Abstract
To date, the term peri-implantitis has been mostly associated with bacterial or foreign body reaction as primary factors of its development. Because of this, researchers’ and clinicians’ attention regarding treatment possibilities were directed into the solutions on the basis of surface modifications, debridement, and antibiotics. After years of clinical observations and poor results in treatment of peri-implantitis, a new proposal of this condition is presented, shifting our way of thinking regarding bone and implant interactions. In the second part of the paper presenting a new definition of peri-implantitis, we focused on a biological explanation of the bone behavior at the bone–implant interface. The main conclusion is that PI is not an “infectious disease”, but rather the result of natural changes of the bone’s morphology in response to implant such as a decrease in convexity of the outer surface of the bone and subsequently a decrease in concavity of the inner bone.
1. Introduction
Historically, peri-implantitis (originally coined by Levignac et al. [1]) became an accepted term at the first European Workshop on Periodontology in 1993. The term peri-implantitis (PI) was then agreed upon as a general name for destructive peri-implant inflammatory processes. Accordingly, with a progression of this inflammation, significant bone loss was observed, which is a result of an unfavorable disruption of the balance between the foreign body response and host internal immunological response factors [2]. It means that if a purulent infection is present, microorganisms may be involved but not necessarily cause marginal bone resorption, as pointed out by Mombelli and Décaillet, who concluded that “microorganisms are involved in the disease process. However, this is not a proof that they are always the origin of the condition” [3].
In 1987, in the study by Mombelli et al., PI was first described as an infectious disease with many features common to periodontitis and, on the basis of their findings, it was suggested that “periimplantitis can be regarded as a site-specific infection in which microbial pathogens, mainly belonging to the group of gram-negative anaerobic rods, may play an important role” [4]. Since then, a growing interest in defining peri-implant disease as a clinical entity and proposing a treatment approach for it has been observed. The multifaceted etiology and varied characteristics of the disease, however, from the clinical perspective resulted in lack of consensus in defining peri-implant disease (more than 33 definitions) and its treatment. Peri-implantitis is one of the most burning issues that modern dental implantology has been facing recently. After more than 30 years since the first description of this “disease”, its causes and proper treatment protocols are unknown. Recently published papers indicate that peri-implant microbiota present a different bacterial ecosystem compared to the periodontal microbiota [5,6], as well as critical histopathological differences between periodontitis and peri-implantitis [7]. As a result, the researchers have become interested in looking for immunological mechanisms that may play the key role in peri-implantitis development [8].
According to the literature, the prevalence of peri-implantitis ranges from 1.1% to 85% [9]. This means that if every year, on average, 12 million implants are placed—in the worst case scenario—there are more than 10 million patients facing this problem every year and they cannot be successfully treated [10].
2. Physio-Biological Explanation of Clinical Observations
The outer layer of every bone consists of cortical bone. In other words, all our bones are surrounded by corticals, i.e., bones are areas with low metabolism and high mineralization [11,12]. Additional cortical-type bone areas inside the bones are found only under very selected circumstances, such as around bone cysts, in the vicinity of teeth (lamina cribrosa), and around osseo-integrated implants (as described in part I [13]). If, for example, due to sudden changes in the bone morphology the outer cortical of the bone becomes covered by an additional new bone of periosteal origin (through modelling), the old cortical will be removed from the other (inner) side through remodeling because the perfusion through the additional outer layer will not be enough to nourish additional thickness of the cortical. In cases of infection close to the bone, highly mineralized areas will be formed under the periosteum or even inside the jaw bones. Moreover, in cases of progressing periodontal disease, highly mineralized barrier layers form below that apex of the involved teeth [12]. A functional explanation states that once the original cortical bone is lost due to peri-implantitis, the remaining apical trabecular bone is reinforced and transformed into cortical bone that might take over the functional load [13].
Such highly mineralized bone layers in some cases may later lead to the atrophy of the outer layer (old outer cortical, OC), as will be presented in this paper.
All corticals are surrounded by outer (periost) and inner (endost) membranes. The purpose of the periosteum is to form a reliable barrier against high levels of oxygen and blood supply as it is typically given in the soft tissues and to regulate the inflow of blood through the vessels of the haversian canals [14]. Whenever the periost is missing between bone tissue and soft tissues, granulation is the result. As previously pointed out, the term “osseointegration” is not synonymous with “bonding”, and the word only describes direct contact between the living bone and the endosseous implant surface [15]. Hence, if the convexity changes in the coupling area between outer cortical (OC) and inner cortical (IC), no “dissolving chemical activity” is required—a process that consists of “normal” bone resorption and bone apposition (inside) is observed [16]. As in the case of periodontitis and the associated granulation, no periosteum is formed in the gap between the exfoliating implant and the retracting bone during the development of PI [17]. In this context, it has to be mentioned that if osteons tunnel along implant surfaces and consolidate (mineralized) layers, the outer layer of each osteon remains (at least initially) non-mineralized [18]. This envelope around the osteons is one of the reasons why (cortical) bone shows elasticity, and it is called the “cement line”. Osteons can even be pulled out of their envelope through the tension, which is called the “osteonal pulled-out” effect [19,20]. As a result, the bone’s elasticity vanishes in the vicinity of stiff endosseous implant designs (especially once the implants are functionally loaded and mineralization increases along the IC) [21].
