Hydrogeochemical Survey along the Northern Coastal Region of Ramanathapuram District, Tamilnadu, India
Abstract
:1. Introduction
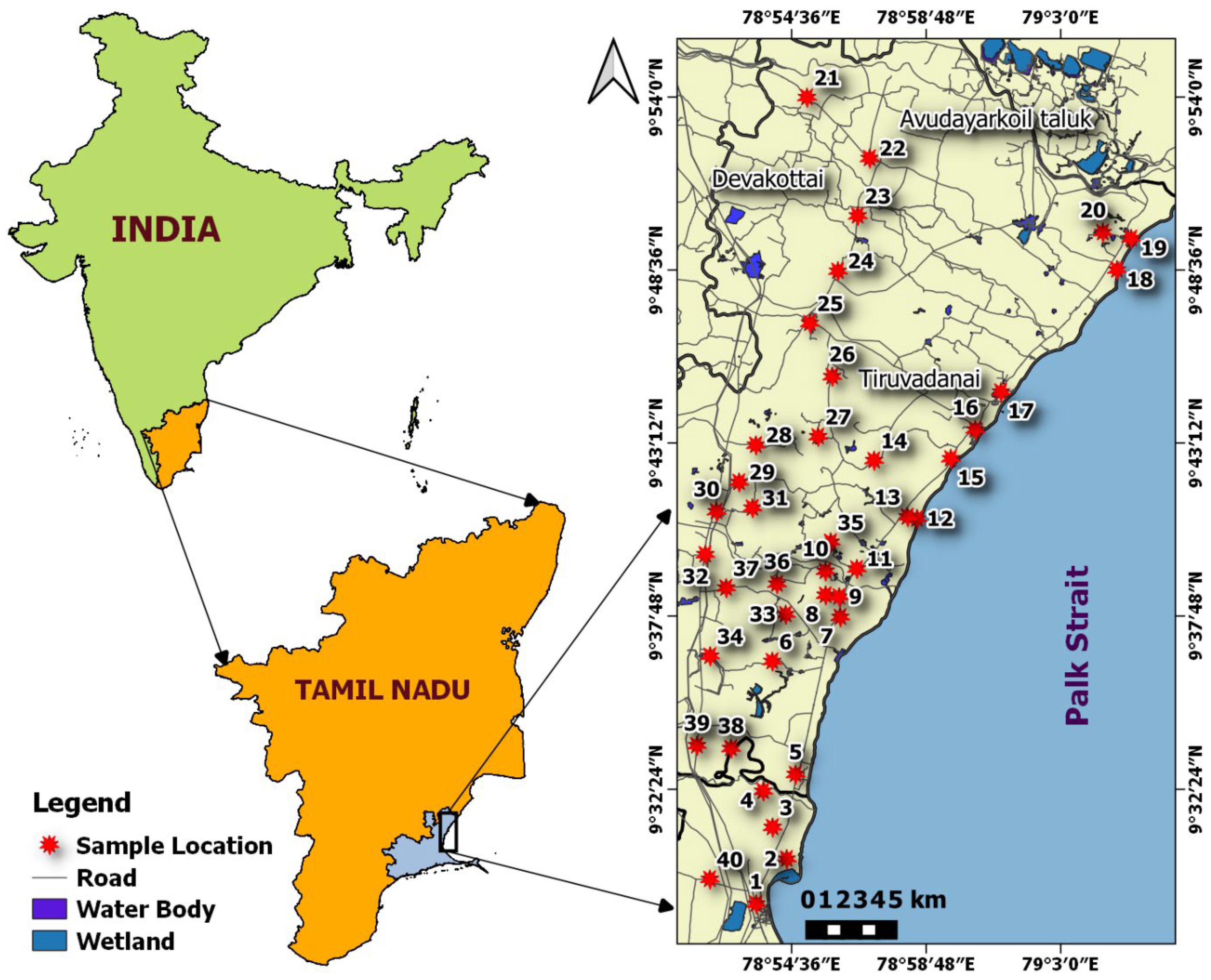
2. Study Site Description
3. Hydrogeological Setup
4. Methodology
5. Results and Discussions
5.1. Physico-Chemical Parameters
5.2. Cations and Anions
6. Potability of Groundwater
Drinking Water Quality Index (DWQI)
7. Irrigation Suitability of Groundwater
7.1. Percent Sodium vs. EC Plot (Na%)
7.2. US Salinity Diagram
7.3. Doneen’s Permeability Index Plot (PI)
7.4. Irrigation Water Quality Index (IRWQI)
8. Hydrogeochemical Facies
9. Gibbs Plot
10. Chadha’s Plots
11. Scatter Plot
12. Cl−/HCO3− Ratio
13. Multivariate Statistical Analysis
13.1. Correlation Matrix
13.2. Principal Component Analysis (PCA)
13.3. Cluster Analysis
14. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chidambaram, S.; Karmegam, U.; Prasanna, M.V.; Sasidhar, P.; Vasan-thavigar, M. A study on hydrochemical elucidation of coastal groundwater in and around Kalpakkam region, Southern India. Environ. Earth Sci. 2011, 64, 1419–1431. [Google Scholar] [CrossRef]
- Belkhiri, L.; Mouni, L. Geochemical modeling of groundwater in the El Eulma area, Algeria. Desalin Water Treat. 2012, 51, 1468–1476. [Google Scholar] [CrossRef]
- Lim, J.W.; Lee, E.; Moon, S.H.-N.; Lee, K.K. Integrated investigation of seawater intrusion around oil storage caverns in a coastal fractured aquifer using hydrogeochemical and isotopic data. J. Hydrol. 2013, 486, 202–210. [Google Scholar] [CrossRef]
- Tomaszkiewicz, M.; AbouNajm, M.E.; Fadel, M. Development of a groundwater quality index for seawater intrusion in coastal aquifers. Environ. Model. Softw. 2014, 57, 13–26. [Google Scholar] [CrossRef]
- Han, D.M.; Song, X.F.; Currell, M.J.; Yang, J.L.; Xiao, G.Q. Chemical and isotopic constraints on evolution of groundwater salinization in the coastal plain aquifer of Laizhou Bay, China. J. Hydrol. 2014, 508, 12–27. [Google Scholar] [CrossRef]
- Askri, B.; Ahmed, A.T.; Al-Shanfari, R.A.; Bouhlila, R.; Ben, K.; Al-Farisi, K. Isotopic and geochemical identifications of groundwater salinization processes in Salalah coastal plain, Sultanate of Oman. Chem. Erde. 2016, 76, 243–255. [Google Scholar] [CrossRef]
- Sridhar, S.G.D.; Sakthivel, A.M.; Sangunathan, U.; Balasubramanian, M.; Jenefer, S.; Mohamed Rafik, M.; Kanagaraj, G. Heavy metal concentration in groundwater from Besant Nagar to Sathankuppam, South Chennai, Tamil Nadu, India. Appl. Water Sci. 2017, 7, 4651–4662. [Google Scholar] [CrossRef] [Green Version]
- Dar, I.A.; Sankar, K.; Dar, M.A. Spatial assessment of groundwater quality in Mamundiyar basin, Tamil Nadu, India. Environ. Monit. Assess. 2011, 178, 437–447. [Google Scholar] [CrossRef]
- Sivasubramanian, P.; Balasubramanian, N.; Soundranayagam, J.P.; Chandrasekar, N. Hydrochemical characteristics of coastal aquifers of Kadaladi, Ramanathapuram District, Tamilnadu, India. Appl. Water Sci. 2013, 3, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Werner, A.D.; Bakker, M.; Post, V.E.A.; Vandenbohede, A.; Lu, C.; Ataie-Ashtiani, B.; Simmons, C.T.; Barry, D.A. Seawater intrusion processes, investigation and management: Recent advances and future challenges. Adv. Water Resour. 2013, 51, 3–26. [Google Scholar] [CrossRef]
- Sun, H.; Alexander, J.; Gove, B.; Koch, M. Mobilization of arsenic, lead, and mercury under conditions of sea water intrusion and road deicing salt application. J. Contam. Hydrol. 2015, 180, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Kazemi, H.; Ehtashemi, M.; Rockaway, T. Assessment of groundwater quantity and quality and saltwater intrusion in the Damghan basin, Iran. Chem. Der. Erde. 2016, 76, 227–241. [Google Scholar] [CrossRef]
