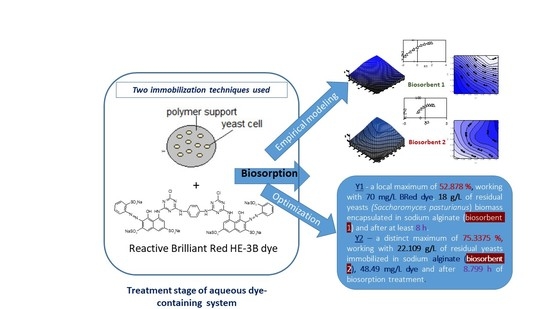
Empirical Modeling and Optimization by Active Central Composite Rotatable Design: Brilliant Red HE-3B Dye Biosorption onto Residual Yeast Biomass-Based Biosorbents
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Batch Biosorption Method
2.2.2. Empirical Modeling and Optimization of Biosorption Process
3. Results
3.1. Biosorption Process Performance
3.2. Empirical Experimental Modeling
1.8257X2X3
3.3. Optimization of the Proposed Mathematical Models
4. Discussions
- (1)
- (2)
- (3)
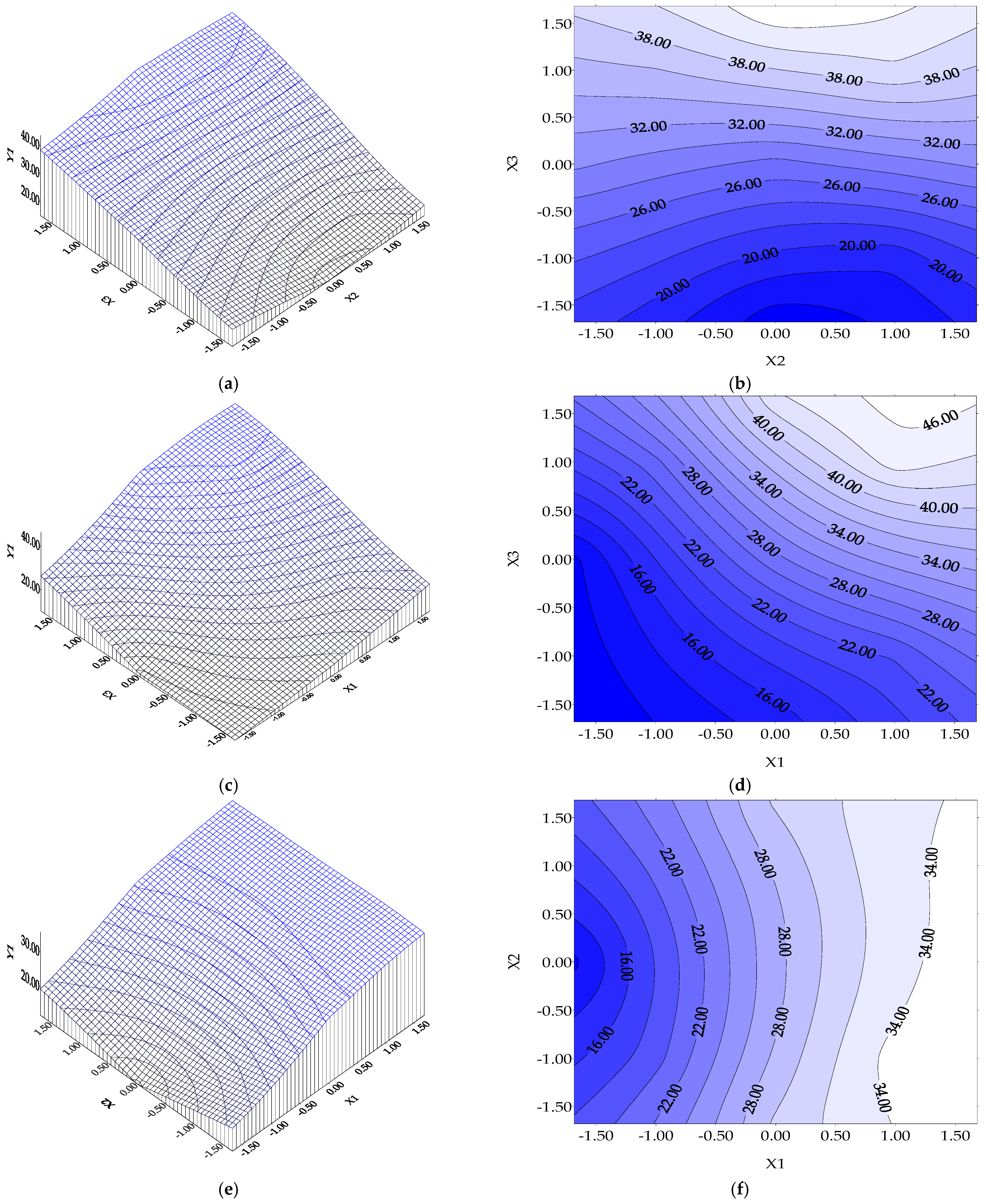
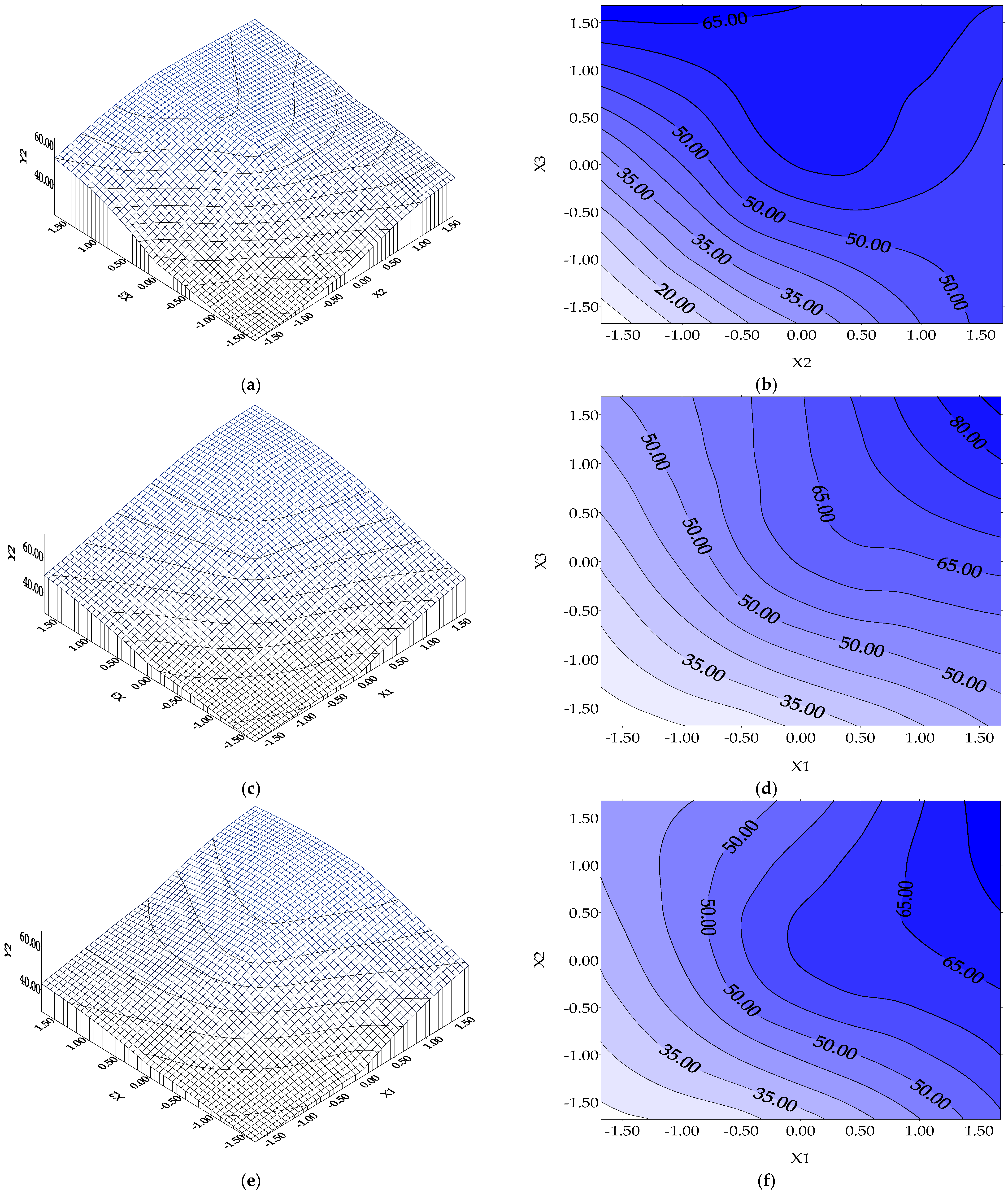
- A biosorption time higher than 8 h (as a possible exchange period at work) is beneficial but at least 6 h (basic value of X3) are obligatory for dye removals of at least 