Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Algal Biomass
2.3. Preparation of Microalgal Extracts
2.4. Extract Fractionation
2.5. Evaluation of Bioactivities
2.5.1. Antioxidant Activity—DPPH Scavenging and Redox Metal Chelation
2.5.2. Calcium Chelating Activity
2.5.3. Anti-Tumoral Activity
Cell Cultivation
In Vitro Cytotoxic Activity
2.5.4. Anti-Inflammatory Activity
Cell Viability Assessment
Inflammatory Assays
2.6. Chemical Characterization
2.7. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Bioactivities of Microalgal Extracts
3.1.1. Antioxidant Activity
DPPH Scavenging
Redox-Metal Chelation
3.1.2. Calcium Chelating Activity
3.1.3. Anti-Tumoral Activity
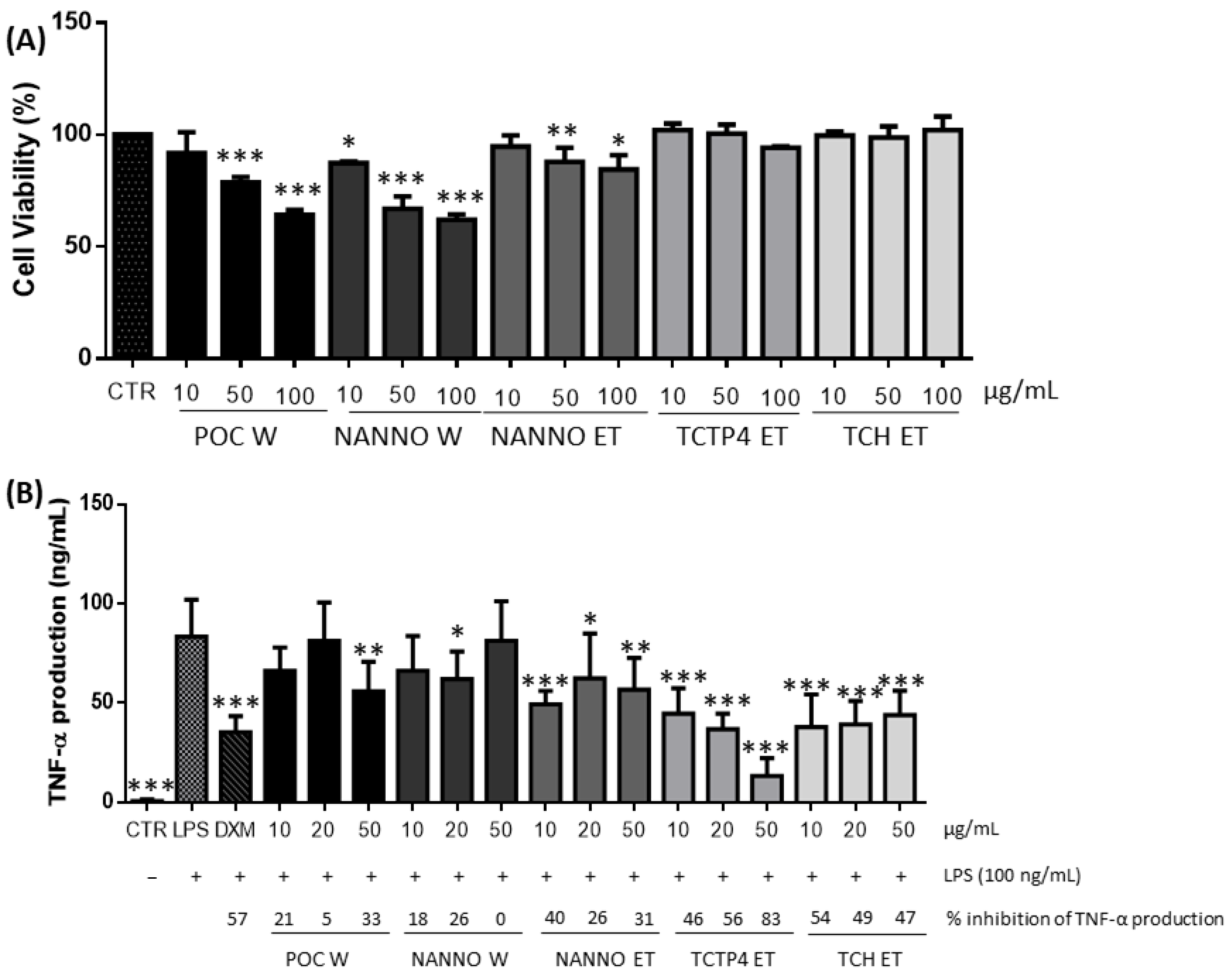
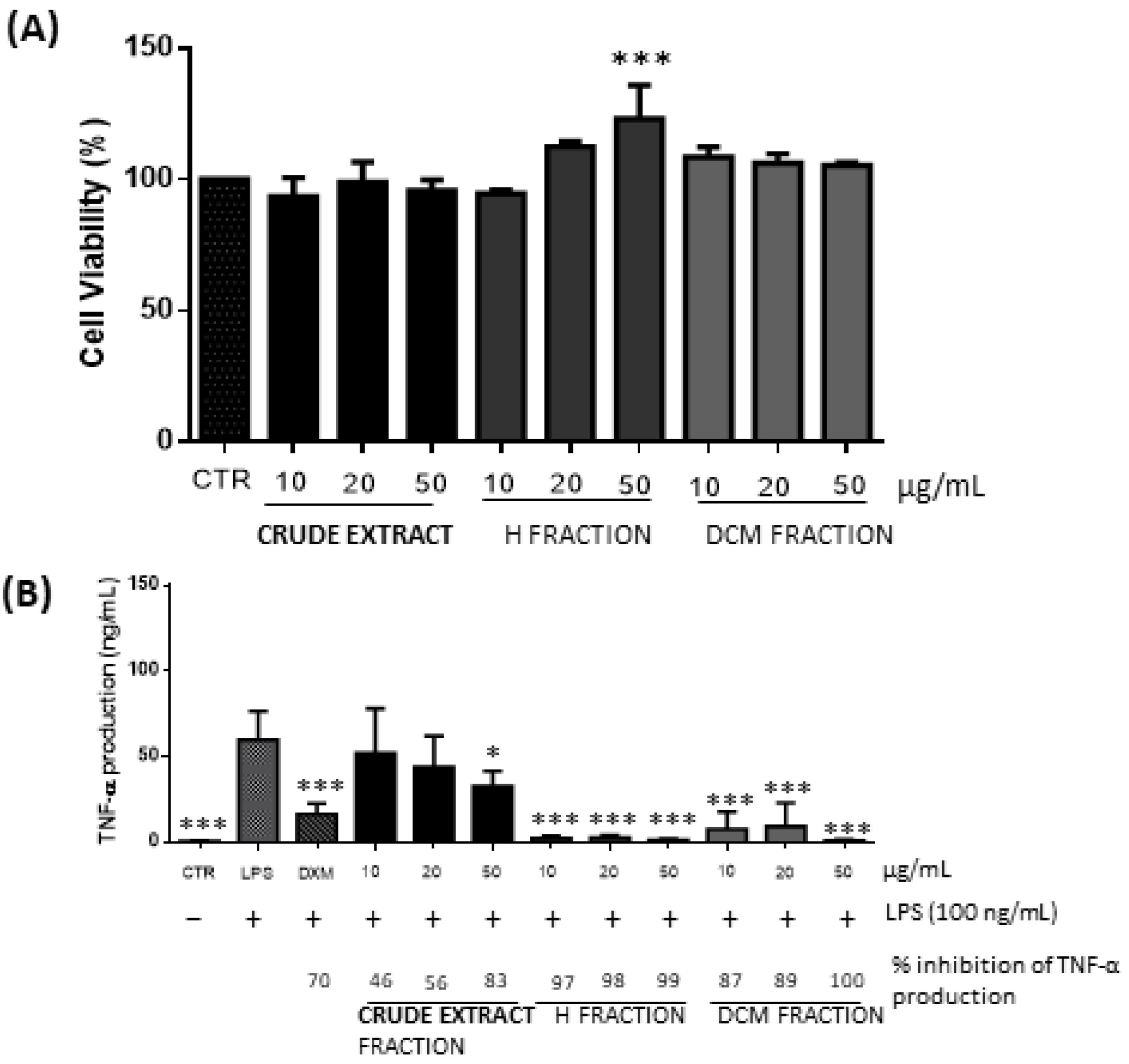
3.1.4. Anti-Inflammatory Activity
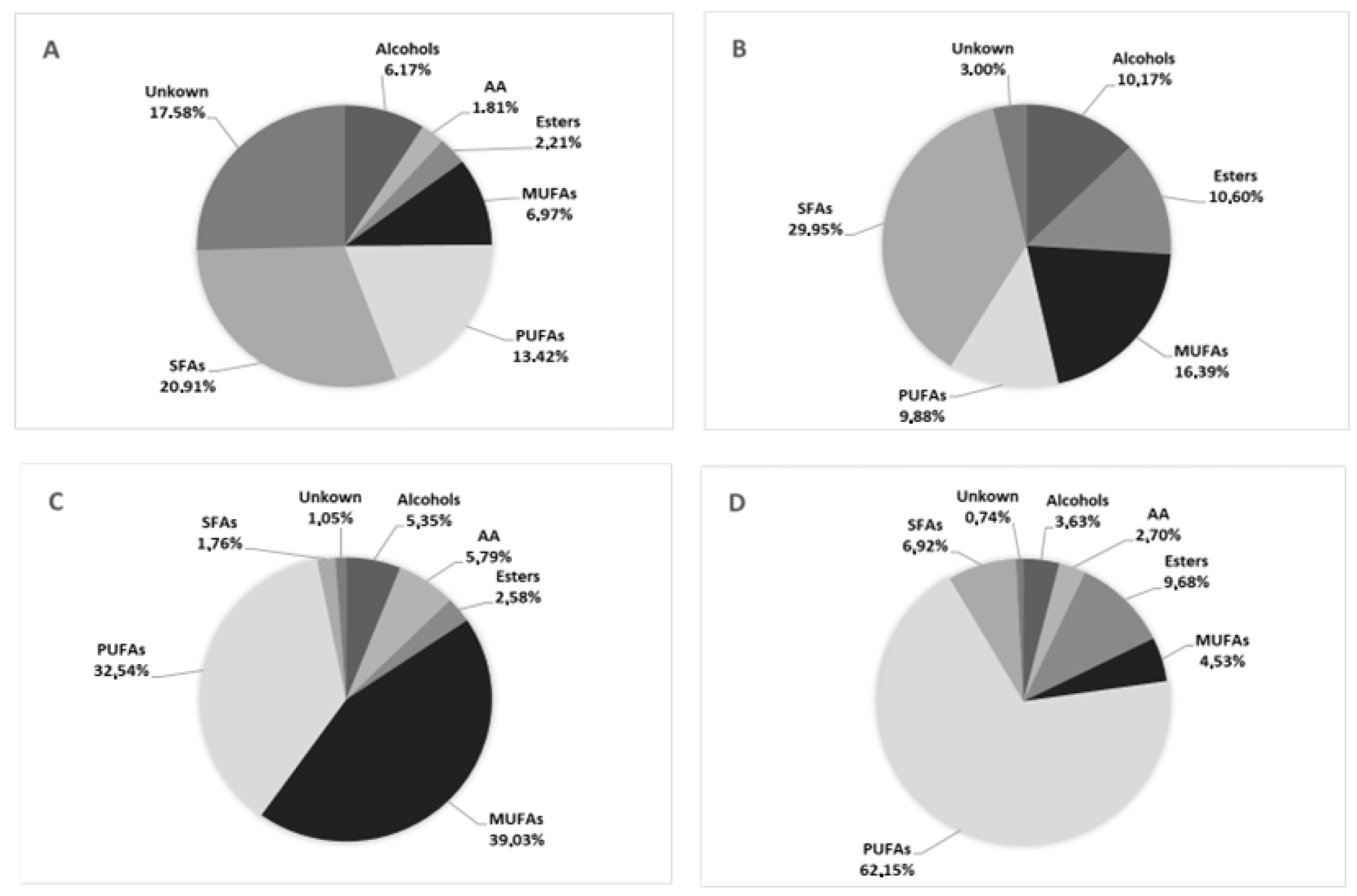
3.2. Chemical Characterization of Promising Microalgal Extracts’ Fractions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89–96. [Google Scholar] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-M.; Song, B.C.; Yeum, K.-J. Impact of volatile anesthetics on oxidative stress and inflammation. BioMed Res. Int. 2015, 2015, 242709. [Google Scholar] [CrossRef]
- Dales, J.-P.; Desplat-Jégo, S. Metal imbalance in neurodegenerative diseases with a specific concern to the brain of multiple sclerosis patients. Int. J. Mol. Sci. 2020, 21, 9105. [Google Scholar] [CrossRef]
- Prakash, A.; Dhaliwal, G.K.; Kumar, P.; Majeed, A.B.A. Brain biometals and Alzheimer’s disease—Boon or bane? Int. J. Neurosci. 2017, 127, 99–108. [Google Scholar] [CrossRef]
- Sheikh, S.; Safia; Haque, E.; Mir, S.S. Neurodegenerative diseases: Multifactorial conformational diseases and their therapeutic interventions. J. Neurodegener. Dis. 2013, 2013, 563481. [Google Scholar] [CrossRef] [Green Version]
- Ferdous, U.T.; Yusof, Z.N.B. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a Source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef]
- Wahab, M.; Zahoor, M.; Muhammad Salman, S.; Kamran, A.W.; Naz, S.; Burlakovs, J.; Kallistova, A.; Pimenov, N.; Zekker, I. Adsorption-membrane hybrid approach for the removal of azithromycin from water: An attempt to minimize drug resistance problem. Water 2021, 13, 1969. [Google Scholar] [CrossRef]
- Morais, E.G.; Cristofoli, N.L.; Maia, I.B.; Magina, T.; Cerqueira, P.R.; Teixeira, M.R.; Varela, J.; Barreira, L.; Gouveia, L. Microalgal systems for wastewater treatment: Technological trends and challenges towards waste recovery. Energies 2021, 14, 8112. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Vázquez, M.; Navarro, F.; Fuentes, J.L.; Bermejo, E.; Garbayo, I.; Vílchez, C.; Cuaresma, M. Chemically-Induced production of anti-inflammatory molecules in microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporgno, M.P.; Mathys, A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef]
- Rotter, A.; Bacu, A.; Barbier, M.; Bertoni, F.; Bones, A.M.; Cancela, M.L.; Carlsson, J.; Carvalho, M.F.; Cegłowska, M.; Dalay, M.C.; et al. A new network for the advancement of marine biotechnology in Europe and beyond. Front. Mar. Sci. 2020, 7, 278. [Google Scholar] [CrossRef]
- Jareonsin, S.; Pumas, C. Advantages of heterotrophic microalgae as a host for phytochemicals production. Front. Bioeng. Biotechnol. 2021, 9, 628597. [Google Scholar] [CrossRef]
- Kumar, G.; Shekh, A.; Jakhu, S.; Sharma, Y.; Kapoor, R.; Sharma, T.R. Bioengineering of microalgae: Recent advances, perspectives, and regulatory challenges for industrial application. Front. Bioeng. Biotechnol. 2020, 8, 914. [Google Scholar] [CrossRef]
- Moreira, D.; Pires, J.C.M. Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresour. Technol. 2016, 215, 371–379. [Google Scholar] [CrossRef]