- Kanagaraj, G.; Elango, L.; Sridhar, S.G.D.; Gowrisankar, G. Hydrogeochemical pro- cesses and influence of seawater intrusion in coastal aquifers south of Chennai, Tamil Nadu, India. Environ. Sci. Pollut. Res. 2018, 25, 8989–9011. [Google Scholar] [CrossRef]
- Muthusamy, S.; Sivakumar, K.; Shanmugasundharam, A.; Jayaprakash, M. Appraisal on water chemistry of Manakudy estuary, south west coast, India. Shengtai Xuebao/Acta Ecol. Sin. 2021, 41, 463–478. [Google Scholar] [CrossRef]
- Huang, G.; Sun, J.; Zhang, Y.; Chen, Z.; Liu, F. Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, South China. Sci. Total Environ. 2013, 463, 209–221. [Google Scholar] [CrossRef]
- Huang, G.; Liu, C.; Sun, J.; Zhang, M.; Jing, J.; Li, L. A regional scale investigation on factors controlling the groundwater chemistry of various aquifers in a rapidly urbanized area: A case study of the Pearl River Delta. Sci. Total Environ. 2018, 625, 510–518. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, M.; Liu, C.; Li, L.; Chen, Z. Heavy metal(loid)s and organic contaminants in groundwater in the Pearl River Delta that has undergone three decades of urbanization and industrialization: Distributions, sources, and driving forces. Sci. Total Environ. 2018, 635, 913–925. [Google Scholar] [CrossRef]
- Mondal, N.C.; Singh, V.P. Hydrochemical analysis of salinization for a tannery belt in Southern India. J. Hydrol. 2011, 405, 235–247. [Google Scholar] [CrossRef]
- Srinivas, Y.; Hudson Oliver, D.; Stanley Raj, A.; Chandrasekar, N. Evaluation of groundwater quality in and around Nagercoil town, Tamil Nadu, India: An integrated geochemical and GIS approach. Appl. Water Sci. 2013, 3, 631–651. [Google Scholar] [CrossRef] [Green Version]
- Boluda-Botella, N.; Valdes-Abellan, J.; Pedraza, R. Applying reactive models to column experiments to assess the hydrogeochemistry of seawater intrusion: Optimizing Acuaintrusion and selecting cation exchange coefficients with PHREEQC. J. Hydrol. 2014, 510, 59–69. [Google Scholar] [CrossRef]
- Sivakumar, K.; Priya, J.; Muthusamy, S.; Saravanan, P.; Jayaprakash, M. Spatial Diversity of Major Ionic Absorptions in Groundwater Recent study from the Industrial region of Tuticorin, Tamil Nadu, India. EnviroGeoChimica Acta 2016, 3, 138–147. [Google Scholar]
- Ataie-Ashtiani, B.; Volker, R.E.; Lockington, D.A. Tidal effects on sea water intrusion in unconfined aquifers. J. Hydrol. 1999, 216, 17–31. [Google Scholar] [CrossRef]
- Cardona, A.; Carrillo-Rivera, J.J.; Huizar-Alvarez, R.; Graniel-Castro, E. Salinization in coastal aquifers of arid zones: An example from Santo Domingo, Baja California Sur, Mexico. Environ. Geol. 2004, 45, 350–366. [Google Scholar] [CrossRef]
- Raju, N.J.; Shukla, U.K.; Ram, P. Hydrogeochemistry for the assessment of groundwater quality in Varanasi: A fast-urbanizing center in Uttar Pradesh, India. Environ. Monit. Assess. 2011, 173, 279–300. [Google Scholar] [CrossRef]
- Anil Kumar, K.S.; Prijub, C.P.; Narasimha Prasad, N.B. Study on saline water in- trusion into the shallow coastal aquifers of Periyar River Basin, Kerala using hydro- chemical and electrical resistivity methods. Aquat Procedia 2015, 4, 32–40. [Google Scholar] [CrossRef]
- Chidambaram, S.; Sarathidasan, J.; Srinivasamoorthy, K.; Thivya, C.; Thilagavathi, R.; Prasanna, M.V.; Singaraja, C.; Nepolian, M. Assessment of hydrogeochemical status of groundwater in a coastal region of Southeast coast of India. Appl. Water Sci. 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- World Bank Report. Deep Wells and Prudence: Towards Pragmatic Action for Addressing Groundwater Overexploitation in India; Report No. 51676; The World Bank: Washington, DC, USA, 2010. [Google Scholar]
