Development of Screen-Printable Nafion Dispersion for Electrochemical Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Nafion pH Adjustment
2.3. Nafion Solvent Replacement
2.4. Screen Printing and Stencil Coating
2.5. Characterization of Nafion
2.6. Electrochemical Sensor Performance Tests
3. Results
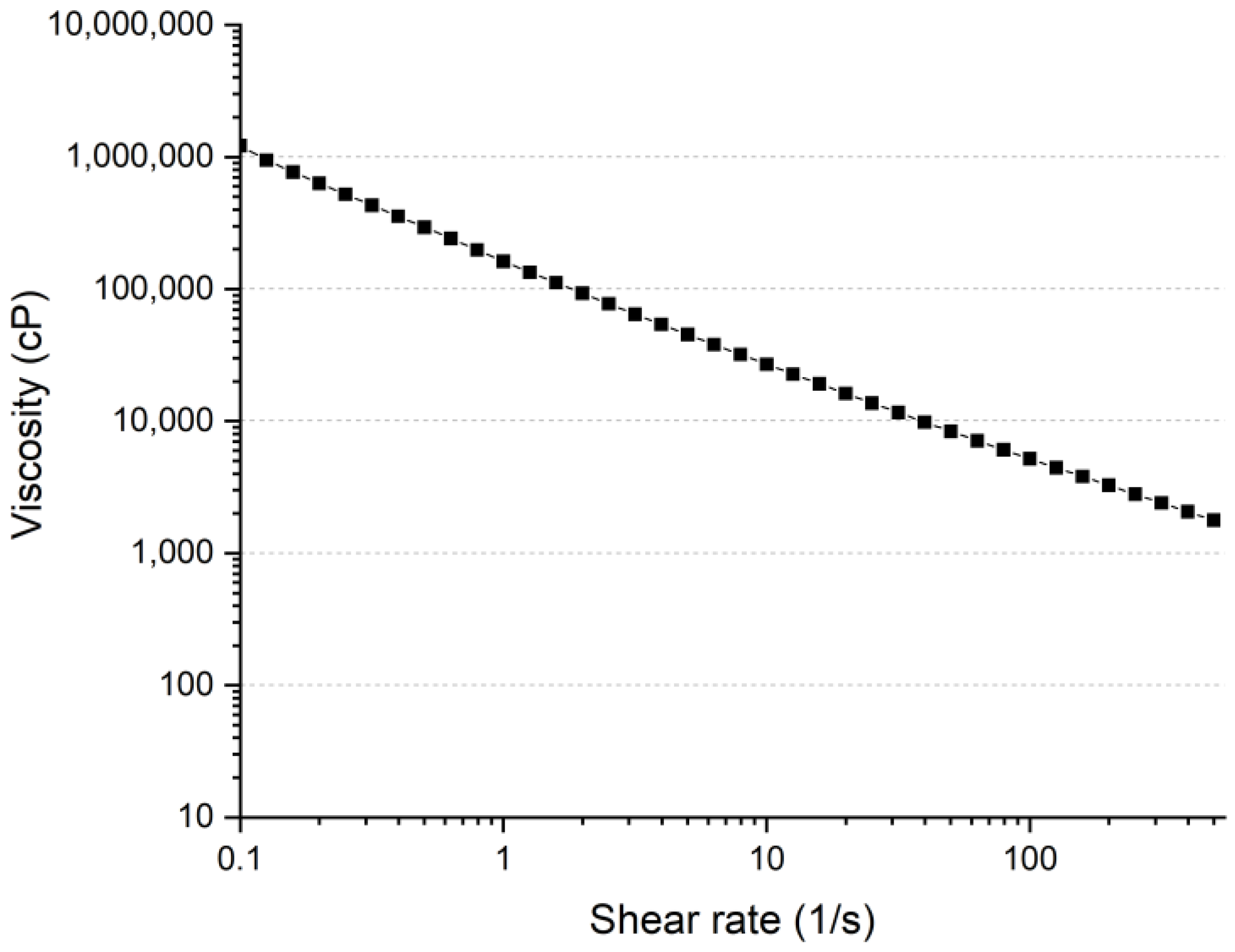
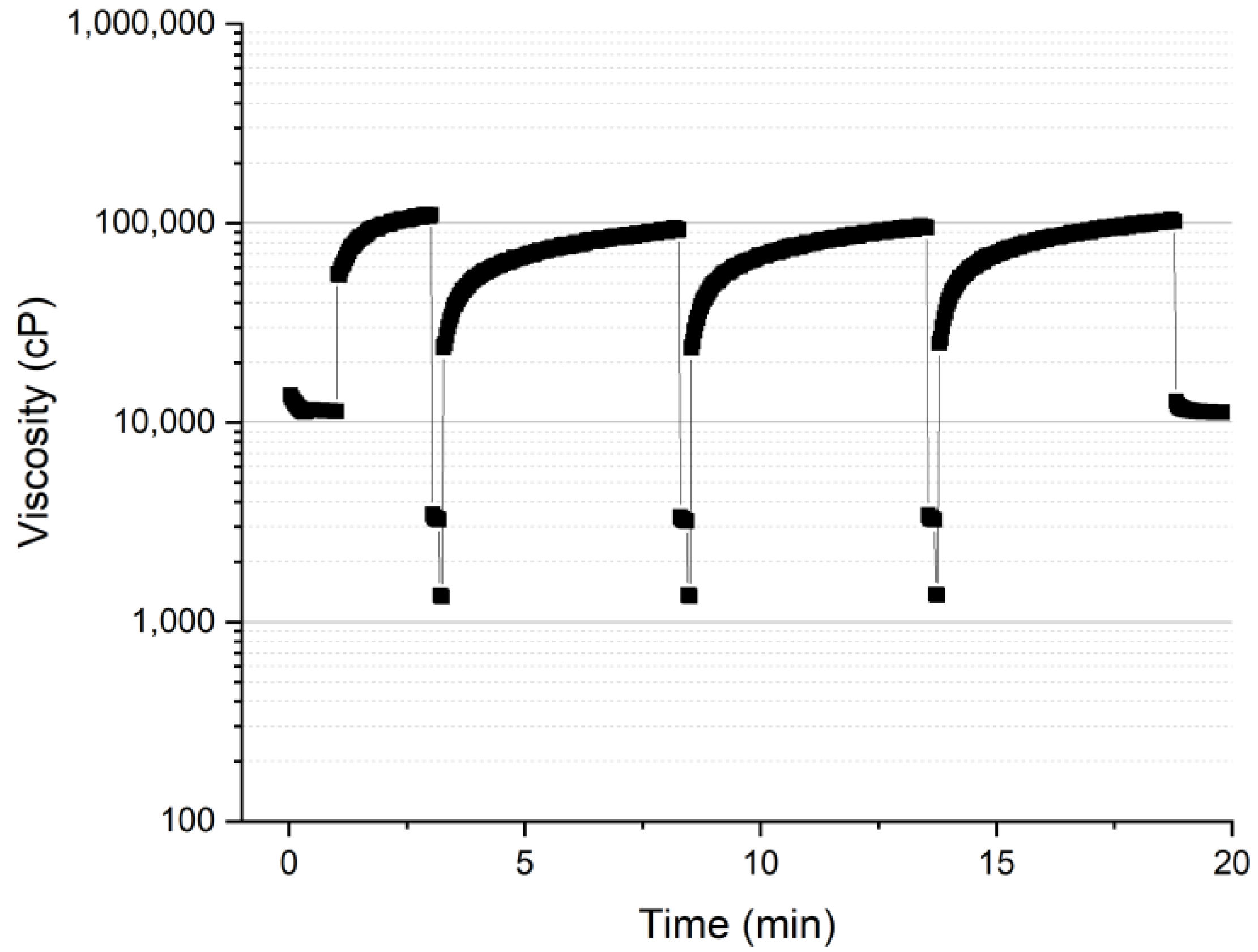
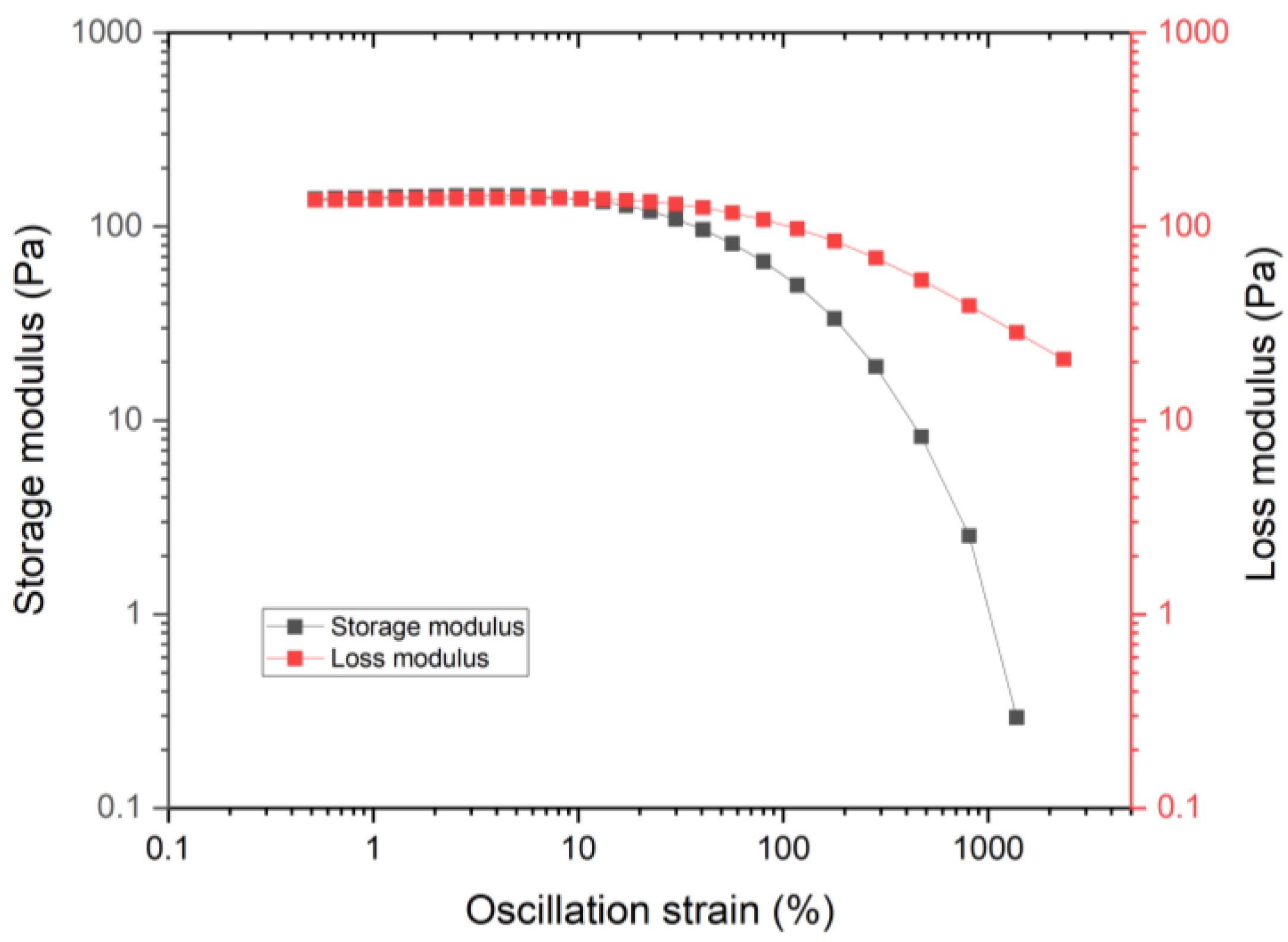
3.1. Rheology Properties
3.1.1. Shear Thinning
3.1.2. Thixotropy
3.1.3. Yield Stress
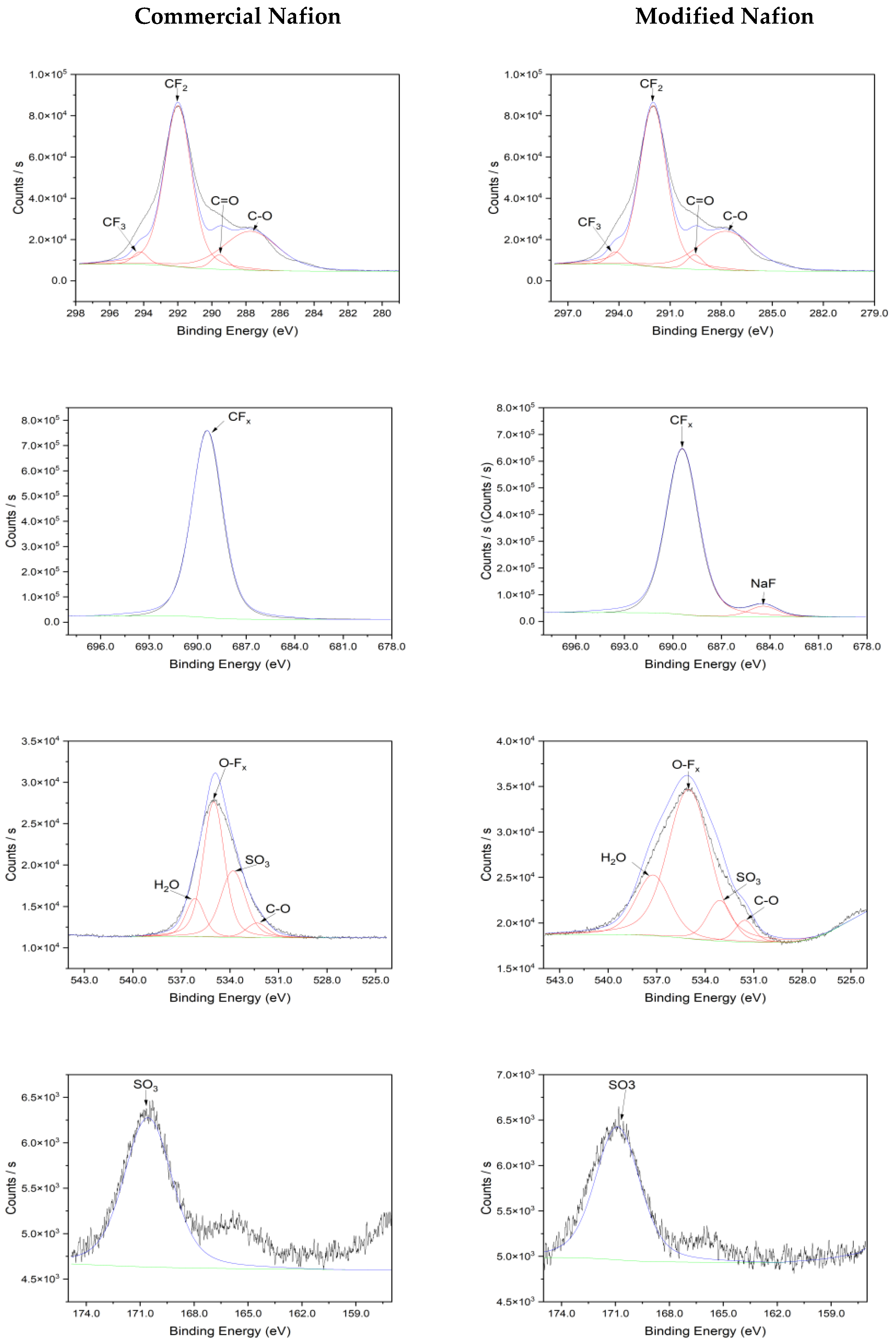
3.2. Characterization of Nafion Membrane
3.3. Detection of Heavy Metals
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, K.; Shukla, A.K. Polymer Electrolyte Membrane Fuel Cells: Principles and Advances. Rev. Environ. Sci. Biotechnol. 2004, 3, 273–280. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yi, J.-Y.; Jung, C.-Y.; Jeong, E.; Yi, S.-C. Solvent Effect on the Nafion Agglomerate Morphology in the Catalyst Layer of the Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 478–485. [Google Scholar] [CrossRef]
- Dai, J.; Teng, X.; Song, Y.; Ren, J. Effect of Casting Solvent and Annealing Temperature on Recast Nafion Membranes for Vanadium Redox Flow Battery. J. Membr. Sci. 2017, 522, 56–67. [Google Scholar] [CrossRef]