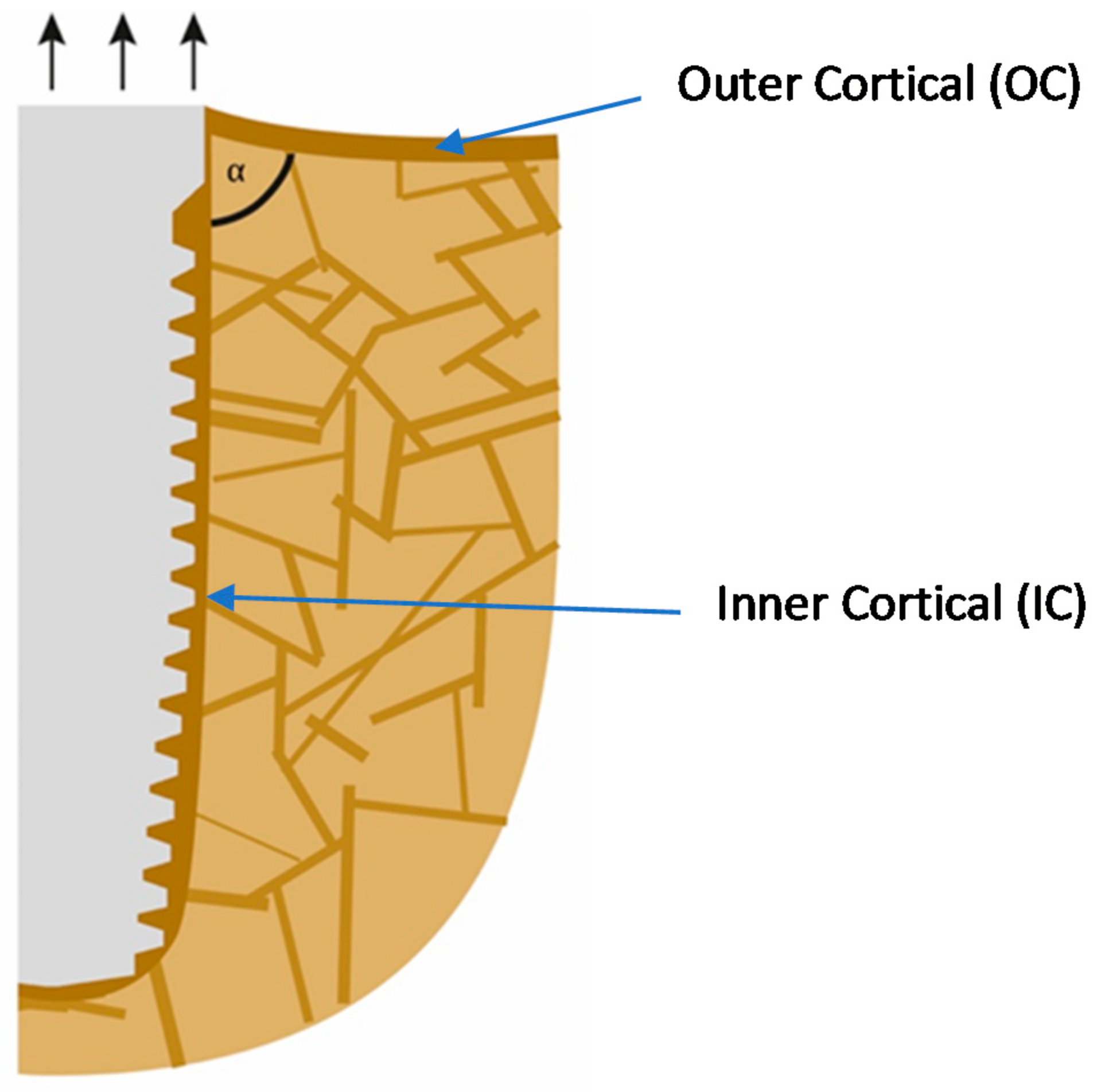
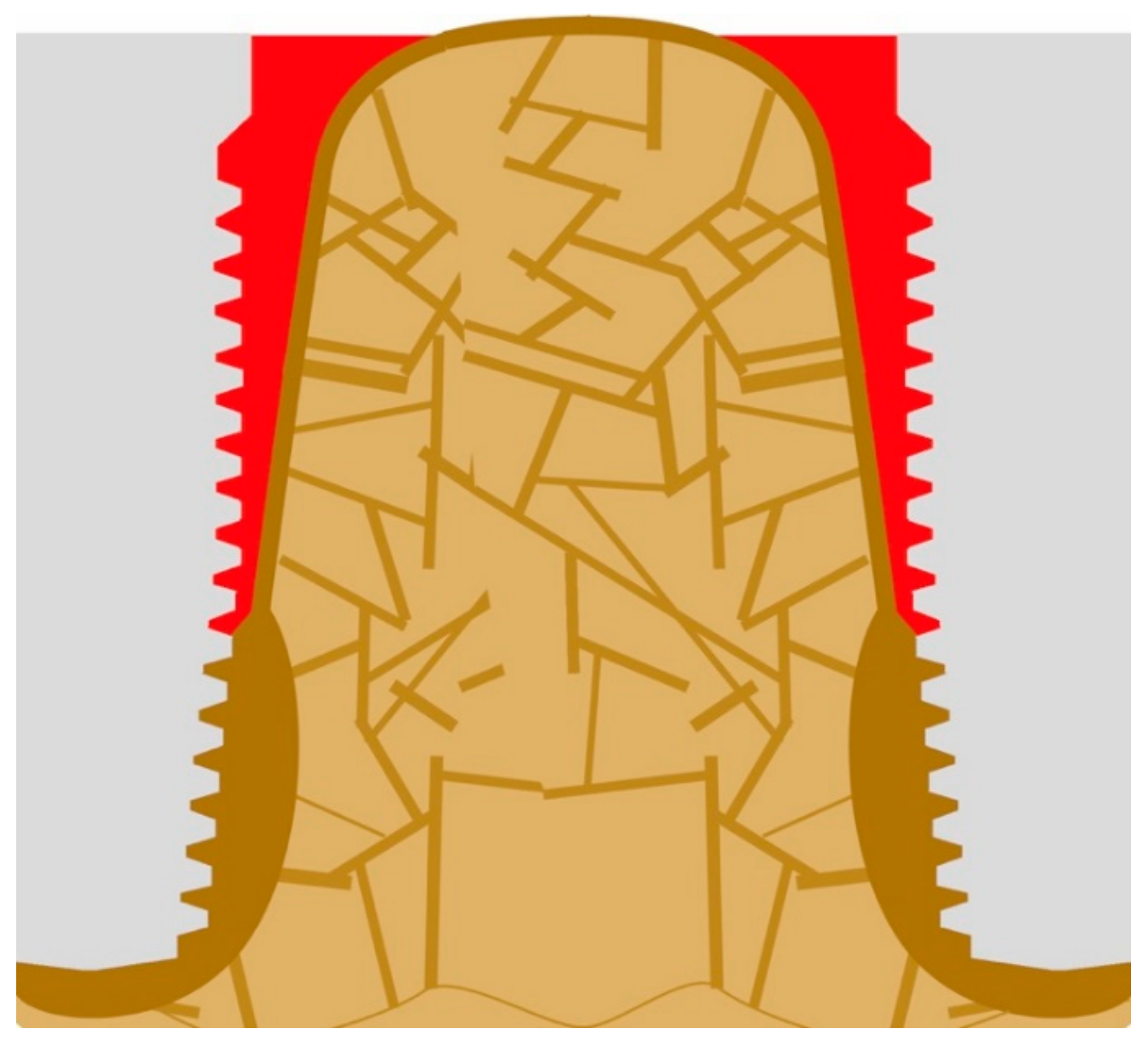
If implants are loaded predominantly on extrusion, as shown in Figure 1 (i.e., on the non-chewing side of the implant-supported prosthodontics), the inter-cortical angle becomes rather sharper, and this leads to the resorption on the outside of the cortical. In addition, bone areas under pull become osteoporotic as a consequence of incomplete remodeling and checkerboarding (it may be referred to as a localized disuse-atrophy) and, additionally, the corticals (IC and OC) become thin. Deformation in the direction of the occlusal surface starts to occur, and thereby the angle will become “sharper”, i.e., the convexity becomes even larger. It has to be noted that after the IC is mechanically coupled to the OC, the whole assembly is considered by the body as an outer cortical. Both corticals become functionally one cortical. Only after this occurs, the angle between OC and IC becomes significant and influences the behavior of the bone.
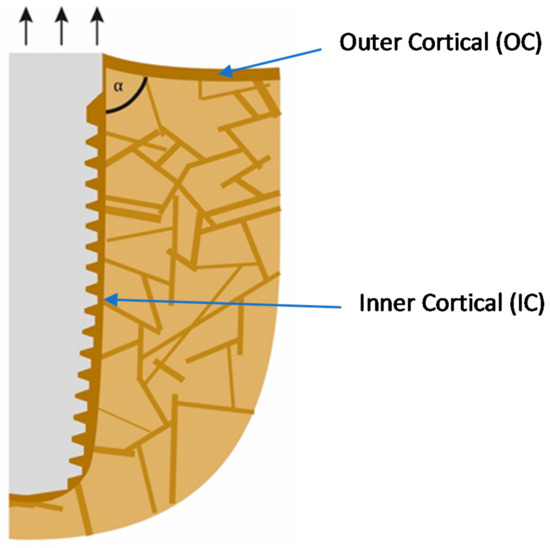
Figure 1.