- Srinivasamoorthy, K.; Nanthakumar, C.; Vasanthavigar, M.; Vijayaraghavan, K.; Rajivgandhi, R.; Chidambaram, S.; Anandhan, P.; Manivannan, R.; Vasudevan, S. Groundwater quality assessment from a hard rock terrain, Salem district of Tamil Nadu, India. Arab. J. Geosci. 2011, 4, 91–102. [Google Scholar] [CrossRef]
- Venkatramanan, S.; Chung, S.Y.; Selvam, S.; Lee, S.Y.; Elzain, H.E. Factors controlling groundwater quality in the Yeonjegu District of Busan City, Korea, using the hydrogeochemical processes and fuzzy GIS. Environ. Sci. Pollut. Res. 2017, 24, 23679–23693. [Google Scholar] [CrossRef]
- Sivakumar, K.; Shanmugasundaram, A.; Jayaprakash, M.; Prabakaran, K.; Muthusamy, S.; Ramachandran, A.; Venkatramanan, S.; Selvam, S. Causes of heavy metal contamination in groundwater of Tuticorin industrial block, Tamil Nadu, India. Environ. Sci. Pollut. Res. 2021, 28, 18651–18666. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, World Health Organization, 4th ed.; Recommendations: Geneva, Switzerland, 2016; Volume 1, p. 631.
- Gopinath, S.; Srinivasamoorthy, K.; Saravanan, K.; Suma, C.S.; Prakash, R.; Senthilnathan, D.; Chandrasekaran, N.; Srinivas, Y.; Sarma, V.S. Modeling saline water intrusion in Nagapattinam coastal aquifers, Tamilnadu. India. Model. Earth Syst. Environ. 2016, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Senthilkumar, M.; Elango, L. Geochemical processes controlling the groundwater quality in lower Palar river basin, Southern India. J. Earth Syst. Sci. 2013, 122, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, A.; Sivakumar, K.; Shanmugasundharam, A.; Sangunathan, U.; Krishnamurthy, R.R. Evaluation of potable groundwater zones identification based on WQI and GIS techniques in Adyar River basin, Chennai, Tamilnadu, India. Shengtai Xuebao/Acta Ecol. Sin. 2021, 41, 285–295. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Tan, H.; Zhang, Y.; Cao, J. Geochemical and isotopic data for restricting seawater intrusion and groundwater circulation in a series of typical volcanic islands in the South China Sea. Mar. Pollut. Bul. 2015, 93, 53–162. [Google Scholar] [CrossRef] [PubMed]
- Zenhom, E.; Salem, A.M.A.; Temamy, M.; Salah, K.; Kassa, M. Origin and characteristics of brackish groundwater in Abu Madi coastal area, Northern Nile Delta. Egypt. Estu. Coast. Shelf Sci. 2016, 178, 21–35. [Google Scholar] [CrossRef]
- Sarath Prasanth, S.V.; Magesh, N.S.; Jitheshlal, K.V.; Chandrasekar, N.; Gangadhar, K. Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl. Water Sci. 2012, 2, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Muruganantham, A.; Sivakumar, K.; Kongeswaran, T.; Prabakaran, K.; Bangaru Priyanga, S.; Karikalan, R.; Agastheeswaran, V.; Perumal, V. Hydrogeochemical Analysis for Groundwater Suitability Appraisal in Sivagangai, an Economically Backward District of Tamil Nadu. J. Geol. Soc. India 2021, 97, 789–798. [Google Scholar] [CrossRef]
- Krishna Kumar, S.; Bharani, R.; Magesh, N.S.; Godson, P.S.; Chandrasekar, N. Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Appl. Water Sci. 2014, 4, 341–350. [Google Scholar] [CrossRef] [Green Version]
- KrishnaKumar, S.; Chandrasekar, N.; Seralathan, P.; Prince, S.; Godson, M.N.S. Hydrogeochemical study of shallow carbonate aquifers, Rameswaram Island, India. Environ. Monit. Assess. 2012, 184, 4127–4138. [Google Scholar] [CrossRef]
- Selvam, S.; Jesuraja, K.; Venkatramanan, S.; Chidambaram, S.; Prasanna, M.V.; Sivakumar, K. Delineating saline and fresh water aquifers in Tuticorin of southern India by using geophysical techniques. Environ. Dev. Sustain. 2021, 23, 17723–17744. [Google Scholar] [CrossRef]
- Central Ground Water Board (CGWB). District Ground Water Brochure Ramanathapuram District, Tamil Nadu; Central Ground Water Board South Eastern Coastal Region: Chennai, India, 2009. Available online: http://cgwb.gov.in/district_profile/tamilnadu/ramanathapuram.pdf (accessed on 5 February 2020).