- Bagwell, C.E.; Abernathy, A.; Barnwell, R.; Milliken, C.E.; Noble, P.A.; Dale, T.; Beauchesne, K.R.; Moeller, P.D.R. Discovery of bioactive metabolites in biofuel microalgae that offer protection against predatory bacteria. Front. Microbiol. 2016, 7, 516. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimzadeh, M.A.; Khalili, M.; Dehpour, A.A. Antioxidant activity of ethyl acetate and methanolic extracts of two marine algae, Nannochloropsis oculata and Gracilaria gracilis—An in vitro assay. Braz. J. Pharm. Sci. 2018, 54, 1. [Google Scholar] [CrossRef] [Green Version]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as sustainable bio-factories of healthy lipids: Evaluating fatty acid content and antioxidant activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef] [PubMed]
- Gangadhar, K.N.; Pereira, H.; Rodrigues, M.J.; Custódio, L.; Barreira, L.; Malcata, F.X.; Varela, J. Microalgae-based unsaponifiable matter as source of natural antioxidants and metal chelators to enhance the value of wet Tetraselmis chuii biomass. Open Chem. 2016, 14, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.R.; Hamouda, R.A.; Tayel, A.A.; Al-Saman, M.A. Anti-cholesterol and antioxidant activities of independent and combined microalgae aqueous extracts in vitro. Waste Biomass Valorization 2021, 12, 4845–4857. [Google Scholar] [CrossRef]
- Casas-Arrojo, V.; Decara, J.; de Los Ángeles Arrojo-Agudo, M.; Pérez-Manríquez, C.; Abdala-Díaz, R.T. Immunomodulatory, antioxidant activity and cytotoxic effect of sulfated polysaccharides from Porphyridium cruentum. (S.F.Gray) Nägeli. Biomolecules 2021, 11, 488. [Google Scholar] [CrossRef]
- Nacer, W.; Baba Ahmed, F.Z.; Merzouk, H.; Benyagoub, O.; Bouanane, S. Evaluation of the anti-inflammatory and antioxidant effects of the microalgae Nannochloropsis gaditana in streptozotocin-induced diabetic rats. J. Diabetes Metab. Disord. 2020, 19, 1483–1490. [Google Scholar] [CrossRef]
- Saranya, C.; Hemalatha, A.; Chermapandi, P.; Anantharaman, P. Evaluation of antioxidant properties, total phenolic and carotenoid content of Chaetoceros calcitrans, Chlorella salina and Isochrysis galbana. Int. J. Curr. Microbiol. App.Sci. 2014, 3, 365–377. [Google Scholar]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, A carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2012, 131, 134–140. [Google Scholar] [CrossRef]
- Bhattacharjee, M. Pharmaceutically valuable bioactive compounds of algae. Asian J. Pharm. Clin. Res. 2016, 9, 43–47. [Google Scholar] [CrossRef]
- Manrique, R.; Moreno, J.L.; Villagracia, A.R.; Ubando, A.; Kasai, H.; Arboleda, N.; David, M.; Culaba, A. Effects of salinity on the CO2 permeation across lipid bilayer for microalgae biofixation: A molecular dynamics study. J. Appl. Phycol. 2018, 30, 55–61. [Google Scholar] [CrossRef]
- Ismael, A.; Henriques, M.S.C.; Marques, C.; Rodrigues, M.; Barreira, L.; Paixão, J.A.; Fausto, R.; Cristiano, M.L.S. Exploring saccharinate-tetrazoles as selective Cu(II) ligands: Structure, magnetic properties and cytotoxicity of copper(II) complexes based on 5-(3-aminosaccharyl)-tetrazoles. RSC Adv. 2016, 6, 71628–71637. [Google Scholar] [CrossRef]
- Araújo, N.; Viegas, C.; Pontes, J.F.; Marreiros, C.; Raimundo, P.; Macedo, A.L.; Alves de Matos, A.; Grenha, A.; Vermeer, C.; Costa Simes, D. Nanoencapsulation as a novel delivery approach for therapeutic applications of gla-rich protein (GRP). Ann. Med. 2021, 53, S23–S24. [Google Scholar] [CrossRef]
- Araújo, N.; Viegas, C.S.B.; Zubía, E.; Magalhães, J.; Ramos, A.; Carvalho, M.M.; Cruz, H.; Sousa, J.P.; Blanco, F.J.; Vermeer, C.; et al. Amentadione from the alga Cystoseira usneoides as a novel osteoarthritis protective agent in an ex vivo co-culture OA model. Mar. Drugs 2020, 18, 624. [Google Scholar] [CrossRef] [PubMed]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Martín-Álvarez, P.J.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Aminina, N.M.; Karaulova, E.P.; Vishnevskaya, T.I.; Yakush, E.V.; Kim, Y.-K.; Nam, K.-H.; Son, K.-T. Characteristics of polyphenolic content in brown algae of the Pacific coast of Russia. Mol. Basel Switz. 2020, 25, 3909. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Yau, S.K.; Khong, N.M.H.; Chan, K.W.; Ebrahimi, M. Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. J. Biotechnol. 2017, 241, 175–183. [Google Scholar] [CrossRef]