35% for Y1 (or > 52.878% experimentally performed, Figure 3e,f) and 50% for Y2, or much more than the obtained experimental value of 72.4868% (Figure 4e,f);
- (4)
- -
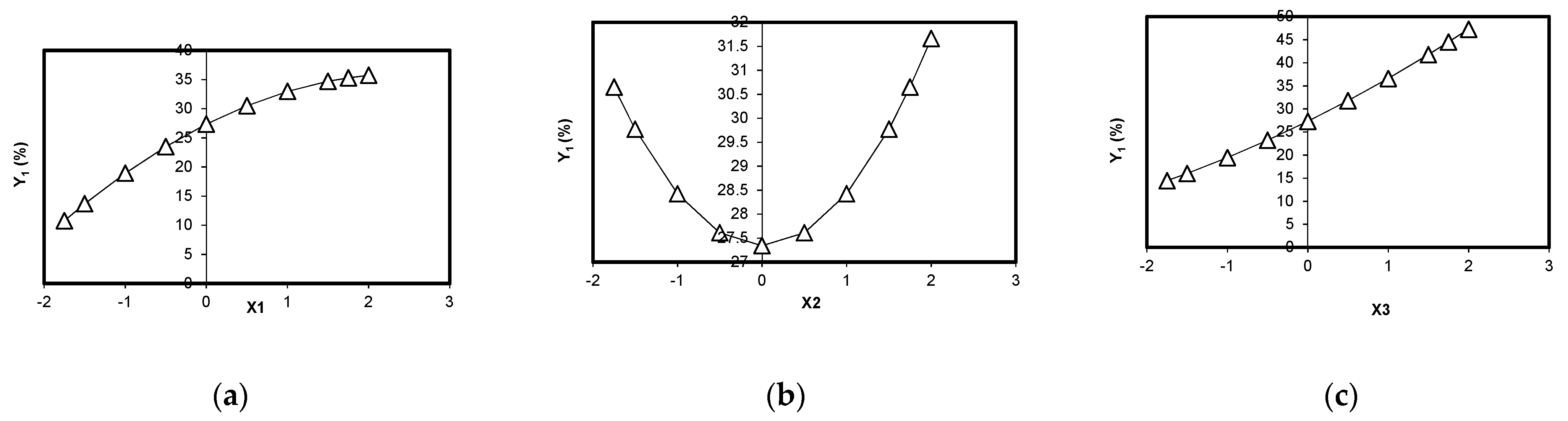
- for Y1 function (BRed dye biosorption efficiency onto biosorbent 1): the influence of initial dye concentration (X1) vs. Y1 is illustrated in Figure 5a. It seems that it is no distinct maximum but only an extreme local maximum for BRed dye removal (Y1* = 35.724%) at X1*= +2.00 corresponding to a BRed dye concentration of 100.00 mg/L, for a biosorbent 1 concentration of 12 g/L and tbiosorption of 6 h (X2 and X3 kept constant at their basic values). The dependence of BRed dye removal (Y1) vs. X2 (biosorbent 1 concentration) (X1 and X3 coded values are 0) indicates no distinct minimum of dye removal degree; thus, an extreme local maximum can be considered (Y = 31.661%) at X2* = +2.00, corresponding to a biosorbent concentration of 24 g/L (0.60 g per 25 mL), a dye concentration of 50.00 mg/L and tsorption of 6 h (Figure 5b). The dependence of reactive BRed dye removal (Y1) vs. X3 (biosorption time) (X1 and X2 coded values are 0) indicates no distinct maximum dye removal degree, thus only an extreme local maximum can be considered (Y = 47.291%) for X3*= +2.00 (i.e., a local maximum point after a S/W contact time in biosorption step of 12 h, a dye concentration of 50 mg/L and a biosorbent concentration of 12 g/L (i.e., around 0.3 g per 25 mL) (Figure 5c). The X3 variable (biosorption time) has the most significant influence on the maximum value Y1.
