalid A, Yaqub T, Alomar SY, Gohar H. Synthesis of Gold-PVP Nanostructured Composites by Microplasma: A Test to Study Their Inhibiting Tendency of Avian Influenza Virus Activity. Applied Sciences. 2022; 12(11):5352. https://doi.org/10.3390/app12115352
Chicago/Turabian StyleZubair, Muhammad, Muhammad Shahid Rafique, Afshan Khalid, Tahir Yaqub, Suliman Yousef Alomar, and Huma Gohar. 2022. "Synthesis of Gold-PVP Nanostructured Composites by Microplasma: A Test to Study Their Inhibiting Tendency of Avian Influenza Virus Activity" Applied Sciences 12, no. 11: 5352. https://doi.org/10.3390/app12115352