- Zullaikah, S.; Jessinia, M.C.P.; Rinaldi; Yasmin, M.; Rachimoellah, M.; Wu, D.W. Lipids extraction from wet and unbroken microalgae Chlorella vulgaris using subcritical water. Mater. Sci. Forum 2019, 964, 103–108. [Google Scholar] [CrossRef]
- Ardiles, P.; Cerezal-Mezquita, P.; Salinas-Fuentes, F.; Órdenes, D.; Renato, G.; Ruiz-Domínguez, M.C. Biochemical composition and phycoerythrin extraction from red microalgae: A comparative study using green extraction technologies. Processes 2020, 8, 1628. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Barra, L.; Carillo, S.; Caruso, T.; Corsaro, M.M.; Dal Piaz, F.; Graziani, G.; Corato, F.; Pepe, D.; Manfredonia, A.; et al. Light modulation of biomass and macromolecular composition of the diatom Skeletonema marinoi. J. Biotechnol. 2014, 192, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Kranthi, K.R.; Sardar, U.R.; Bhargavi, E.; Devi, I.; Bhunia, B.; Tiwari, O.N. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: A critical review. Carbohydr. Polym. 2018, 199, 353–364. [Google Scholar] [CrossRef]
- Priatni, S.; Ratnaningrum, D.; Warya, S.; Audina, E. Phycobiliproteins production and heavy metals reduction ability of Porphyridium sp. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012006. [Google Scholar] [CrossRef]
- Pratiwi, D.C.; Pratiwi, N.; Yona, D.; Sasmita, R.D.; Pratiwi, I.A. Microalgae Skeletonema costatum for Cd and Cu remediation. Asian J. Water Environ. Pollut. 2020, 17, 43–48. [Google Scholar] [CrossRef]
- Canabady-Rochelle, L.L.S.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J.-M. Determination of reducing power and metal chelating ability of antioxidant peptides: Revisited methods. Food Chem. 2015, 183, 129–135. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, W.; Auckloo, B.N.; Wu, B. Several classes of natural products with metal ion chelating ability. Curr. Org. Chem. 2015, 19, 1935–1953. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Kheeree, N.; Kuptawach, K.; Puthong, S.; Sangtanoo, P.; Srimongkol, P.; Boonserm, P.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. Discovery of calcium-binding peptides derived from defatted lemon basil seeds with enhanced calcium uptake in human intestinal epithelial cells, caco-2. Sci. Rep. 2022, 12, 4659. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, J.; Chen, S.; Huang, Y.; Chen, W.; He, L.; Zhang, Y. Role of calcium homeostasis in Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2022, 18, 487–498. [Google Scholar] [CrossRef]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2018, 70, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Vizetto-Duarte, C.; Custódio, L.; Acosta, G.; Lago, J.H.G.; Morais, T.R.; Bruno de Sousa, C.; Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Lima, R.T.; et al. Can macroalgae provide promising anti-tumoral compounds? A closer look at Cystoseira tamariscifolia as a source for antioxidant and anti-hepatocarcinoma compounds. PeerJ 2016, 4, e1704. [Google Scholar] [CrossRef] [PubMed]
- Castell, J.V.; Jover, R.; Martnez-Jimnez, C.P.; Gmez-Lechn, M.J. Hepatocyte cell lines: Their use, scope and limitations in drug metabolism studies. Expert Opin. Drug Metab. Toxicol. 2006, 2, 183–212. [Google Scholar] [CrossRef]
- Saide, A.; Damiano, S.; Ciarcia, R.; Lauritano, C. Promising Activities of marine natural products against hematopoietic malignancies. Biomedicines 2021, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.M.M.; Sousa, E.P.; Barreira, L.; Marques, C.; Rodrigues, M.J.; Pinho e Melo, T.M.V.D. Synthesis and anti-cancer activity of chiral tetrahydropyrazolo[1,5-a]pyridine-fused steroids. Steroids 2017, 122, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Morales, S.A.T.; Aguilar, M.G.d.; Pereira, R.C.G.; Duarte, L.P.; Sousa, G.F.; Oliveira, D.M.d.; Evangelista, F.C.G.; Sabino, A.P.; Viana, R.O.; Alves, V.S.; et al. Constituents from roots of Maytenus distichophylla, antimicrobial activity and toxicity for cells and Caenorhabditis elegans. Quím. Nova 2020, 43, 1066–1073. [Google Scholar] [CrossRef]