Extrusion forces working on the implant lead to a sharper angle between the outer and the inner corticals.
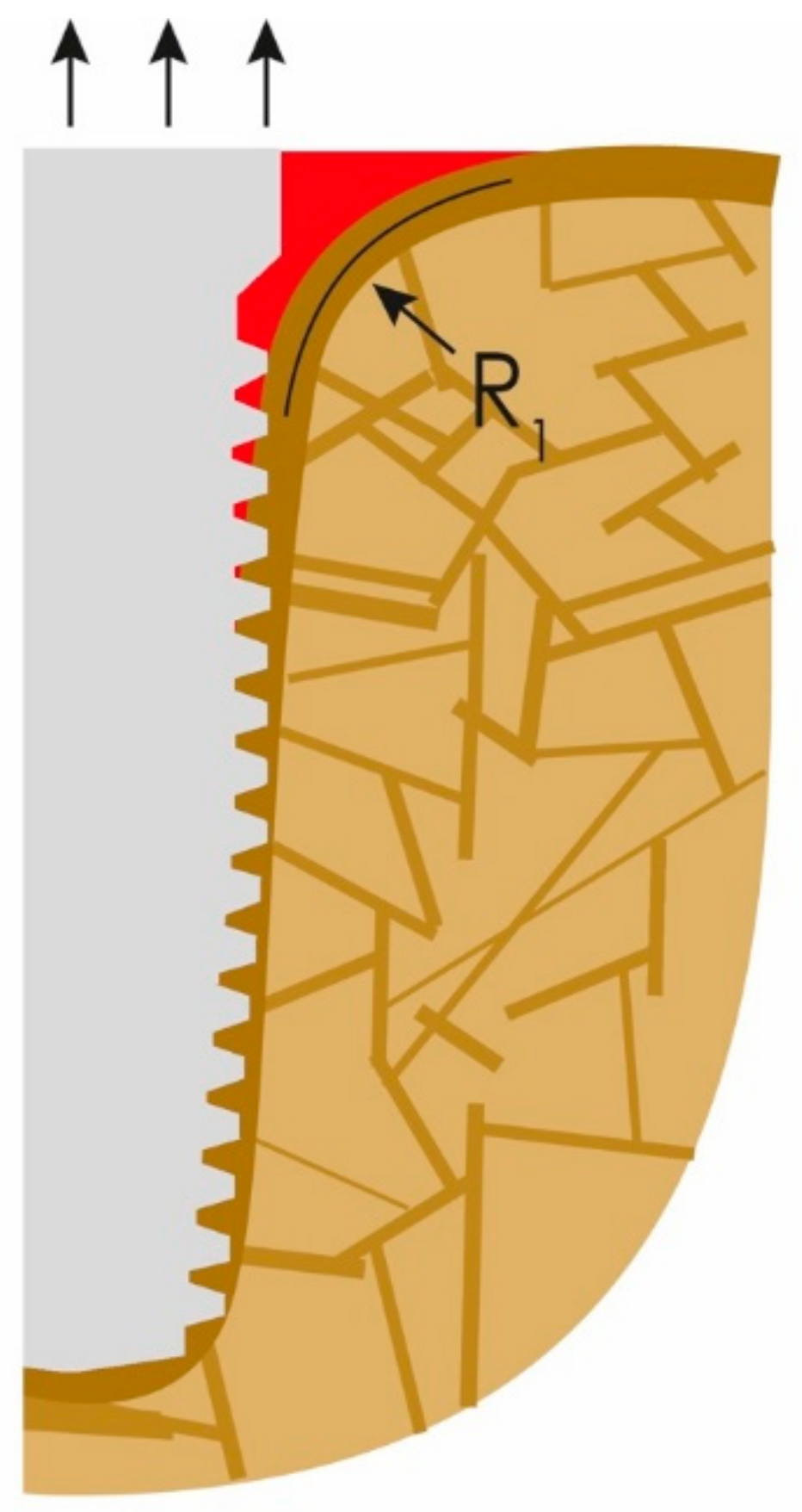
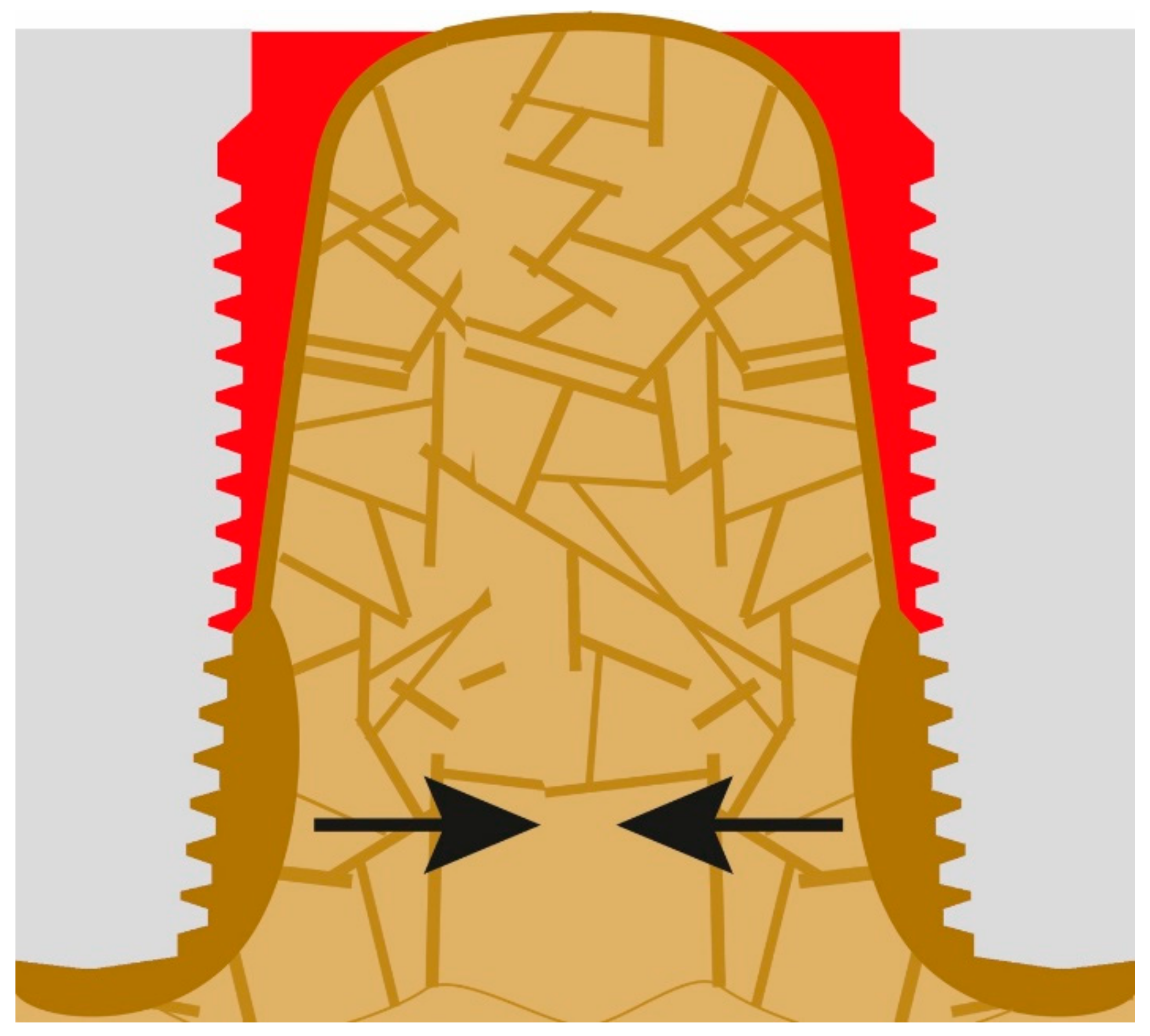
What clinicians in the dental field consider to be the onset of PI is nothing else than normal bone reaction to an unacceptable convexity in the junction area between the IC and OC. This angle and subsequent change in morphology occurs all around the implants (figures show only the two-dimensional aspect), and hence a crater forms. Instead of a sharp angle, the corticals now form an acceptable radius “R1” presented in Figure 2. Immediate (opportunistic) bacterial colonialization of the exposed rough implant surface leads to the development of signs of the infection with all the inflammation symptoms, which, in turn, leads to diagnosing this condition as “PI”. In other words, the infection is the result of the morphological changes of the bone and the roughness of the implant surface. Subsequently, during function, the load transmission zone between the bone and the implant from now on slips downwards towards the apex of the implant.