- -
- for Y2 function (BRed dye biosorption onto biosorbent 2): the influence of biosorbent 2 concentration (X1) vs. Y2 is evidenced in Figure 6a. It seems that there exists a distinct maximum of the BRed dye removal (Y2* = 67.189%) at X1* = +1.4048 corresponding to a biosorbent 2 concentration of 20.4288 g/L (0.5107 g per 25 mL), for a BRed dye concentration of 50 mg/L and tbiosorption of 6 h (X2 and X3 kept constant at their basic values). The dependence of BRed dye removal (Y2) vs. X2 (dye concentration) (X1 and X3 coded values are 0) indicates a distinct maximum dye removal degree (Y = 61.852%) at X2* = +0.4402, corresponding to a biosorbent concentration of 14.6412 g/L (0.366 g per 25 mL), a dye concentration of 50.00 mg/L and tsorption of 6 h (Figure 6b). The dependence of reactive BRed dye removal (Y2) vs. X3 (biosorption time) (X1 and X2 coded values are 0) indicates a distinct maximum dye removal degree (Y = 66.207%) for X3*= +1.1133 (i.e., a maximum point after a S/W contact time in biosorption step of 8.226 h, a dye concentration of 50 mg/L and a biosorbent concentration of 12 g/L (i.e., around 0.30 g per 25 mL) (Figure 6c).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Suteu, D.; Zaharia, C.; Blaga, A.C. Biosorption-current bioprocess for wastewater treatment (chapter 10). In Current Topics, Concepts and Research Priorities in Environmental Chemistry; Zaharia, C., Ed.; ‘Al.I. Cuza’ University Ed.: Iasi, Romania, 2012; Volume I, pp. 221–244. [Google Scholar]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Daza-López, E.V.; Fernández-Andrade, K.J.; Nóbrega, R.Q.; Zambrano-Intriago, L.A.; Ramos, G.V.; Quiroz-Fernández, L.S.; Montenegro, M.C.B.S.M.; Rodríguez-Díaz, J.M. Modified or Functionalized Natural Bioadsorbents: New Perspectives as Regards the Elimination of Environmental Pollutants. In Advances in the Domain of Environmental Biotechnology. Environmental and Microbial Biotechnology; Maddela, N.R., García Cruzatty, L.C., Chakraborty, S., Eds.; Springer: Singapore, 2021; pp. 195–225. [Google Scholar] [CrossRef]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Żywicka, A.; Wenelska, K.; Junka, A.; Chodaczek, G.; Szymczyk, P.; Fijałkowski, K. Immobilization pattern of morphologically different microorganisms on bacterial cellulose membranes. World J. Microbiol. Biotechnol. 2019, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as Support for Microbial Biomass-Based Adsorbents with Applications in Removal of Heavy Metals and Dyes. Polymers 2021, 13, 2893. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaban, M.I.; Alharbi, N.K.; Albarakaty, F.M.; Alharthi, S.; Hassan, S.H.A.; Fawzy, M.A. Experimental Modeling Investigations on the Biosorption of Methyl Violet 2B Dye by the Brown Seaweed Cystoseira Tamariscifolia. Sustainability 2022, 14, 5285. [Google Scholar] [CrossRef]