- López-Lázaro, M. A simple and reliable approach for assessing anticancer activity in vitro. Curr. Med. Chem. 2015, 22, 1324–1334. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 1–48. [Google Scholar] [CrossRef]
- Schüler, L.M.; Gangadhar, K.N.; Duarte, P.; Placines, C.; Molina-Márquez, A.M.; Léon-Bañares, R.; Sousa, V.S.; Varela, J.; Barreira, L. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (Chlorophyta). Bioprocess Biosyst. Eng. 2020, 43, 785–796. [Google Scholar] [CrossRef]
- Schüler, L.; Greque de Morais, E.; Trovão, M.; Machado, A.; Carvalho, B.; Carneiro, M.; Maia, I.; Soares, M.; Duarte, P.; Barros, A.; et al. Isolation and characterization of novel Chlorella vulgaris mutants with low chlorophyll and improved protein contents for food applications. Front. Bioeng. Biotechnol. 2020, 8, 469. [Google Scholar] [CrossRef]
- Schüler, L.M.; Bombo, G.; Duarte, P.; Santos, T.F.; Maia, I.B.; Pinheiro, F.; Marques, J.; Jacinto, R.; Schulze, P.S.C.; Pereira, H.; et al. Carotenoid biosynthetic gene expression, pigment and n-3 fatty acid contents in carotenoid-rich Tetraselmis striata CTP4 strains under heat stress combined with high light. Bioresour. Technol. 2021, 337, 125385. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.W. Improvement of chemical composition of Tisochrysis lutea grown mixotrophically under nitrogen depletion towards biodiesel production. Molecules 2020, 25, 4609. [Google Scholar] [CrossRef] [PubMed]
- Andrianasolo, E.H.; Haramaty, L.; Vardi, A.; White, E.; Lutz, R.; Falkowski, P. Apoptosis-inducing galactolipids from a cultured marine diatom, Phaeodactylum tricornutum. J. Nat. Prod. 2008, 71, 1197–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, F.-Z.; Wang, Q.; Zhang, Q.; Chang, L.-L.; Li, W. Omega-3 polyunsaturated fatty acid inhibits the malignant progression of hepatocarcinoma by inhibiting the wnt/β-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4500–4508. [Google Scholar] [CrossRef]
- Hussein, H.A.; Abdullah, M.A. Anticancer compounds derived from marine diatoms. Mar. Drugs 2020, 18, 356. [Google Scholar] [CrossRef]
- Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Gaspar, H.; Malcata, F.X.; Barreira, L.; Varela, J. Anti-hepatocellular carcinoma (HepG2) activities of monoterpene hydroxy lactones isolated from the marine microalga Tisochrysis lutea. Mar. Drugs 2020, 18, 567. [Google Scholar] [CrossRef]
- Le Goff, M.; Delbrut, A.; Quinton, M.; Pradelles, R.; Bescher, M.; Burel, A.; Schoefs, B.; Sergent, O.; Lagadic-Gossmann, D.; Le Ferrec, E.; et al. Protective action of Ostreococcus tauri and Phaeodactylum tricornutum extracts towards benzo[a]pyrene-induced cytotoxicity in endothelial cells. Mar. Drugs 2020, 18, 3. [Google Scholar] [CrossRef] [Green Version]
- Cayot, N.; Lafarge, C.; Bou-Maroun, E.; Cayot, P. Substitution of carcinogenic solvent dichloromethane for the extraction of volatile compounds in a fat-free model food system. J. Chromatogr. A 2016, 1456, 77–88. [Google Scholar] [CrossRef]
- Li, Y.; Ghasemi Naghdi, F.; Garg, S.; Adarme-Vega, T.C.; Thurecht, K.J.; Ghafor, W.A.; Tannock, S.; Schenk, P.M. A Comparative study: The impact of different lipid extraction methods on current microalgal lipid research. Microb. Cell Factories 2014, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Xia, D.; Qiu, W.; Wang, X.; Liu, J. Recent advancements and future perspectives of microalgae-derived pharmaceuticals. Mar. Drugs 2021, 19, 703. [Google Scholar] [CrossRef]
- Ulusoy Erol, H.B.; Menegazzo, M.L.; Sandefur, H.; Gottberg, E.; Vaden, J.; Asgharpour, M.; Hestekin, C.N.; Hestekin, J.A. Porphyridium cruentum grown in ultra-filtered swine wastewater and its effects on microalgae growth productivity and fatty acid composition. Energies 2020, 13, 3194. [Google Scholar] [CrossRef]
- Sharmin, T.; Monirul Hasan, C.M.; Aftabuddin, S.; Rahman, M.A.; Khan, M. Growth, fatty acid, and lipid composition of marine microalgae Skeletonema costatum available in bangladesh coast: Consideration as biodiesel feedstock. J. Mar. Biol. 2016, 2016, e6832847. [Google Scholar] [CrossRef] [Green Version]