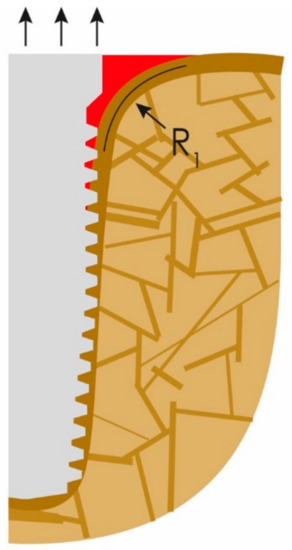
Figure 2.
The onset of peri-implantitis: the crestal cortical detaches from the implant’s surface and the angle (α) (Figure 1) changes to a radius (R1).
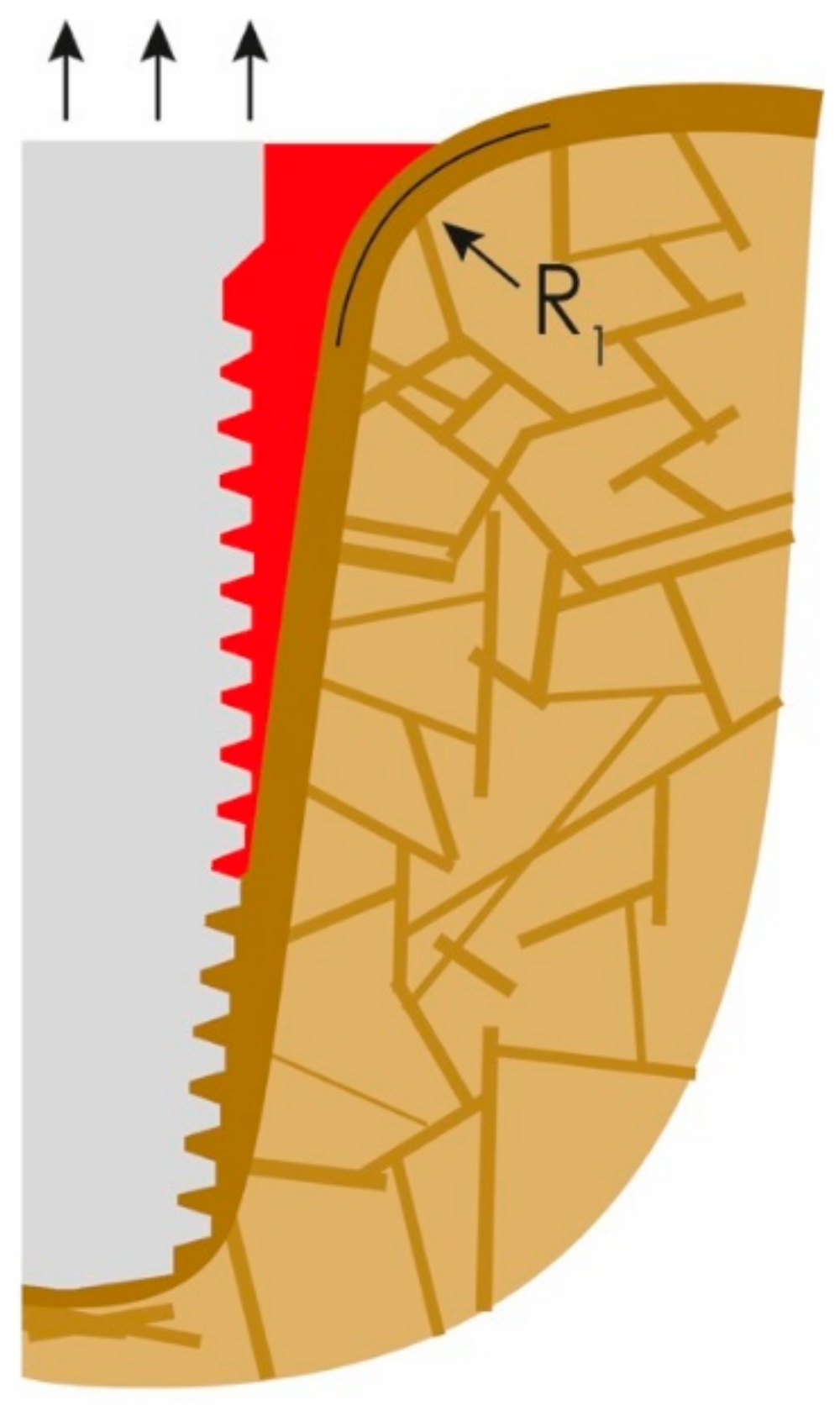
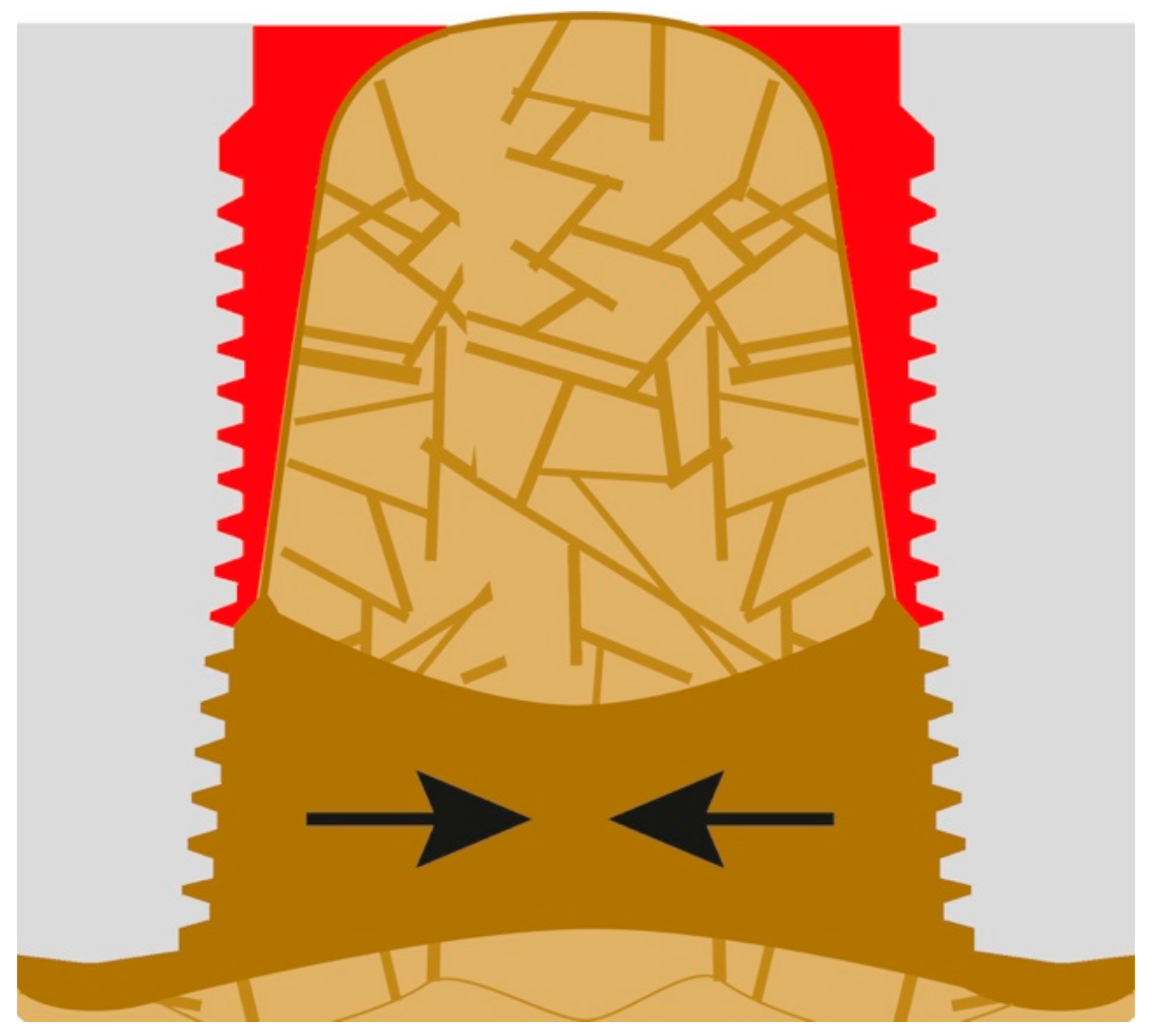
The bone-to-implant contact (BIC) is then further reduced until the soft-tissue front reaches resorption stable bone areas. At this stage, quite a bit of bone is resorbed and, as a result, masticatory forces in the interface to the bone increase due to the created lever. In other words, the magnitude and distribution of the stress transferred to the peri-implant bone correlate with the bone loss [22]. In the case of advanced loss of the cortical bone, the remaining peri-implant trabecular bone is subjected to masticatory forces, so it will