- Jayakrishnan, M.P.; Vena, A.; Meghit, A.; Sorli, B.; Perret, E. Nafion-Based Fully Passive Solid-State Conductive Bridging RF Switch. IEEE Microw. Wirel. Compon. Lett. 2017, 27, 1104–1106. [Google Scholar] [CrossRef]
- Cele, N.; Ray, S.S. Recent Progress on Nafion-Based Nanocomposite Membranes for Fuel Cell Applications: Recent Progress on Nafion-Based Nanocomposite Membranes. Macromol. Mater. Eng. 2009, 294, 719–738. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Kang, B.-C.; Ha, T.-J. Flexible PH Sensors Based on Printed Nanocomposites of Single-Wall Carbon Nanotubes and Nafion. Appl. Surf. Sci. 2020, 514, 145956. [Google Scholar] [CrossRef]
- Keawkim, K.; Chuanuwatanakul, S.; Chailapakul, O.; Motomizu, S. Determination of Lead and Cadmium in Rice Samples by Sequential Injection/Anodic Stripping Voltammetry Using a Bismuth Film/Crown Ether/Nafion Modified Screen-Printed Carbon Electrode. Food Control 2013, 31, 14–21. [Google Scholar] [CrossRef]
- Frutos-Puerto, S.; Miró, C.; Pinilla-Gil, E. Nafion-Protected Sputtered-Bismuth Screen-Printed Electrode for On-Site Voltammetric Measurements of Cd(II) and Pb(II) in Natural Water Samples. Sensors 2019, 19, 279. [Google Scholar] [CrossRef] [Green Version]