- Chwastowski, J.; Staroń, P. Influence of Saccharomyces cerevisiae yeast cells immobilized on Cocos nucifera fibers for the adsorption of Pb(II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127735. [Google Scholar] [CrossRef]
- Suteu, D.; Blaga, A.C.; Cimpoesu, R.; Puițel, A.C.; Tataru-Farmus, R.-E. Composites Based on Natural Polymers and Microbial Biomass for Biosorption of Brilliant Red HE-3B Reactive Dye from Aqueous Solutions. Polymers 2021, 13, 4314. [Google Scholar] [CrossRef] [PubMed]
- Suteu, D.; Blaga, A.C.; Zaharia, C.; Cimpoesu, R.; Puițel, A.C.; Tataru-Farmus, R.-E.; Tanasă, A.M. Polysaccharides Used in Biosorbents Preparation for Organic Dyes Retaining from Aqueous Media. Polymers 2022, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- Iacob Tudose, E.T.; Zaharia, C. Textile Wastewater Treatment on a Spinning Disc Reactor: Characteristics, Performances and Empirical Modeling. Appl. Sci. 2020, 10, 8687. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D. A preliminary modeling and optimization study of a homogenous advanced oxidation process applied for an industrial colored effluent. J. Environ. Prot. Ecol. 2014, 15, 1680–1689. [Google Scholar]
- Macoveanu, M.; Nicu, V. Basis of the chemical technology. Methodology of mathematical modeling in the chemical industry. In Romanian: Bazele Tehnologiei Chimice. Metodologia Modelarii Matematice in Industria Chimica; “Gheorghe Asachi” Technical University Publishing House: Iasi, Romania, 1987. [Google Scholar]
- Cojocaru, C.; Cretu, V.C.; Preda, C.; Macoveanu, M.; Cretescu, I. Response surface methodology applied for investigation of diesel oil spill removal from surface water using textile sorbents. J. Environ. Prot. Ecol. 2010, 11, 643–656. [Google Scholar]
- Cojocaru, C.; Macoveanu, M.; Cretescu, I. Peat-based sorbents for the removal of oil spills from water surface: Application of artificial neural network modeling. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 675–684. [Google Scholar] [CrossRef]
- Betianu, C.; Caliman, F.A.; Gavrilescu, M.; Cretescu, I.; Cojocaru, C.; Poulios, I. Response surface methodology applied for Orange II photocatalytic degradation in TiO2 aqueous suspensions. J. Chem. Technol. Biotechnol. 2008, 83, 1454–1465. [Google Scholar] [CrossRef]