be reinforced as functional adaptation [23]. As a result of the above and chronic infection in some cases, horizontal bone apposition can be observed (Figure 3). The three black arrows in Figure 3 show the dominating extrusive forces.
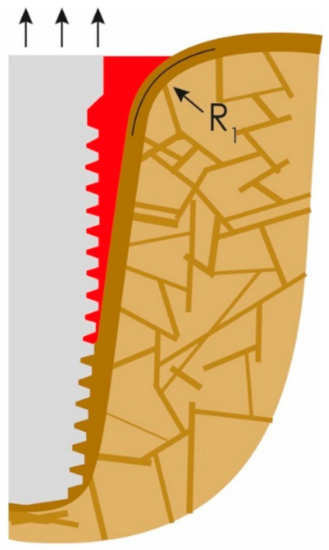
Figure 3.
The loss of attachment progresses, PI slips deeper until more mineralized “basal” bone is reached.
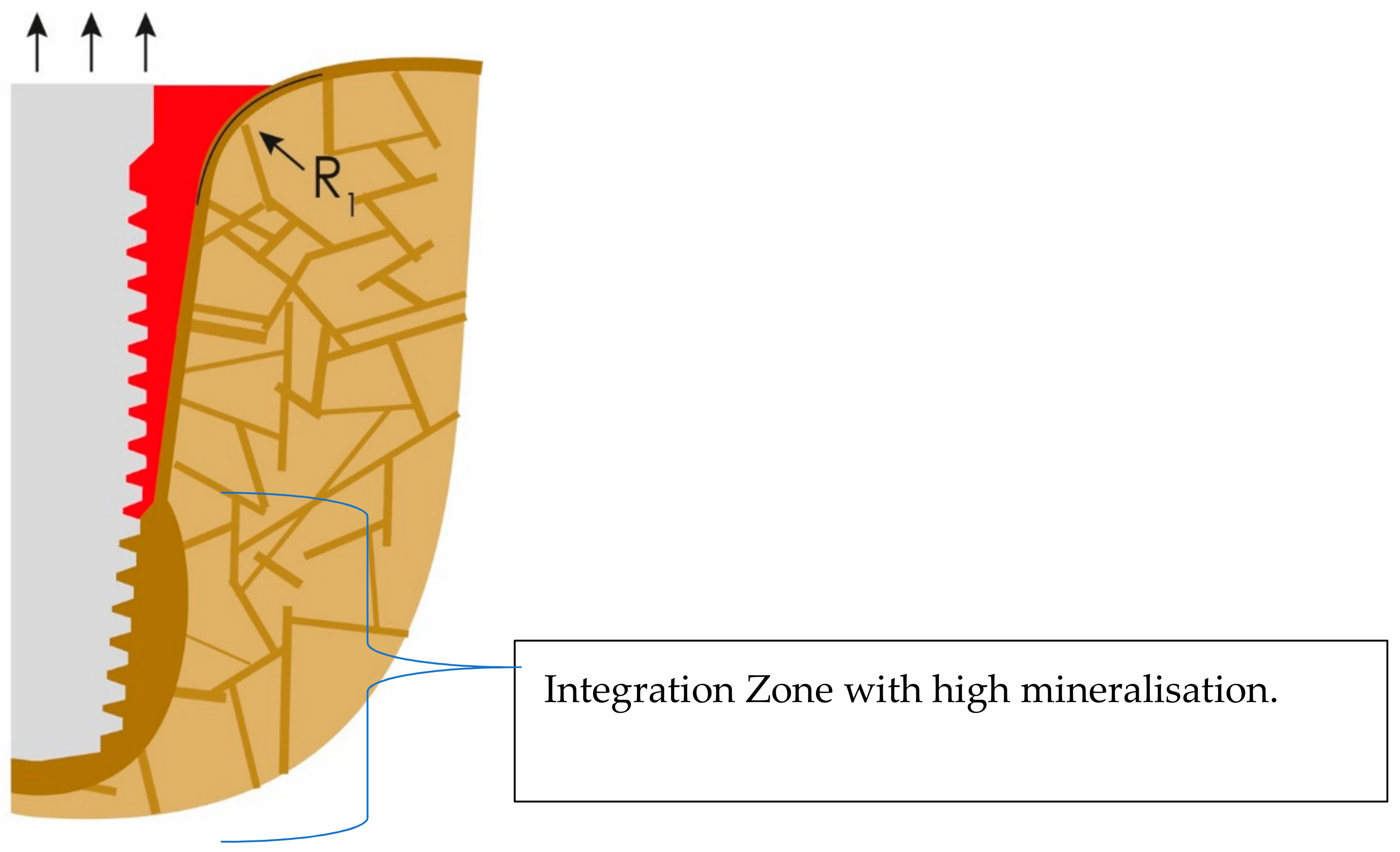
If lateral forces are considerable, additional cortical formation and mineralization will occur around the apical part of the implant, as presented in Figure 4. Thereby, the crestal and vertical cortical above the integration zone will lose more and more functional stimulation, and this zone may collapse sooner or later, as a result of a process that could be subsumed as “disuse atrophy”.