- Branco-Vieira, M.; Martin, S.S.; Agurto, C.; Santos, M.A.; Freitas, M.A.V.; Caetano, N.S. Analyzing Phaeodactylum tricornutum lipid profile for biodiesel production. Energy Procedia 2017, 136, 369–373. [Google Scholar] [CrossRef]
- Cardoso, C.; Pereira, H.; Franca, J.; Matos, J.; Monteiro, I.; Pousão-Ferreira, P.; Gomes, A.; Barreira, L.; Varela, J.; Neng, N.; et al. Lipid composition and some bioactivities of 3 newly isolated microalgae (Tetraselmis sp. IMP3, Tetraselmis sp. CTP4, and Skeletonema sp.). Aquac. Int. 2020, 28, 711–727. [Google Scholar] [CrossRef]
- Calviello, G.; Su, H.-M.; Weylandt, K.H.; Fasano, E.; Serini, S.; Cittadini, A. Experimental evidence of -3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: Their potential role in inflammatory, neurodegenerative, and neoplastic diseases. BioMed Res. Int. 2013, 2013, e743171. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.A.; Delattre, A.M.; Carabelli, B.; Pudell, C.; Bortolanza, M.; Staziaki, P.V.; Visentainer, J.V.; Montanher, P.F.; Del Bel, E.A.; Ferraz, A.C. Neuroprotective effect of omega-3 polyunsaturated fatty acids in the 6-OHDA model of Parkinson’s disease is mediated by a reduction of inducible nitric oxide synthase. Nutr. Neurosci. 2018, 21, 341–351. [Google Scholar] [CrossRef] [PubMed]
- El-Fayoumy, E.A.; Shanab, S.M.M.; Gaballa, H.S.; Tantawy, M.A.; Shalaby, E.A. Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complement. Med. Ther. 2021, 21, 51. [Google Scholar] [CrossRef]
- Kang, M.-K.; Nielsen, J. Biobased production of alkanes and alkenes through metabolic engineering of microorganisms. J. Ind. Microbiol. Biotechnol. 2017, 44, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Enema, O.; Umoh, U.; Thomas, P.; Adesina, S.; Eseyin, O. Phytochemical and antioxidant studies of leaf of Tetrapleura tetraptera (schum and thon) taubert (mimosaceae). Brifish J. Pharm. Med. Res. 2019, 4, 1865–1875. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Jayalakshmi, M.; Pushpabharathi, N.; Varadharaj, V. Identification of bioactive components in Enhalus acoroides seagrass extract by gas chromatography–mass spectrometry. Asian J. Pharm. Clin. Res. 2018, 11, 313. [Google Scholar] [CrossRef]
- Salaün, J. Cyclopropane derivatives and their diverse biological activities. In Small Ring Compounds in Organic Synthesis VI; de Meijere, A., Ed.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2000; Volume 207, pp. 1–67. ISBN 978-3-540-66471-0. [Google Scholar]
- Yehye, W.A.; Abdul Rahman, N.; Alhadi, A.A.; Khaledi, H.; Weng, N.S.; Ariffin, A. Butylated hydroxytoluene analogs: Synthesis and evaluation of their multipotent antioxidant activities. Molecules 2012, 17, 7645–7665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi, S.M.; Habibi, A.; Dolati, H.; Shahcheragh, S.M.; Sardari, S.; Azerang, P. Synthesis and antimicrobial evaluation of 4H-pyrans and schiff bases fused 4H-pyran derivatives as inhibitors of Mycobacterium bovis (BCG). Iran. J. Pharm. Res. IJPR 2018, 17, 1229–1239. [Google Scholar] [PubMed]
- Luo, X.; Su, P.; Zhang, W. Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Ham, Y.-M.; Heo, S.-J.; Yoon, S.-A.; Cho, S.-H.; Kwon, S.-H.; Jeong, M.S.; Jeon, Y.-J.; Sanjeewa, K.; Yoon, W.-J.; et al. Anti-inflammation effects of 8-Oxo-9-octadecenoic acid isolated from Undaria peterseniana in lipopolysaccharide-stimulated macrophage cells. EXCLI J. 2018, 17, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; D’Ambrosio, M.; Cinci, L.; Niccolai, A.; Biondi, N.; Rodolfi, L.; Dos Santos Nascimiento, L.B.; Tredici, M.R.; Luceri, C. A Comparative in vitro evaluation of the anti-inflammatory effects of a Tisochrysis lutea extract and fucoxanthin. Mar. Drugs 2021, 19, 334. [Google Scholar] [CrossRef] [PubMed]
| Microalga/Positive Control | Extract | DPPH Scavenging Activity (%) | IC50 (mg/mL) |
|---|---|---|---|
| Porphyridium sp. (POC) | W | 87.5 ± 10.0 | 0.535 ± 0.001 d |
| ET | 59.1 ± 1.0 | 0.566 ± 0.001 d | |
| EA | 105 ± 3 | 0.524 ± 0.001 d | |
| Nannochloropsis sp. (NANNO) | W | 51.0 ± 5.2 | n.d. |