- IMD. Rainfall of Ramanathapuram District; Regional Meterological Centre: Chennai, India, 2013.
- ENVIS. Ramanathapuram District. ENVIS Centre Tamilnadu, 2015. Available online: tnenvis.nic.in/files/RAMANATHAPURAM.pdf (accessed on 15 March 2020).
- CCC&AR and TNSCCC. Climate Change Projection (Rainfall) for Ramanathapuram. District-Wise Climate Change Information for the State of Tamil Nadu. Centre for Climate Change and Adaptation Research (CCC&AR), Anna University and Tamil Nadu State Climate Change Cell (TNSCCC), Department of Environment (DoE); Government of Tamil Nadu: Chennai, India, 2015. Available online: www.tnsccc.in (accessed on 10 February 2020).
- Central Water Commission. Problems of Salination of Land in Coastal Areas of India and Suitable ProtectionMeasures. In Ministry of Water Resources, River Development & Ganga Rejuvenation; Government of India: New Delhi, India, 2017. [Google Scholar]
- Central Ground Water Board (CGWB). Report on Status of Ground Water Quality in Coastal Aquifers of India. In Ministry of Water Resources; Government of India: Faridabad, India, 2014. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association Inc., American Water Works Association, Water Environment Federation: New York, NY, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- Prabakaran, K.; Sivakumar, K.; Aruna, C. Use of GIS-AHP tools for potable groundwater potential zone investigations—a case study in Vairavanpatti rural area, Tamil Nadu, India. Arab. J. Geosci. 2020, 13, 866. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rammohan, V.; Sahayam, J.D.; Jeevanandam, M. Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ. Monit. Assess. 2009, 159, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Magesh, N.S.; Chandrasekar, N.; Soundranayagam, J.P. Delineation of groundwater potential zones in Theni district, Tamil Nadu, using remote sensing, GIS and MIF techniques. Geosci. Front. 2012, 3, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Krishnakumar, P.; Lakshumanan, C.; Kishore, V.P. Assessment of groundwater quality in and around Vedaraniyam, South India. Environ. Earth Sci. 2014, 71, 2211–2225. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water, 3rd ed.; Scientific Publishers: Jodhpur, India, 1985; p. 2254. [Google Scholar] [CrossRef] [Green Version]
- BIS (IS 10500: 2012); Drinking Water Specifications 2nd Revision. Bureau of Indian Standards: New Delhi, India, 2012. Available online: https://law.resource.org/pub/in/bis/S06/is.10500.2012.pdf (accessed on 29 September 2020).
- Sawyer, C.N.; McCarty, P.L. Chemistry for Environmental Engineering, 3rd ed.; McGraw-Hill Book Co.: New York, NY, USA, 1978. [Google Scholar]
- Sivakumar, K.; Prabakaran, K.; Saravanan, P.K.; Muthusamy, S.; Kongeswaran, T.; Muruganantham, A.; Gnanachandrasamy, G. Agriculture Drought Management in Ramanathapuram District of Tamil Nadu, India. J. Clim. Chang. 2022, 8, 59–65. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcott, D.W. Water Quality for Agriculture (No. 29); Food and Agriculture Organization of the United Nations: Rome, Italy, 1985. [Google Scholar]
- University of California Committee of Consultants (UCCC). Guidelines for Interpretations of Water Quality for Irrigation; University of California Committee of Consultants: Berkeley, CA, USA, 1974. [Google Scholar]
- Wilcox. Classification and Use of Irrigation Waters; US Department of Agriculture: Washington, DC, USA, 1955; p. 969.