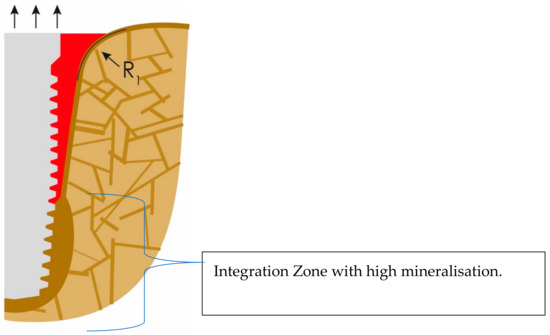
Figure 4.
Cortical bone formation at the apical part of the implant. This cortical will later compete and win against the outer cortical (OC) for functional stimulation.
If load transmission moves more caudally, cantilever forces become larger, while the BIC becomes smaller, and thus increased local forces are balanced by more cortical bone formation in the area of the apex of the implant. Infections, which persist or form nearby, will increase the amount of highly mineralized bone and the bone mass at the same time [24], as shown in Figure 5. The approximation between highly mineralized corticals takes place towards the adjacent implant in longitudinal direction of the jaw bone (black arrows), but it also takes place towards the outer cortical of the jaw bone (Figure 6).
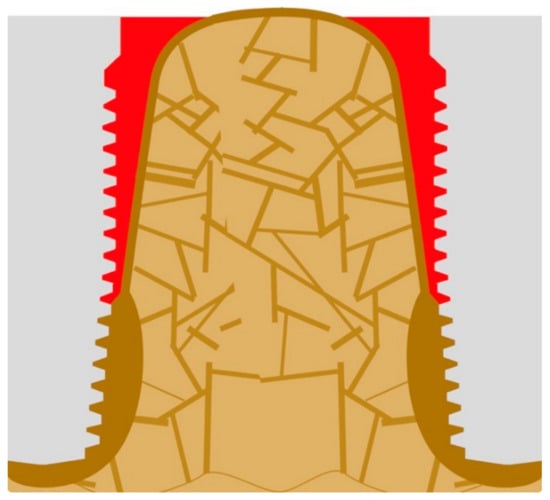
Figure 5.
If several implants have been placed in the same jaws and close to each other, all of their basal corticals begin to grow both under the aspect of the nearby chronic infection and due to the high cantilever forces.
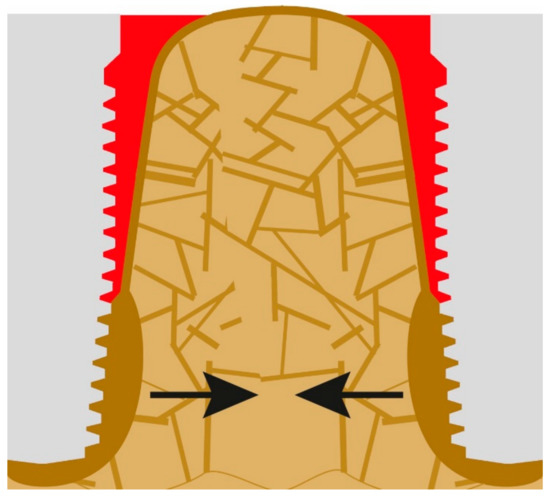
Figure 6.
Cortical bone approximation.
Likewise, the zone of the increased cortical bone mass at the apex of the implant increases in all directions, i.e., also towards the lingual and vestibular cortical, and thereby the already weak bone crestal to that mineralized bone mass will lose all functional stimuli. These stimuli concentrate in the peri-apical areas around the implants. It is only a matter of time until the (e.g., vestibular) functionally not cortical (without function) of the jaw segment is lost. If bone mass decreases too fast, additional bone volume might be formed in vertical direction (Figure 7).
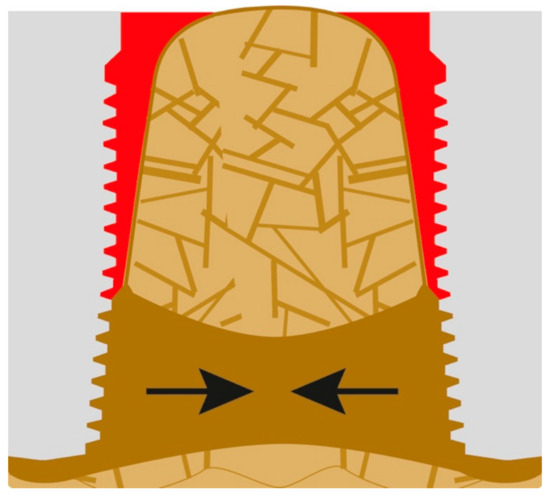
Figure 7.
After the newly formed cortical areas at the apex of the implant unite with either the outer cortical or the new cortical at the apex of the adjacent implant and additional cortical has been formed, this triggers the dismantling of the old (crestal) first cortical.
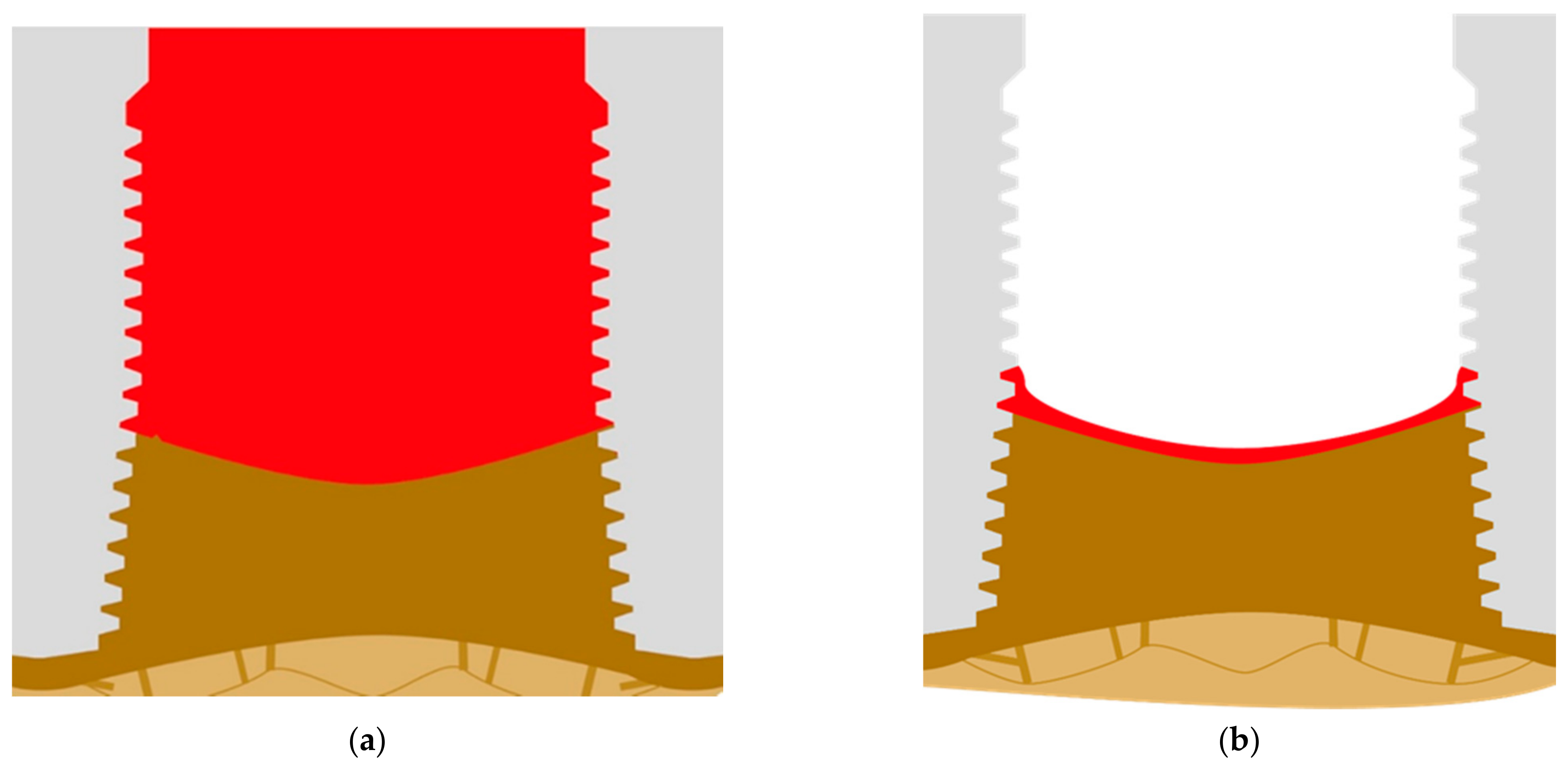
The basal cortical then develops in order to be the dominating cortical, and all the bone that is left on the top may resorb in short period of time (Figure 8a). The connection between the implants and the bone is now stable, and this situation can persist for years. The quality of life for patients with such deep pockets is strongly affected by constant pus development. As an alternative to deep pockets also (massive) shrinkage of the sort tissues, and thinning of keratinized mucosa [25] around the implants can take place and this leads to visible exposure of implants (Figure 8b).