| ET | 80.3 ± 2.3 | 0.539 ± 0.030 d | |
| EA | 81.7 ± 2.2 | 0.990 ± 0.024 b | |
| Tetraselmis striata CTP4 (TCTP4) | W | 23.2 ± 3.7 | n.d. |
| ET | 58.1 ± 1.5 | 1.17 ± 0.01 a | |
| EA | 37.8 ± 3.2 | n.d. | |
| Tisochrysis lutea (TIL) | W | 42.2 ± 3.3 | n.d. |
| ET | 41.6 ± 0.2 | n.d. | |
| EA | 97.8 ± 2.1 | 0.794 ± 0.001 c | |
| Phaeodactylum tricornutum (PHA) | W | 26.8 ± 2.2 | n.d. |
| ET | 61.8 ± 1.3 | 0.575 ± 0.004 d | |
| EA | 94.9 ± 3.3 | 0.452 ± 0.002 d | |
| Skeletonema sp. (SKE) | W | 24.1 ± 3.1 | n.d. |
| ET | 71.1 ± 1.2 | 0.478 ± 0.054 d | |
| EA | 73.3 ± 7.25 | 0.447 ± 0.060 d | |
| Spirulina (SPR) | ET | 71.5 ± 0.3 | 0.923 ± 0.001 b |
| Tetraselmis chui (TCH) | ET | 66.8 ± 1.0 | 1.14 ± 0.01 a |
| Haematococcus pluvialis (HPL) | ET | 103 ± 58 | 0.408 ± 0.007 d |
| BHT * | - | 94.1 ± 2.8 | n.d. |
| Microalga | Extract | % Cu2+ CA | IC50 (mg/mL) for Cu2+CA | % Fe2+ CA | IC50 (mg/mL) for Fe2+CA |
|---|---|---|---|---|---|
| Porphyridium sp. (POC) | W | 52.5 ± 3.1 | n.d. | 34.7 ± 0.2 | n.d. |
| ET | 62.5 ± 0.3 | 0.144 ± 0.004 a | 71.7 ± 0.1 | 0.0854 ± 0.0140 a | |
| EA | 82.4 ± 2.0 | 0.337 ± 0.013 c | 47.6 ± 0.5 | n.d. | |
| Nannochloropsis sp. (NANNO) | W | 72.0 ± 0.3 | 0.621 ± 0.018 e | 83.2 ± 0.1 | 0.279 ± 0.014 c |
| ET | 43.5 ± 0.6 | n.d. | 19.7 ± 0.5 | n.d. | |
| EA | 3.6 ± 0.40 | n.d. | 4.03 ± 0.1 | n.d. | |
| Tetraselmis striata CTP4 (TCTP4) | W | 31.6 ± 0.7 | n.d. | 96.6 ± 0.7 a | 0.385 ± 0.003 d |
| ET | 54.4 ± 6.5 | n.d. | 3.23 ± 0.15 | n.d. | |
| EA | 39.0 ± 5.6 | n.d. | 33.2 ± 0.4 | n.d. | |
| Tisochrysis lutea (TIL) | W | 72.6 ± 0.4 | 0.184 ± 0.003 a | 79.0 ± 1.9 | 0.403 ± 0.013 d |
| ET | 85.5 ± 0.1 | 0.228 ± 0.008 b | 75.0 ± 0.1 | 0.207 ± 0.006 b | |
| EA | 67.8 ± 0.5 | 0.297 ± 0.009 c | 42.7 ± 1.0 | n.d. | |
| Phaeodactylum tricornutum (PHA) | W | 62.8 ± 1.5 | n.d. | 75.2 ± 0.9 | 0.508 ± 0.005 e |
| ET | 74.4 ± 0.6 | 0.358 ± 0.008 c | 58.8 ± 1.4 | n.d. | |
| EA | 65.0 ± 0.9 | 0.737 ± 0.025 f | 11.8 ± 0.1 | n.d. | |
| Skeletonema sp. (SKE) | W | 74.0 ± 0.5 | 0.434 ± 0.012 d | 63.8 ± 0.9 | n.d. |
| ET | 87.1 ± 0.1 | 0.195 ± 0.007 a | 53.4 ± 0.1 | n.d. | |
| EA | 76.3 ±0.1 | 0.487 ± 0.030 d | 42.4 ± 0.6 | n.d. | |
| Spirulina (SPR) | W | 74.0 ± 0.5 | n.d. | 1.71 ± 0.21 | n.d. |
| ET | 60.0 ± 1.7 | n.d. | 6.04 ± 0.62 | n.d. | |
| EA | 52.3 ± 0.1 | n.d. | 4.46 ± 0.27 | n.d. | |
| Tetraselmis chui (TCH) | W | 19.8 ± 4.8 | n.d. | 4.90 ± 0.23 | n.d. |
| ET | 54.4 ± 6.5 | n.d. | 16.6 ± 0.7 | n.d. | |
| EA | 39.0 ± 5.6 | n.d. | 15.8 ± 0.3 | n.d. | |
| Haematococcus pluvialis (HPL) | W | 20.3 ± 0.3 | n.d. | 1.90 ± 1.20 | n.d. |
| ET | 51.9 ± 0.2 | n.d. | 6.60 ± 0.73 | n.d. | |
| EA | 59.5 ± 0.1 | n.d. | 4.71 ± 0.48 | n.d. | |
| EDTA * | - | 91.0 ± 0.3 | n.d. | 96.1 ± 0.2 | n.d. |
| Microalgal Extract | Fractionating Solvent | % Cu2+ CA | IC50 (mg/mL) for Cu2+CA | % Fe2+ CA | IC50 (mg/mL) for Fe2+CA |
|---|---|---|---|---|---|
| Porphyridium sp. (POC ET) | Crude ET extract | 74.1 ± 0.6 | 0.272 ± 0.010 b | 77.4 ± 0.1 | 0.047 ± 0.002 a |
| H | 81.2 ± 0.1 | 0.046 ± 0.004 a | 85.2 ± 0.3 | 0.026 ± 0.001 a | |
| DCM | 61.1 ± 1.1 | 0.648 ± 0.043 c | 61.1 ± 1.0 | n.d. | |
| EA | 46.6 ± 1.2 | 0.912 ± 0.023 d | 46.6 ± 1.2 | 0.384 ± 0.019 c | |
| Skeletonema sp. (SKE ET) | Crude ET extract | 83.4 ± 0.4 | 0.240 ±0.010 b | 83.5 ± 1.6 | 0.055 ± 0.002 a |
| H | 92.6 ± 0.3 | 0.036 ± 0.001 a | 97.9 ± 0.3 | 0.024 ± 0.001 a | |
| DCM | 61.4 ± 0.1 | 0.725 ± 0.038 c | 61.4 ± 0.1 | 0.386 ± 0.015 c | |
| EA | 51.0 ± 0.1 | 0.917 ± 0.056 d | 51.0 ± 0.1 | 0.246 ± 0.018 b |
| Microalga | Extract | % Ca2+ CA | IC50 (mg/mL) for Ca2+ CA |
|---|---|---|---|
| Porphyridium sp. (POC) | W | 28.6 ± 0.01 | n.d. |
| ET | 75.8 ± 0.08 | 0.0519 ± 0.0019 a | |
| EA | 67.3 ± 1.30 | 0.150 ± 0.004 c | |
| Nannochloropsis sp. (NANNO) | W | 13.2 ± 1.48 | n.d. |
| ET | 15.5 ± 0.59 | n.d. | |
| EA | 7.98 ± 0.15 | n.d. | |
| Tetraselmis striata CTP4 (TCTP4) | W | 58.6 ± 1.50 | n.d. |
| ET | 54.7 ± 1.07 | n.d. | |
| EA | 12.3 ± 0.26 | n.d. | |
| Tisochrysis lutea (TIL) | W | 6.67 ± 0.46 | n.d. |
| ET | 52.8 ± 0.63 | n.d. | |