- Cretescu, I.; Diaconu, M.; Cojocaru, C.; Benchea, R.E.; Pohontu, C. Removal of Dunkel Blau dye from aqueous solutions by fungal and peat biomass in batch mode. Environ. Eng. Manag. J. 2010, 9, 107–112. [Google Scholar] [CrossRef]
- Secula, M.S.; Cagnon, B.; Creţescu, I.; Diaconu, M.; Petrescu, S. Removal of an acid dye from aqueous solutions by adsorption on a commercial granular activated carbon: Equilibrium, kinetic and thermodynamic study. Sci. Study Res.-Chem. Eng. Biotechnol. Food Ind. 2011, 12, 307–322. [Google Scholar]
- Cojocaru, C.; Cretescu, I.; Macoveanu, M.; Diaconu, M.; Kicsi, A. Studies on diesel oil spills removal from water surface using peat. Process modelling and optimization. Environ. Eng. Manag. J. 2004, 3, 247–257. [Google Scholar] [CrossRef]
- Rodrigues, M.I.; Iemma, A.F. Experimental Design and Process Optimization, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781482299557-CAT# K24472. [Google Scholar]

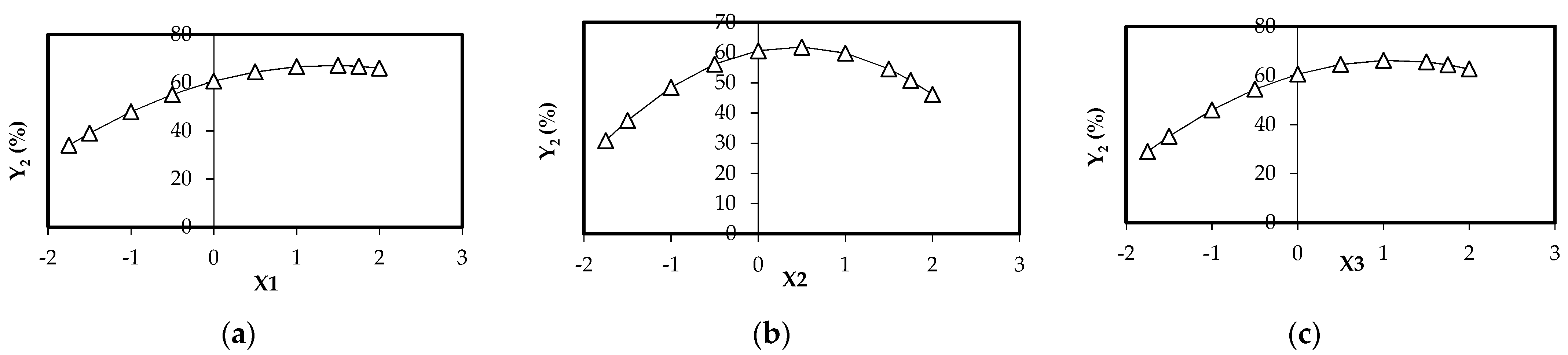
| Variable/Value | Real Variable (Zi) | Coded Variable (Xi) | Real Basic Variable (Zi0) | Variation Step (∆Zi0) |
|---|---|---|---|---|
| Biosorbent 1: Residual yeast biomass prepared by using a Buchi microencapsulator | ||||
| Dye concentration, (mg/L) | Z1 | X1 | 50 | 20 |
| Residual encapsulated biomass, (g/L) | Z2 | X2 | 12 | 6 |
| Biosorption (S/L) contact time, (h) | Z3 | X3 | 6 | 2 |
| Biosorbent 2: Residual biomass immobilized in sodium alginate by simple dropping technique | ||||
| Residual immobilized biomass, (g/ L) | Z1 | X1 | 12 | 6 |
| Dye concentration, (mg/L) | Z2 | X2 | 50 | 20 |
| Biosorption (S/L) contact time, (h) | Z3 | X3 | 6 | 2 |
| Exp. No. | Z1, (mg/L) | Z2, (g/L) | Z3, (h) | X1 | X2 | X3 | Yei (%) | Yci (%) | Deviation (A) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 6 | 4 | −1 | −1 | −1 | 13.624 | 16.023 | −0.176 |
| 2 | 30 | 18 | 4 | 1 | −1 | −1 | 31.878 | 25.887 | 0.188 |
| 3 | 70 | 6 | 4 | −1 | 1 | −1 | 14.362 | 13.828 | 0.037 |
| 4 | 70 | 18 | 4 | 1 | 1 | −1 | 16.795 | 20.779 | −0.237 |
| 5 | 30 | 6 | 8 | −1 | −1 | 1 | 31.217 | 23.939 | 0.233 |
| 6 | 30 | 18 | 8 | 1 | −1 | 1 | 47.751 | 44.991 | 0.058 |
| 7 | 70 | 6 | 8 | −1 | 1 | 1 | 26.350 | 29.047 | −0.102 |
| 8 | 70 | 18 | 8 | 1 | 1 | 1 | 52.878 | 47.186 | 0.108 |
| 9 | 50 | 1.908 | 6 | −1.682 | 0 | 0 | 11.557 | 11.581 | −0.002 |
| 10 | 50 | 22.092 | 6 | 1.682 | 0 | 0 | 30.498 | 35.132 | −0.152 |
| 11 | 16.36 | 12 | 6 | 0 | −1.682 | 0 | 31.266 | 29.240 | 0.064 |
| 12 | 83.64 | 12 | 6 | 0 | 1.682 | 0 | 31.270 | 30.907 | 0.012 |
| 13 | 50 | 12 | 2.64 | 0 | 0 | −1.682 | 19.823 | 14.881 | 0.249 |
| 14 | 50 | 12 | 9.36 | 0 | 0 | 1.682 | 49.278 | 40.721 | 0.174 |
| 15 | 50 | 12 | 6 | 0 | 0 | 0 | 23.756 | 27.342 | −0.151 |