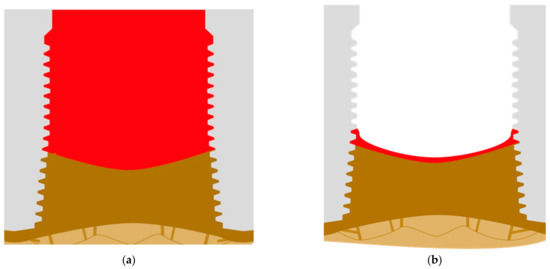
Figure 8.
(a) Full formation of the basal cortical bone and development of large pockets along the vertical part of the implant after the crestal part of the alveolar bone has been resorbed. (b) As soon as the pockets resorb, a large part of the implant’s thread becomes visible, but the development output is reduced or has stopped.
3. Discussion
According to the literature, oral peri-implant diseases may present as mucositis and peri-implantitis, where both the soft and hard tissue destruction is “caused by bacteria” [26,27]. Real life experience casts doubt on the truthfulness of the statement regarding the bacteria causing bone destruction, as many antimicrobial approaches (antibiotics, bactericidal substances, etc.) are not effective, and because we can observe that even after decades in graves around, the world the soft tissues have been dissolved and disappeared, whereas bones remain intact. It seems that bacteria are not so much interested in consuming bones nor have enough tools to degrade them. It should be highlighted that the large amount of minerals in bone guarantees a bacteria-unfriendly pH, and hence mineralized bone can be considered to be auto-sterile. Bacteria even refuse to consume bones in the case of osteomyelitis, while they are located in the true center of the bone, i.e., inside the Haversian canals [28]. The true reason why bacteria bother the peri-implant bone is rather the answer of the body itself: the presence of bacteria (adhering to the exposed rough surfaces of the implant) triggers and increases blood supply in order to increase the number of defense cells. Associated to this the blood supply and the oxygen levels are increased (e.g., in the phase of peri-implant mucositis), and this by itself will lead to a de-mineralization of the bone to make space for vessel-rich granuloma-like tissue (just as in the case of periodontitis). This event should not be mixed up with an immunological reaction, e.g., as an answer to foreign bodies or immune-activating substances. Derks et al. [29] stated that PI starts 2–3 years after the implant placement and that a precursor to PI is peri-implant mucositis (PIM). In the explanation section of this article, we explain why this process lasts so long and why it occurs only after sucessful osseointegration.
Passoni et al. reported [30], on the contrary, that PI is oberved already after 12 months and occurs more often if more than five implants per jaw are placed. In their study, they grouped patients into two groups: G1 with up to five implants placed per jaw and G2 with more than five implants placed per jaw. G1 showed PI in 50% of the implants, whereas G2 showed PI in 81.8% of the implants. Assuming that all parameters were equal in both groups, the only difference between them were more jaw bone under stronger remodelling because more implants had been placed. The volume ratio between bone and implants is in such a situation strongly changing in favor of the implants, and for the jaw bone, it becomes functionally impossible to maintain its bone mass and the pre-operative macro-trajectories (i.e., the bone volume, dimensions, and structural architecture). As a result of the increased remodelling, the bone is more “optimized” [16], and hence the crestal bone level receses away from the “bone level” of the implants. As a result, the rough (formerly endosseous) surface of the implant is exposed very early and there is not even time for this part of the surface to undergo osseointegration. Infection starts right away.
It may be concluded that in the regular case (with up to five implants per jaw placed), it takes 2–3 years until PI begins to occur. If, however, simultaneously more than five rough-surfaced endosseouse implants are placed into the same jaw, the overall remodelling can reach an extent (compared to the amount of non-remodelled bone) where vertical bone loss is extensively observable already after 12 months.
Moreover, peri-implantitis was originally defined as “an inflammatory reaction with the loss of supporting bone in the tissues surrounding a functioning implant” and this definition was coined by Albrektsson and Isidor in their consensus report of session IV, Proceedings of the First European Workshop on Periodontology [31]. On the basis of the etiology of PI, as explained in our two publications, we have to revise this description drastically: PI is rather a phenomenon where bone, following its own rules, i.e., in reducing the amount of the outer cortical (OC), does so in order to abolish non-physiological angles between outer (OC) and inner cortical (IC). As this process exposes instantly rough implant surfaces to the oral cavity, an opportunistic colonialization of the implant surfaces by regular oral bacteria takes place, and only this leads to the signs of an infection [32]. The infection propagates the bone loss due to its associated high level of blood and oxygen supply, which is known to weaken bones in general [33]. The progression of bone loss stops at a basal cortical bone or inside the jaw bones where it can result in an increase in size and mineralization of the bone. This is in agreement with the histological results presented in the paper of Galárraga-Vinueza et al. where it is called functional corticalisation of trabecular bone [34].
4. Conclusions
Within the limits of this article and the observations considered, it may be stated that
- PI is not an “infectious disease”, it is the result of natural changes of the bone’s morphology (after the incorporation of an oral implant with a considerable diameter), such as a decrease in convexity of the outer surface of the bone and subsequently a decrease in concavity of the inner bone.
- PI occurs because bone retracts along the vertical axis of an implant from the first cortical downwards. This process starts after the inner cortical (IC) and the outer cortical (OC) come to a mechanical coupling.
- The fact that implants that are affected by PI show vertical bone loss while their stability and mineralization of the apical bone part rather increases (functional corticalisation) questions the demand for “large implant surfaces and lengths” as is traditionally set up in conventional dental implantology.