- Richard, L.A. Diagnosis and improvement of saline and alkali soils. USDA Handb. 1954, 60, 160. [Google Scholar] [CrossRef]
- Doneen, L.D. Notes on Water Quality in Agriculture; Water Science and Engineering Paper 4001; Department of Water Science and Engineering, University of California: Davis, CA, USA, 1964. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, K.V. Irrigation with Saline Water, Monogram No. 2 (New Series); IARI: New Delhi, India, 1972; p. 198. [Google Scholar]
- Eaton, F.M. Significance of carbonates in irrigation waters. Soil Sci. 1950, 69, 123–134. [Google Scholar] [CrossRef]
- Kelley, W.P. Use of saline irrigation water. Soil Sci. 1963, 95, 385–391. [Google Scholar] [CrossRef]
- Agastheeswaran, V.; Udayaganesan, P.; Sivakumar, K.; Venkatramanan, S.; Prasanna, M.V.; Selvam, S. Identification of groundwater potential zones using geospatial approach in Sivagangai district, South India. Arab. J. Geosci. 2021, 14, 8. [Google Scholar] [CrossRef]
- Bagyaraj, M.; Tenaw, M.A.; Gnanachandrasamy, G.; Chung, S.Y.; Venkatramanan, S.; Selvam, S.; Hussam, E.E.; Sivakumar, K. Site selection of check dams using geospatial techniques in Debre Berhan region, Ethiopia—water management perspective. Environ. Sci. Pollut. Res. 2021, 29, 1–20. [Google Scholar] [CrossRef]
- Kongeswaran, T.; Sivakumar, K. Application of Remote Sensing and GIS in Floodwater Harvesting for Groundwater Development in the Upper Delta of Cauvery River Basin, Southern India. In Water Resources Management and Sustainability, Advances in Geographical and Environmental Sciences; Pankaj, K., Gaurav, K.N., Manish Kumar, S., Anju, S., Eds.; Springer Nature: Berlin, Germany, 2022; pp. 257–280. [Google Scholar] [CrossRef]
- Varma, V.K.; Malhotra, S.; Yoo, E.S.; Jiloha, R.C.; Finnerty, M.T.; Susser, E. Course and outcome of acute non-organic psychotic states in India. Psychiatr. Q. 1996, 67, 195–207. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water analysis. Trans. Am. Geophys. Union 1944, 25, 914–923. [Google Scholar] [CrossRef]
- Richter, B.C.; Kreitler, C.W. Geochemical Techniques for Identifying Sources of Ground-Water Salinization; CRC: Boca Raton, FL, USA, 1993. [Google Scholar]
- Jeen, S.W.; Kim, J.M.; Ko, K.S. Hydrogeochemical characteristics of groundwater in a mid-western coastal aquifer system, Korea. Geosci. J. 2001, 5, 339–348. [Google Scholar] [CrossRef]
- Michael, H.A.; Russoniello, C.J.; Byron, L.A. Global assessment of vulnerability to sea-level rise in topography-limited and recharge- limited coastal groundwater systems. Water Resour. Res. 2013, 49, 2228–2240. [Google Scholar] [CrossRef]
- Shanmugam, D.; Krishnamu, R.R.; Sivakumar, K.; Nethaji, S. An Integrated Study on the Impact of Anthropogenic Influenced Coastal Erosion in Puducherry and Villupuram Coasts, Bay of Bengal, South India. EnviroGeoChemica Acta 2014, 1, 437–445. [Google Scholar]
- Kongeswaran, T.; Sivakumar, K. Assessment of shoreline positional uncertainty using remote sensing and GIS techniques: A case study from the east coast of India. J. Geogr. Inst. Jovan Cvijic SASA 2021, 71, 249–263. [Google Scholar] [CrossRef]
- Pulido-Leboeuf, P. Seawater intrusion and associated processes in a small coastal complex aquifer (Castell de Ferro, Spain). Appl. Geochem. 2004, 19, 1517–1527. [Google Scholar] [CrossRef]
- Vasanthavigar, M.; Srinivasamoorthy, K.; Vijayaragavan, K.; Rajiv Ganthi, R.; Chidambaram, S.; Anandhan, P.; Manivannan, R.; Vasudevan, S. Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ. Monit. Assess. 2010, 171, 595–609. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world’s water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Volker, A. Source of brackish ground water in pleistocene formations beneath the dutch polderland. Economic. Geol. 1961, 56, 1045–1057. [Google Scholar] [CrossRef]