- Kokkinos, C.; Economou, A. Disposable Nafion-Modified Micro-Fabricated Bismuth-Film Sensors for Voltammetric Stripping Analysis of Trace Metals in the Presence of Surfactants. Talanta 2011, 84, 696–701. [Google Scholar] [CrossRef]
- Palisoc, S.; Sow, V.A.; Natividad, M. Fabrication of a Bismuth Nanoparticle/Nafion Modified Screen-Printed Graphene Electrode for in Situ Environmental Monitoring. Anal. Methods 2019, 11, 1591–1603. [Google Scholar] [CrossRef]
- Liu, M.; Xiang, J.; Zhou, J.; Ding, H. A Disposable Amperometric Sensor for Rapid Detection of Serotonin in the Blood and Brain of the Depressed Mice Based on Nafion Membrane-Coated Colloidal Gold Screen-Printed Electrode. J. Electroanal. Chem. 2010, 640, 1–7. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wang, S.-C.; Yuan, C.-J.; Wen, M.-F.; Chang, K.-S. Comparison of Amperometric Biosensors Fabricated by Palladium Sputtering, Palladium Electrodeposition and Nafion/Carbon Nanotube Casting on Screen-Printed Carbon Electrodes. Biosens. Bioelectron. 2007, 22, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Montes, C.; Contento, A.M.; Villaseñor, M.J.; Ríos, Á. A Screen-Printed Electrode Modified with Silver Nanoparticles and Carbon Nanofibers in a Nafion Matrix for Ionic Liquid-Based Dispersive Liquid-Liquid Microextraction and Voltammetric Assay of Heterocyclic Amine 8-MeIQx in Food. Microchim. Acta 2020, 187, 190. [Google Scholar] [CrossRef] [PubMed]