- The usage of crestal bone for the fixation of an oral implant as the most optimal solution should be reconsidered. This bone is prone to atrophy throughout all life simply as a result of disuse atrophy and age-dependent bone remodeling after tooth loss.
Author Contributions
Conceptualization, S.I. and A.I.; methodology, O.S.; validation, S.I., A.I., O.S. and Ł.P.; formal analysis, S.I. and Ł.P.; investigation, A.I. and O.S.; resources, S.I. and A.I.; data curation, S.I., A.I. and O.S.; writing—original draft preparation, S.I. and Ł.P.; writing—review and editing, S.I., A.I., Ł.P. and O.S.; visualization, S.I. and A.I.; supervision, Ł.P.; project administration, S.I., A.I. and O.S.; funding acquisition, S.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levignac, J. L’ostéolyse periimplantaire, périimplantose—Périimplantite [Periimplantation osteolysis—Periimplantosis—Periimplantitis]. Rev. Fr. Odontostomatol. 1965, 12, 1251–1260. [Google Scholar] [PubMed]
- Jemt, T.; Gyzander, V.; Britse, A.Ö. Incidence of surgery related to problems with peri-implantitis: A retrospective study on patients followed up between 2003 and 2010 at one specialist clinic. Clin. Implant Dent. Relat. Res. 2015, 17, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Décaillet, F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 203–213. [Google Scholar] [CrossRef] [Green Version]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Polymeri, A.; van der Horst, J.; Buijs, M.J.; Zaura, E.; Wismeijer, D.; Crielaard, W.; Loos, B.G.; Laine, M.L.; Brandt, B.W. Submucosal microbiome of peri-implant sites: A cross-sectional study. J. Clin. Periodontol. 2021, 48, 1228–1239. [Google Scholar] [CrossRef]
- Eick, S. Oral Biofilms. Monogr. Oral Sci. 2021, 29, 98–104. [Google Scholar]
- Giro, G.; Tebar, A.; Franco, L.; Racy, D.; Bastos, M.F.; Shibli, J.A. Treg and TH17 link to immune response in individuals with peri-implantitis: A preliminary report. Clin. Oral Investig. 2021, 25, 1291–1297. [Google Scholar] [CrossRef]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal. Res. 2018, 53, 657–681. [Google Scholar] [CrossRef]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef]
- Bilgiç, E.; Boyacıoğlu, Ö.; Gizer, M.; Korkusuz, P.; Korkusuz, F. Architecture of bone tissue and its adaptation to pathological conditions. In Comparative Kinesiology of the Human Body; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–90. [Google Scholar]
- Ihde, S. Principles of BOI, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Ihde, S.; Ihde, A.; Sipic, O.; Pałka, Ł. Peri-Implantitis: A New Definition Proposal Based on Unnatural Spatial Arrangement and Late Mechanical Coupling between Two Cortical Bone Layers during Osseointegration Phase. Part I. Appl. Sci. 2022, 12, 4317. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, N.; Yang, M.; Sun, T.; Zhang, J.; Zhao, Y.; Huo, N.; Li, Z. Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J. Orthop. Transl. 2022, 33, 41–54. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar] [PubMed]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Laass, M.; Günzl, H.J. The histopathology of different foreign-body reactions in oral soft tissue and bone tissue. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 420, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Albrektsson, B. Osseointegration of bone implants. A review of an alternative mode of fixation. Acta Orthop. Scand. 1987, 58, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Skedros, J.G.; Keenan, K.E.; Williams, T.J.; Kiser, C.J. Secondary osteon size and collagen/lamellar organization (“osteon morphotypes”) are not coupled, but potentially adapt independently for local strain mode or magnitude. J. Struct. Biol. 2013, 181, 95–107. [Google Scholar] [CrossRef]
- Chen, Y.G.; Wang, W.S.; Li, X. Fracture analysis of cortical bone under the condition of cement line debonding and osteon pullout. Int. J. Biomath. 2018, 11, 1850023. [Google Scholar] [CrossRef]
- Pérez-Pevida, E.; Chávarri-Prado, D.; Diéguez-Pereira, M.; Estrada-Martínez, A.; Montalbán-Vadillo, O.; Jiménez-Garrudo, A. Consequences of Peri-Implant Bone Loss in the Occlusal Load Transfer to the Supporting Bone in terms of Magnitude of Stress, Strain, and Stress Distribution: A Finite Element Analysis. BioMed Res. Int. 2021, 2021, 3087071. [Google Scholar] [CrossRef]
- Frost, M.L.; Fogelman, I.; Blake, G.M.; Marsden, P.K.; Cook, G., Jr. Dissociation between global markers of bone formation and direct measurement of spinal bone formation in osteoporosis. J. Bone Miner. Res. 2004, 19, 1797–1804. [Google Scholar] [CrossRef]
- Ogiso, M.; Tabata, T.; Kuo, P.T.; Borgese, D. A histologic comparison of the functional loading capacity of an occluded dense apatite implant and the natural dentition. J. Prosthet. Dent. 1994, 71, 581–588. [Google Scholar] [CrossRef]
- Ihde, S.; Ihde, A. Considerations regarding implantological treatment in patients with aggressive periodontal involvement. CMF Implant Dir. 2021, 15, 202–212. [Google Scholar]
- Wang, Q.; Tang, Z.; Han, J.; Meng, H. The width of keratinized mucosa around dental implants and its influencing factors. Clin. Implant Dent. Relat. Res. 2020, 22, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J. Peri-implant diseases: Consensus report of the sixth European workshop on periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, P.; Clem, D.; Cochran, D.; Froum, S.; McAllister, B.; Renvert, S.; Wang, H.L. Peri-implant mucositis and peri- implantitis: A current understanding of their diagnoses and clinical implications. J. Periodontol. 2013, 84, 436–443. [Google Scholar]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Peri-implantitis—Onset and pattern of progression. J. Clin. Periodontol. 2016, 43, 383–388. [Google Scholar] [CrossRef]
- Passoni, B.B.; Dalago, H.R.; Schuldt Filho, G.; Oliveira de Souza, J.G.; Benfatti, C.A.; Magini Rde, S.; Bianchini, M.A. Does the number of implants have any relation with peri-implant disease? J. Appl. Oral Sci. 2014, 22, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Isidor, F. Consensus Report of Session IV. In Proceedings of the First European Workshop on Periodontology, Thurgau, Switzerland, 1–4 February 1993; Lang, N.P., Karring, T., Eds.; Quintessence Publishing: London, UK, 1994; pp. 365–369. [Google Scholar]
- Ihde, S.; Pałka, Ł.; Janeczek, M.; Kosior, P.; Kiryk, J.; Dobrzyński, M. Bite Reconstruction in the Aesthetic Zone Using One-Piece Bicortical Screw Implants. Case. Rep. Dent. 2018, 29, 4671482. [Google Scholar] [CrossRef] [Green Version]
- Hannah, S.S.; McFadden, S.; McNeilly, A.; McClean, C. “Take My Bone Away?” Hypoxia and bone: A narrative review. J. Cell. Physiol. 2021, 236, 721–740. [Google Scholar] [CrossRef]
- Galárraga-Vinueza, M.; Tangl, S.; Bianchini, M.; Magini, R.; Obreja, K.; Gruber, R.; Schwarz, F. Histological characteristics of advanced peri-implantitis bone defects in humans. Int. J. Implant Dent. 2020, 6, 12. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).