- Stuyfzand, P.J. Hydrochemistry and Hydrology of the Coastal Dune Area of the Western Netherlands; Vrije Universiteit: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Chadha, D.K. A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol. J. 1999, 7, 431–439. [Google Scholar] [CrossRef]
- Nazzal, Y.; Ahmed, I.; Al-Arifi, N.S.; Ghrefat, H.; Zaidi, F.K.; El-Waheidi, M.M.; Batayneh, A.; Zumlot, T. A pragmatic approach to study the groundwater quality suitability for domestic and agricultural usage, Saq aquifer, northwest of Saudi Arabia. Environ. Monit. Assess. 2014, 186, 4655–4667. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.S.; Bhattacharya, S.K.; Tyagi, S.K. 18O studies on recharge of phreatic aquifers and groundwater flow-paths of mixing in the Delhi area. J. Hydrol. 1996, 176, 25–36. [Google Scholar] [CrossRef]
- Papazotos, P.; Koumantakis, I.; Vasileiou, E. Hydrogeochemical assessment and suitability of groundwater in a typical Mediterranean coastal area: A case study of the Marathon basin, NE Attica, Greece. Hydrol. Res. 2019, 2, 49–59. [Google Scholar] [CrossRef]
- Papazotos, P.; Vasileiou, E.; Perraki, M. The synergistic role of agricultural activities in groundwater quality in ultramafic environments: The case of the Psachna basin, central Euboea, Greece. Environ. Monit. Assess. 2019, 191, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Papazotos, P.; Vasileiou, E.; Perraki, M. Elevated groundwater concentrations of arsenic and chromium in ultramafic environments controlled by seawater intrusion, the nitrogen cycle, and anthropogenic activities: The case of the Gerania Mountains, NE Peloponnese, Greece. Appl. Geochem. 2020, 121, 104697. [Google Scholar] [CrossRef]
- Revelle, R. Criteria for recognition of the sea water in ground-waters. Eos Trans. Am. Geophys. Union 1941, 22, 593–597. [Google Scholar] [CrossRef]
- Aris, A.Z.; Abdullah, M.H.; Kim, K.W. Hydrogeochemistry of groundwater in Manukan island, Sabah. Malays. J. Anal. Sci. 2007, 11, 407–413. [Google Scholar]
- Liu, W.X.; Li, X.D.; Shen, Z.G.; Wang, D.C.; Wai, O.W.; Li, Y.S. Multivariate statistical study of heavy metal enrichment in sediments of the Pearl River Estuary. Environ. Pollut. 2003, 121, 377–388. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, G.; Hou, Q.; Liu, C.; Zhang, Y.; Zhang, Q. Groundwater quality in the Pearl River Delta after the rapid expansion of industrialization and urbanization: Distributions, main impact indicators, and driving forces. J. Hydrol. 2019, 577, 124004. [Google Scholar] [CrossRef]
- Huang, G.; Liu, C.; Li, L.; Zhang, F.; Chen, Z. Spatial distribution and origin of shallow groundwater iodide in a rapidly urbanized delta: A case study of the Pearl River Delta. J. Hydrol. 2020, 585, 124860. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, Q.; Huang, G.; Liu, C.; Zhang, Y. Elevated manganese concentrations in shallow groundwater of various aquifers in a rapidly urbanized delta, south China. Sci. Total Environ. 2020, 701, 134777. [Google Scholar] [CrossRef] [PubMed]
- Alberto, W.D.; del Pilar, D.M.; Valeria, A.M.; Fabiana, P.S.; Cecilia, H.A.; de Los Ángeles, B.M. Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquía River Basin (Córdoba–Argentina). Water Res. 2001, 35, 2881–2894. [Google Scholar] [CrossRef]
- Meng, S.X.; Maynard, J.B. Use of statistical analysis to formulate conceptual models of geochemical behavior: Water chemical data from the Botucatu aquifer in Sao Paulo state, Brazil. J. Hydrol. 2001, 250, 78–97. [Google Scholar] [CrossRef]
- Huang, G.; Liu, C.; Zhang, Y.; Chen, Z. Groundwater is important for the geochemical cycling of phosphorus in rapidly urbanized areas: A case study in the Pearl River Delta. Environ. Pollut. 2020, 260, 114079. [Google Scholar] [CrossRef]