| EA | 38.0 ± 0.12 | n.d. | |
| Phaeodactylum tricornutum (PHA) | W | 47.4 ± 0.49 | n.d. |
| ET | 35.2 ± 0.97 | n.d. | |
| EA | 48.1 ± 0.50 | n.d. | |
| Skeletonema sp. (SKE) | W | 24.3 ± 0.75 | n.d. |
| ET | 87.8 ± 1.66 | 0.0638 ± 0.0038 a | |
| EA | 86.7 ± 1.58 | 0.0906 ± 0.0001 b | |
| Spirulina (SPR) | W | 7.12 ± 0.30 | n.d. |
| ET | 48.8 ± 0.37 | n.d. | |
| EA | 19.4 ± 0.30 | n.d. | |
| Tetraselmis chui (TCH) | W | 23.1 ± 1.98 | n.d. |
| ET | 48.9 ± 0.44 | n.d. | |
| EA | 57.0 ± 1.98 | n.d. | |
| Haematococcus pluvialis (HPL) | W | 8.12 ± 0.76 | n.d. |
| ET | 0.70 ± 0.12 | n.d. | |
| EA | 2.40 ± 0.76 | n.d. | |
| EGTA * | - | 85.9 ± 0.42 | n.d. |
| Microalgal Extract | Fractionating Solvent | % Ca2+ CA | IC50 (mg/mL) for Ca2+ CA |
|---|---|---|---|
| Porphyridium sp. (POC ET) | Crude ET extract | 80.0 ± 0.3 | 0.0663 ± 0.0050 b |
| H | 95.4 ± 0.1 | 0.0281 ± 0.0001 a | |
| DCM | 52.5 ± 0.1 | 0.277 ± 0.016 d | |
| EA | 62.5 ± 0.1 | 0.189 ± 0.069 c | |
| Skeletonema sp. (SKE ET) | Crude ET extract | 88.6 ± 0.7 | 0.0850 ± 0.0030 b |
| H | 97.1 ± 0.1 | 0.0113 ± 0.0001 a | |
| DCM | 80.0 ± 0.5 | 0.104 ± 0.003 b | |
| EA | 81.5 ± 0.9 | 0.0844 ± 0.0025 b |
| Cell Line | Selectivity Index | |||||
|---|---|---|---|---|---|---|
| Microalgae | Extract | HepG2 | S17 | THP-1 | HepG2 vs. S17 | THP1 vs. S17 |
| Phaeodactylum tricornutum (PHA) | EA | 34.6 ± 3.5 a | 107 ± 7.0 c | n.d. | 3.09 ± 0.12 b | n.d. |
| Porphyridium sp. (POC) | EA | 42.3 ± 2.7 b | >125 c | n.d. | >2.7 a | n.d. |
| Tisochrysis lutea (TIL) | EA | 44.7 ± 3.1 b | 79.2 ± 2.7 b | n.d. | 1.77 ± 0.09 c | n.d. |
| Skeletonema sp. (SKE) | EA | 37.2 ± 3.6 a | 34.8 ± 5.4 a | n.d. | n.s | n.d. |
| Nannochloropsis sp. (NANNO) | EA | >125 a | >125 c | n.d. | n.s | n.d. |
| Tetraselmis striata CTP4 (TCTP4) | EA | >125 a | >125 c | n.d. | n.s | n.d. |
| Phaeodactylum tricornutum (PHA) | ET | 19.4 ± 2.2 c | 85.6 ± 4.4 c | 102.0 ± 7.00 | 4.40 ± 0.15 a | 0.844 ± 0.072 a |
| Tisochrysis lutea (TIL) | ET | 67.8 ± 2.6 a | 104 ± 4.0 b | n.d. | 1.53 ± 0.08 c | n.d. |
| Etoposide | - | 29.0 ± 3.2 c | 45.4 ± 0.5 a | 0.924 ± 0.241 | 1.56 ± 0.11 c | 49.4 ± 1.2 b |
| Microalgal Extract | Fractioning Solvent | HepG2 | S17 | THP-1 | HepG2 vs. S17 | THP1 vs. S17 |
|---|---|---|---|---|---|---|
| Phaeodactylum tricornutum (PHA ET) | Crude ET extract | 39.8 ± 4.3 b | >125 b | >125 d | >3.14 b | n.s |
| H | >125 a | >125 b | >125 d | n.s | n.s | |
| DCM | 27.5 ± 1.6 c | >125 b | 22.3 ± 1.8 c | >4.54 a | >5.60 b | |
| EA | 84.2 ± 3.7 a | >125 b | 81.9 ± 2.0 b | 1.48 ± 0.13 c | 1.53 ± 0.15 a | |
| W + ET | >125 a | >125 b | >125 d | n.s | n.s | |
| Etoposide | 29.0 ± 3.2 c | 45.4 ± 0.5 a | 0.924 ± 0.241 a | 1.56 ± 0.11 c | 49.4 ± 1.2 c |
| Relative Abundance (%) | ||||
|---|---|---|---|---|
| Compounds | Porphyridium sp. (POC ET) -Hexane Fraction | Skeletonema sp. (SKE ET) -Hexane Fraction | Phaeodactylum tricornutum (PHA ET) -Dichloromethane Fraction | Tetraselmis striata CTP4 (TCTP4) -Hexane Fraction |
| Cyclohexanol, 1-butyl | 2.61 | |||
| 2H-Pyran, 2-[(5cyclopropyl idene pentyl)oxy]tetrahydro | 2.01 | |||
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 0.88 | 7.01 | 5.35 | 1.51 |
| 4-Hexen-3-ol, 2,5-dimethyl | 0.80 | |||
| 2,3-Dimethyl-undec-1-en-3-ol | 0.73 | |||
| Phytol | 0.59 | 1.52 | ||
| 2-Octene-2-ol, 2-methoxy | 0.31 | |||
| 9,12-Octadecadieol | 0.15 | |||
| 1-Tetradecanol, 14-chloro | 0.10 | |||
| 3-[2-pentenyl]-4-methyl-tetrahydrofuran-2-one | 0.11 | 0.48 | ||
| Octadecane | 1.3 | |||
| Tetratetracontane | 0.92 | |||
| Nonadecane | 0.86 | |||
| 9,12-Octadecadien chloride, (Z,Z) | 0.16 | |||
| 9-Eicosyne | 0.79 | 1.51 | ||
| 2-hydroxy-2-methylbutane-1,4-dioic acid | 1.00 | |||
| Tetrahydropyranyl ether of citronellol | 10.60 | 1.6 | 2.96 | |
| 2-Pentadecanone, 6,10,14-trimethyl | 3.07 | |||
| trans-13-Octadecenoic acid | 3.21 | |||
| Methyl (11R,12R,13S)-(Z)-12,13-epoxy-11-ol-9-octadecenoic acid | 2.14 | |||
| 8-Octadecenoic acid | 1.90 | |||
| 15-Tetracosenoic acid, (Z) | 0.82 | |||
| 9-Hexadecenoic acid | 19.6 | 1.61 | ||
| n-Hexadecanoic acid | 17.4 | |||