- Lazouskaya, M.; Tamm, M.; Scheler, O.; Uppuluri, K.; Zaraska, K. Nafion as a Protective Membrane for Screen-Printed PH-Sensitive Ruthenium Oxide Electrodes. In Proceedings of the 2020 17th Biennial Baltic Electronics Conference (BEC), Tallinn, Estonia, 6–8 October 2020; IEEE: Tallinn, Estonia, 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Hsiao, F.-R.; Liao, Y.-C. Printed Micro-Sensors for Simultaneous Temperature and Humidity Detection. IEEE Sens. J. 2018, 18, 6788–6793. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Situ, B.; Dai, Z.; Liu, Q.; Wang, Q.; Gu, D.; Zheng, L. Sensitive HIV-1 Detection in a Homogeneous Solution Based on an Electrochemical Molecular Beacon Coupled with a Nafion–Graphene Composite Film Modified Screen-Printed Carbon Electrode. Biosens. Bioelectron. 2014, 52, 330–336. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Ye, J. Sensitive and Selective Sensing of Hydrogen Peroxide with Iron-Tetrasulfophthalocyanine-Graphene-Nafion Modified Screen-Printed Electrode. Electroanalysis 2012, 24, 1212–1219. [Google Scholar] [CrossRef]
- Dai, Z.; Aboukeila, H.; Ansaloni, L.; Deng, J.; Giacinti Baschetti, M.; Deng, L. Nafion/PEG Hybrid Membrane for CO2 Separation: Effect of PEG on Membrane Micro-Structure and Performance. Sep. Purif. Technol. 2019, 214, 67–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Zheng, S.; Zhang, L.; Shi, X.; He, J.; Chou, X.; Wu, Z.-S. Ink Formulation, Scalable Applications and Challenging Perspectives of Screen Printing for Emerging Printed Microelectronics. J. Energy Chem. 2021, 63, 498–513. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Ise, M.; Fuchs, A.; Maier, J. Proton and Water Transport in Nano-Separated Polymer Membranes. J. Phys. IV Proc. 2000, 10, Pr7-279–Pr7-281. [Google Scholar] [CrossRef]
- Chen, Z.; Fury, J.J.; Neeson, A. Printable Dispersion with Tunable Viscosity. United States Patent Application. U.S. Patent 17/208,386, 23 September 2021. [Google Scholar]
- Xu, C.; Willenbacher, N. How Rheological Properties Affect Fine-Line Screen Printing of Pastes: A Combined Rheological and High-Speed Video Imaging Study. J. Coat. Technol. Res. 2018, 15, 1401–1412. [Google Scholar] [CrossRef]
- Yüce, C.; König, M.; Willenbacher, N. Rheology and Screen-Printing Performance of Model Silver Pastes for Metallization of Si-Solar Cells. Coatings 2018, 8, 406. [Google Scholar] [CrossRef] [Green Version]