- Singh, K.P.; Malik, A.; Singh, V.K.; Mohan, D.; Sinha, S. Chemometric analysis of groundwater quality data of alluvial aquifer of Gangetic plain, North India. Anal. Chim. Acta 2005, 550, 82–91. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]












| S. No | Analytical Parameters | Jan-20 | Jan-21 | WHO & BIS Guidelines | Detection Limit | Quantification Limit | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min-Max | Med | St.dev | Min-Max | Med | St.dev | |||||
| 1 | pH | 7.14–8.15 | 7.4 | 0.3 | 7.5–8.6 | 8.1 | 0.2 | 6.5–8.5 | 0.01 | 14 |
| 2 | EC (µS/Cm) | 266–10,400 | 1156.0 | 2167.6 | 834–6857 | 2526.4 | 1111.4 | Nil | 199 | 20,000 |
| 3 | TDS (mg/L) | 186–7280 | 809.2 | 1517.3 | 503.6–6011 | 1627.0 | 1490.4 | 500–2000 | 99 | 10,000 |
| 4 | Ca2+ (mg/L) | 16–452.8 | 46.4 | 97.8 | 48.7–700 | 122.0 | 152.8 | 75–200 | Nil | Nil |
| 5 | Mg2+ (mg/L) | 1.9–256.3 | 19.7 | 56.6 | 15–450 | 64.5 | 98.6 | 30–100 | Nil | Nil |
| 6 | Na+ (mg/L) | 31–1200 | 139.0 | 261.9 | 20–600 | 150.0 | 181.1 | Nil | 1 | 1000 |
| 7 | K+ (mg/L) | 3–300 | 18.0 | 74.1 | 2–98 | 20.0 | 23.8 | Nil | 1 | 1000 |
| 8 | Cl− (mg/L) | 12–2880 | 158.0 | 599.7 | 50–1674.8 | 315.5 | 371.0 | 250–1000 | Nil | Nil |
| 9 | HCO3− (mg/L) | 48–696 | 190.0 | 146.6 | 10–661.4 | 87.9 | 133.3 | Nil | Nil | NIl |
| 10 | SO42− (mg/L) | 12–750 | 73.0 | 175.5 | 29.8–493 | 143.3 | 114.6 | 200–400 | 3 | 500 |
| 11 | NO3− (mg/L) | 3–74 | 13.5 | 15.3 | 1–140 | 47.0 | 30.7 | 45.0 | 1 | 100 |
| 12 | F− (mg/L) | BDL-1.4 | 0.2 | 0.3 | 0.3–1.3 | 0.5 | 0.3 | 1–1.5 | 0.01 | 10 |
| Parameters | Category | Range | Jan-20 | Jan-21 | ||
|---|---|---|---|---|---|---|
| In. No | In.% | In. No | In.% | |||
| pH | Not potable | <6.5 | Nil | - | Nil | - |
| Permissible | 6.5–8.5 | 40 | 100 | 39 | 97.5 | |
| Not potable | >8.5 | Nil | - | 1 | 2.5 | |
| TDS (mg/L) | Acceptable | 0–500 | 14 | 35.0 | Nil | - |
| Permissible | 500–2000 | 15 | 37.5 | 27 | 67.5 | |
| Not potable | >2000 | 11 | 27.5 | 13 | 32.5 | |
| Ca2+ (mg/L) | Acceptable | <75 | 28 | 70.0 | 6 | 15.0 |
| Permissible | 75–200 | 8 | 20.0 | 27 | 67.5 | |
| Not potable | >200 | 4 | 10.0 | 7 | 17.5 | |
| Mg2+ (mg/L) | Acceptable | <30 | 25 | 62.5 | 9 | 22.5 |
| Permissible | 30–100 | 11 | 27.5 | 21 | 52.5 | |
| Not potable | >100 | 4 | 10.0 | 10 | 25.0 | |
| Cl− (mg/L) | Acceptable | <250 | 24 | 60.0 | 16 | 40.0 |
| Permissible | 250–1000 | 12 | 30.0 | 19 | 47.5 | |
| Not potable | >1000 | 4 | 10.0 | 5 | 12.5 | |
| SO42− (mg/L) | Acceptable | <200 | 33 | 82.5 | 31 | 77.5 |
| Permissible | 200–400 | 2 | 5.0 | 7 | 17.5 | |
| Not potable | >400 | 5 | 12.5 | 2 | 5.0 | |
| NO3− (mg/L) | Acceptable | <45 | 38 | 95.0 | 19 | 47.5 |
| Not potable | >45 | 2 | 5.0 | 21 | 52.5 | |
| F− (mg/L) | Acceptable | <1 | 38 | 95.0 | 37 | 92.5 |
| Permissible | 1–1.5 | 2 | 5.0 | 3 | 7.5 | |
| Not potable | >1.5 | Nil | - | Nil | - | |
| TH as CaCO3 (mg/L) | Acceptable | <200 | 21 | 52.5 | Nil | - |
| Permissible | 200–600 | 11 | 27.5 | 17 | 42.5 | |
| Not potable | >600 | 8 | 20.0 | 23 | 57.5 | |
| Indices with Sources | Range (Class) | No. of Samples (%) | |
|---|---|---|---|
| Jan-20 | Jan-21 | ||
| EC [57] | <250 (Excellent) | 0 (0%) | 0 (0%) |
| 250–750 (Good) | 14 (35%) | 0 (0%) | |
| 750–2000 (Permissible) | 15 (37.5%) | 17 (42.5%) | |
| 2000–3000 (Doubtful) | 1 (2.5%) | 9 (22.5%) | |
| >3000 (Unsuitable) | 10 (25%) | 14 (35%) | |
| TDS [58] | <1000 (Excellent) | 24 (60%) | 10 (25%) |
| 1000–3000 (Suitable) | 11 (27.5%) | 26 (65%) | |
| >3000 (Unsuitable) | 5 (12.5%) | 4 (10%) | |
[55] | <75 (Soft) | 3 (7.5%) | 0 (0%) |
| 75–150 (Moderate) | 12 (30%) | 0 (0%) | |
| 150–300 (Hard) | 10 (25%) | 3 (7.5%) | |
| >300 (Very hard) | 15 (37.5%) | 37 (92.5%) | |
[59] | <20 (Excellent) | 0 (0%) | 13 (32.5) |
| 20–40 (Good) | 3 (7.5%) | 10 (25%) | |
| 40–60 (Permissible) | 24 (60%) | 13 (32.5%) | |
| 60–80 (Doubtful) | 13 (32.5%) | 4 (10%) | |
| >80 (Unsuitable) | 0 (0%) | 0 (0%) | |