| 10-Octadecenoic acid | 0.58 | |||
| 10-Undecenoic acid | 1.15 | |||
| hexadecenic acid | 0.59 | |||
| 9-Hexadecenoic acid, (Z) | 3.48 | 2.47 | ||
| 11-Octadecenoic acid | 2.04 | 5.85 | ||
| 9-Octadecenoic acid (Z) | 0.88 | 1.45 | ||
| 13-Methyltetradecanoic acid | 0.33 | |||
| 9-eicosenoic acid | 0.24 | 1.18 | ||
| 2,4-bis(1,1-dimethylethyl) Phenol/2,4-Di-tertbutylphenol | 0.35 | |||
| Phosphoric acid, monododecyl ester | 0.20 | |||
| 5,8,11,14,17-Eicosapentaenoic acid(EPA) | 2.89 | 16.7 | 0.37 | |
| 7,10-Hexadecadienoic acid | 2.17 | |||
| 6,9,12,15-octadecatetraenoate (Stearidonic acid) | 5.19 | |||
| Octadecatrienoic acid | ||||
| 9,12-Hexadecadienoic acid | 1.95 | |||
| Heneicosapentaenoic Acid | 0.84 | |||
| Arachidonic acid | 0.35 | |||
| 9,12-Octadecadienoic acid (Z,Z) | 7.22 | 6.12 | ||
| 11,13-Eicosadienoic acid | 1.98 | |||
| cis-11,14-Eicosadienoic acid | 0.94 | |||
| 8,11,14-Eicosatrienoic acid, (Z,Z,Z) | 0.87 | 0.3 | ||
| 4,7,10,13,16,19-Docosahexaenoic acid | 0.66 | 0.67 | ||
| 6,9,12-Hexadecatrienoic acid | 0.36 | 4.82 | 1.98 | 13.24 |
| γ-Linolenic acid | 0.29 | 7.2 | 3.4 | |
| 5,8,11,14-Eicosatetraenoic acid, (all-Z) | 0.21 | 0.52 | 2.01 | |
| cis-13,16-Docasadienoic acid | 0.20 | |||
| cis-7,10,13,16-Docosatetraenoic acid | 0.19 | |||
| 4,7,10,13,16,19-Docosahexaenoic acid, (all-Z) | 0.15 | 2.35 | ||
| cis-7,10,13,16-Docosatetraenoic acid | 0.13 | |||
| 12,15-Octadecadienoic acid | 0.12 | |||
| 4,7,10,13,16-Docosapentaenoic acid | 0.10 | |||
| Tridecanoic acid | 16.80 | |||
| Methyl stearate | 1.42 | |||
| Tetracosanoic acid | 0.39 | |||
| Pentadecanoic acid, 14-methyl | 0.81 | 0.38 | ||
| Eicosanoic acid | 1.38 | |||
| Heptadecanoic acid | 2.79 | |||
| Valeric acid | 1.15 | |||
| Myristoleic acid | 0.82 | |||
| Stearic acid | 0.68 | |||
| Heptadecenoic acid | 0.84 | |||
| Hexadecanoic acid | 15.80 | 0.52 | ||
| Tetradecanoic acid | 3.59 | 0.37 | ||
| Stearoic acid | 0.92 | |||
| Tetradecanoic acid, 12-methyl | 0.32 | |||
| Pentadecanoic acid | 0.28 | 9.01 | 0.27 | |
| Chloresterol | 6.66 | |||
| 24-methylcholesta-5,22-dien-3β-ol (Brassicasterol) | 1.21 | |||
| Ergosta-5,22-dien-3-ol, acetate (Brassicasterol acetate) | 0.76 | |||
| Cholesta-5,22-dien-3-ol, (3β) | 2.56 | |||
| Tetrapentacontane, 1,54-dibromo- | 3.04 | |||
| dl-α-Tocopherol | 1.16 | 0.34 | ||
| Pregna-5,9(11)-dien-20-ol-3-one ethylene ketal | 1.03 | 0.34 | ||
| Tetrahydro-2H-pyran | 0.98 | |||
| 3-Hexadecyne | 0.53 | |||
| trans-octadecadienoic acid | 6.12 | |||
| 3,6,6-trimethyl-2-Norpinanol | 2.96 | |||
| 2-methyl-5-pentyl-tetrahydrofuran | 2.96 | |||
| Tetrahydropyran 12-tetradecyn-1-ol ether | 0.6 | |||
| 3,28-bis[(tetrahydro) Lup-20(29)-en-21-ol | 0.6 | |||
| (1-Methoxy-pentyl)-cyclopropane | 0.6 | |||
| 17-chloro7-Heptadecene | 0.59 | |||
| 5.alpha.-Androstan-3-one, 17.beta.-hydroxy-4.alpha.-methyl-, | 0.48 | |||
| Cholestan-3-one, 4,4-dimethyl-, cyclic 3 | 0.48 | |||
| Total identified area | 51.51 | 78.00 | 86.96 | 83.46 |
| Unknown compound | 0.19 | 1.00 | 1.05 | 0.74 |
| Unresolved mixture of compounds | 17.20 | 1.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.-M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Appl. Sci. 2022, 12, 5877. https://doi.org/10.3390/app12125877
Silva M, Kamberovic F, Uota ST, Kovan I-M, Viegas CSB, Simes DC, Gangadhar KN, Varela J, Barreira L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Applied Sciences. 2022; 12(12):5877. https://doi.org/10.3390/app12125877
Chicago/Turabian StyleSilva, Mélanie, Farah Kamberovic, Sisay Tesema Uota, Ismael-Mohammed Kovan, Carla S. B. Viegas, Dina C. Simes, Katkam N. Gangadhar, João Varela, and Luísa Barreira. 2022. "Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals" Applied Sciences 12, no. 12: 5877. https://doi.org/10.3390/app12125877
APA StyleSilva, M., Kamberovic, F., Uota, S. T., Kovan, I.-M., Viegas, C. S. B., Simes, D. C., Gangadhar, K. N., Varela, J., & Barreira, L. (2022). Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Applied Sciences, 12(12), 5877. https://doi.org/10.3390/app12125877