| 16 | 50 | 12 | 6 | 0 | 0 | 0 | 24.318 | 27.342 | −0.124 |
| 17 | 50 | 0.60 | 6 | 0 | 0 | 0 | 23.274 | 27.342 | −0.175 |
| 18 | 50 | 0.60 | 6 | 0 | 0 | 0 | 24.478 | 27.342 | −0.117 |
| 19 | 50 | 0.60 | 6 | 0 | 0 | 0 | 23.596 | 27.342 | −0.159 |
| 20 | 50 | 0.60 | 6 | 0 | 0 | 0 | 23.917 | 27.342 | −0.143 |
| Exp. No. | Z1, (g/L) | Z2, (mg/L) | Z3, (h) | X1 | X2 | X3 | Yei (%) | Yci (%) | Deviation (A) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 30 | 4 | −1 | −1 | −1 | 13.6243 | 17.9134 | −0.3148 |
| 2 | 18 | 30 | 4 | 1 | −1 | −1 | 34.7884 | 33.6922 | 0.0315 |
| 3 | 6 | 70 | 4 | −1 | 1 | −1 | 33.3531 | 38.3054 | −0.1485 |
| 4 | 18 | 70 | 4 | 1 | 1 | −1 | 48.4867 | 54.7038 | −0.1282 |
| 5 | 6 | 30 | 8 | −1 | −1 | 1 | 52.6316 | 44.7842 | 0.1491 |
| 6 | 18 | 30 | 8 | 1 | −1 | 1 | 72.4868 | 65.9042 | 0.0908 |
| 7 | 6 | 70 | 8 | −1 | 1 | 1 | 47.0588 | 46.5250 | 0.0113 |
| 8 | 18 | 70 | 8 | 1 | 1 | 1 | 74.1839 | 68.2646 | 0.0798 |
| 9 | 1.908 | 50 | 6 | −1.682 | 0 | 0 | 36.6774 | 35.3798 | 0.0354 |
| 10 | 22.092 | 50 | 6 | 1.682 | 0 | 0 | 63.3226 | 66.9328 | −0.0570 |
| 11 | 12 | 16.36 | 6 | 0 | −1.682 | 0 | 34.6505 | 41.5375 | −0.1988 |
| 12 | 12 | 83.64 | 6 | 0 | 1.682 | 0 | 51.9091 | 49.6046 | 0.0444 |
| 13 | 12 | 50 | 2.64 | 0 | 0 | −1.682 | 44.9438 | 30.7559 | 0.3157 |
| 14 | 12 | 50 | 9.36 | 0 | 0 | 1.682 | 69.6629 | 65.8538 | 0.0547 |
| 15 | 12 | 50 | 6 | 0 | 0 | 0 | 56.7416 | 60.6002 | −0.0680 |
| 16 | 12 | 50 | 6 | 0 | 0 | 0 | 55.3772 | 60.6002 | −0.0943 |
| 17 | 0.60 | 50 | 6 | 0 | 0 | 0 | 56.8218 | 60.6002 | −0.0665 |
| 18 | 0.60 | 50 | 6 | 0 | 0 | 0 | 58.1059 | 60.6002 | −0.0429 |
| 19 | 0.60 | 50 | 6 | 0 | 0 | 0 | 54.8957 | 60.6002 | −0.1039 |
| 20 | 0.60 | 50 | 6 | 0 | 0 | 0 | 56.5008 | 60.6002 | −0.0726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharia, C.; Suteu, D. Empirical Modeling and Optimization by Active Central Composite Rotatable Design: Brilliant Red HE-3B Dye Biosorption onto Residual Yeast Biomass-Based Biosorbents. Appl. Sci. 2022, 12, 6377. https://doi.org/10.3390/app12136377
Zaharia C, Suteu D. Empirical Modeling and Optimization by Active Central Composite Rotatable Design: Brilliant Red HE-3B Dye Biosorption onto Residual Yeast Biomass-Based Biosorbents. Applied Sciences. 2022; 12(13):6377. https://doi.org/10.3390/app12136377
Chicago/Turabian StyleZaharia, Carmen, and Daniela Suteu. 2022. "Empirical Modeling and Optimization by Active Central Composite Rotatable Design: Brilliant Red HE-3B Dye Biosorption onto Residual Yeast Biomass-Based Biosorbents" Applied Sciences 12, no. 13: 6377. https://doi.org/10.3390/app12136377