[60] | <10 (Excellent) | 37 (92.5%) | 39 (97.5%) |
| 10–18 (Good) | 3 (7.5%) | 1 (2.5%) | |
| 18–26 (Doubtful) | 0 (0%) | 0 (0%) | |
| >26 (Unsuitable) | 0 (0%) | 0 (0%) | |
[61] | 100–75% (Good) | 23 (57.5%) | 1 (2.5%) |
| 75–25% (Moderate) | 17 (42.5%) | 28 (70%) | |
| <25% (Poor) | 0 (0%) | 11 (27.5%) | |
[62] | <50 (Suitable) | 40 (100%) | 27 (67.5%) |
| >50 (Unsuitable) | 0 (0%) | 13 (32.5%) | |
[63] | <1.25 (Safe) | 40 (100%) | 40 (100%) |
| 1.25–2.5 (Moderate) | 0 (0%) | 0 (0%) | |
| >2.5 (Unsuitable) | 0 (0%) | 0 (0%) | |
[64] | <1 (Suitable) | 12 (30%) | 25 (62.5%) |
| >1 (Unsuitable) | 28 (70%) | 15 (37.5%) | |
| Parameters | pH | EC | TDS | Ca2+ | Mg2+ | Na+ | K+ | Cl− | HCO3− | SO42− | NO3− | F− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January-21 | ||||||||||||
| pH | 1.00 | 0.15 | 0.09 | 0.09 | 0.19 | 0.01 | 0.05 | −0.01 | 0.23 | −0.11 | −0.30 | 0.07 |
| EC | −0.16 | 1.00 | 0.91 | 0.73 | 0.85 | 0.52 | 0.49 | 0.54 | 0.82 | 0.49 | −0.09 | 0.45 |
| TDS | −0.16 | 1.00 | 1.00 | 0.61 | 0.74 | 0.66 | 0.61 | 0.83 | 0.53 | 0.42 | 0.05 | 0.47 |
| Ca2+ | 0.01 | 0.91 | 0.91 | 1.00 | 0.72 | −0.09 | −0.11 | 0.36 | 0.68 | 0.18 | −0.07 | 0.40 |
| Mg2+ | 0.02 | 0.91 | 0.91 | 1.00 | 1.00 | 0.14 | 0.12 | 0.46 | 0.77 | 0.24 | −0.17 | 0.38 |
| Na+ | −0.26 | 0.96 | 0.96 | 0.77 | 0.77 | 1.00 | 0.96 | 0.54 | 0.16 | 0.53 | 0.10 | 0.24 |
| K+ | −0.28 | 0.91 | 0.91 | 0.71 | 0.70 | 0.95 | 1.00 | 0.51 | 0.15 | 0.45 | 0.08 | 0.21 |
| Cl− | −0.23 | 0.99 | 0.99 | 0.86 | 0.85 | 0.98 | 0.92 | 1.00 | 0.00 | 0.14 | 0.20 | 0.39 |
| HCO3− | 0.04 | 0.60 | 0.60 | 0.62 | 0.62 | 0.54 | 0.39 | 0.55 | 1.00 | 0.33 | −0.24 | 0.26 |
| SO42− | 0.02 | 0.84 | 0.84 | 0.90 | 0.91 | 0.71 | 0.75 | 0.77 | 0.48 | 1.00 | −0.28 | 0.37 |
| NO3− | −0.12 | 0.82 | 0.82 | 0.75 | 0.75 | 0.79 | 0.70 | 0.79 | 0.81 | 0.67 | 1.00 | −0.18 |
| F− | −0.17 | 0.26 | 0.26 | 0.19 | 0.19 | 0.30 | 0.27 | 0.27 | 0.13 | 0.28 | 0.36 | 1.00 |
| January-20 | ||||||||||||
| Parameters | January 2020 | January 2021 | ||||
|---|---|---|---|---|---|---|
| F_1 | F_2 | F_1 | F_2 | F_3 | F_4 | |
| pH | 0.10 | −0.86 | 0.11 | 0.04 | −0.15 | 0.93 |
| EC | 0.94 | 0.31 | 0.87 | 0.44 | −0.08 | 0.05 |
| TDS | 0.94 | 0.31 | 0.75 | 0.63 | 0.16 | 0.01 |
| Ca2+ | 0.96 | 0.03 | 0.93 | −0.15 | 0.06 | −0.03 |
| Mg2+ | 0.96 | 0.02 | 0.91 | 0.09 | −0.01 | 0.14 |
| Na+ | 0.84 | 0.46 | 0.07 | 0.98 | −0.03 | −0.02 |
| K+ | 0.78 | 0.50 | 0.03 | 0.96 | −0.02 | 0.05 |
| Cl− | 0.89 | 0.39 | 0.44 | 0.60 | 0.48 | −0.02 |
| HCO3− | 0.73 | −0.12 | 0.80 | 0.03 | −0.33 | 0.16 |
| SO42− | 0.89 | 0.08 | 0.26 | 0.51 | −0.62 | −0.36 |
| NO3− | 0.84 | 0.21 | −0.12 | 0.09 | 0.79 | −0.27 |
| F− | 0.18 | 0.49 | 0.49 | 0.27 | −0.21 | −0.17 |
| Total | 7.80 | 1.86 | 4.19 | 3.22 | 1.45 | 1.15 |
| % of Variance | 64.99 | 15.50 | 34.93 | 26.85 | 12.11 | 9.55 |
| Cumulative % | 64.99 | 80.48 | 34.93 | 61.78 | 73.88 | 83.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karthikeyan, S.; Kulandaisamy, P.; Senapathi, V.; Chung, S.Y.; Thangaraj, K.; Arumugam, M.; Sugumaran, S.; Ho-Na, S. Hydrogeochemical Survey along the Northern Coastal Region of Ramanathapuram District, Tamilnadu, India. Appl. Sci. 2022, 12, 5595. https://doi.org/10.3390/app12115595
Karthikeyan S, Kulandaisamy P, Senapathi V, Chung SY, Thangaraj K, Arumugam M, Sugumaran S, Ho-Na S. Hydrogeochemical Survey along the Northern Coastal Region of Ramanathapuram District, Tamilnadu, India. Applied Sciences. 2022; 12(11):5595. https://doi.org/10.3390/app12115595
Chicago/Turabian StyleKarthikeyan, Sivakumar, Prabakaran Kulandaisamy, Venkatramanan Senapathi, Sang Yong Chung, Kongeswaran Thangaraj, Muruganantham Arumugam, Sathish Sugumaran, and Sung Ho-Na. 2022. "Hydrogeochemical Survey along the Northern Coastal Region of Ramanathapuram District, Tamilnadu, India" Applied Sciences 12, no. 11: 5595. https://doi.org/10.3390/app12115595