- Michelarakis, N.; Franz, F.; Gkagkas, K.; Gräter, F. Longitudinal Strand Ordering Leads to Shear Thinning in Nafion. Phys. Chem. Chem. Phys. 2021, 23, 25901–25910. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fies, M.; Willenbacher, N. Impact of Wall Slip on Screen Printing of Front-Side Silver Pastes for Silicon Solar Cells. IEEE J. Photovolt. 2017, 7, 129–135. [Google Scholar] [CrossRef]
- Barnes, H.A. A Handbook of Elementary Rheology; University of Wales, Institute of Non-Newtonian Fluid Mechanics: Aberystwyth, UK, 2000; Volume 1. [Google Scholar]
- Bertola, V.; Bertrand, F.; Tabuteau, H.; Bonn, D.; Coussot, P. Wall Slip and Yielding in Pasty Materials. J. Rheol. 2003, 47, 1211–1226. [Google Scholar] [CrossRef]
- Zhang, W.; Yue, P.-L.; Gao, P. Crystallinity Enhancement of Nafion Electrolyte Membranes Assisted by a Molecular Gelator. Langmuir 2011, 27, 9520–9527. [Google Scholar] [CrossRef]
- del Valle Martínez de Yuso, M.; Arango-Díaz, A.; Bijani, S.; Romero, V.; Benavente, J.; Rodríguez-Castellón, E. Chemical Surface, Thermal and Electrical Characterization of Nafion Membranes Doped with IL-Cations. Appl. Sci. 2014, 4, 195–206. [Google Scholar] [CrossRef] [Green Version]


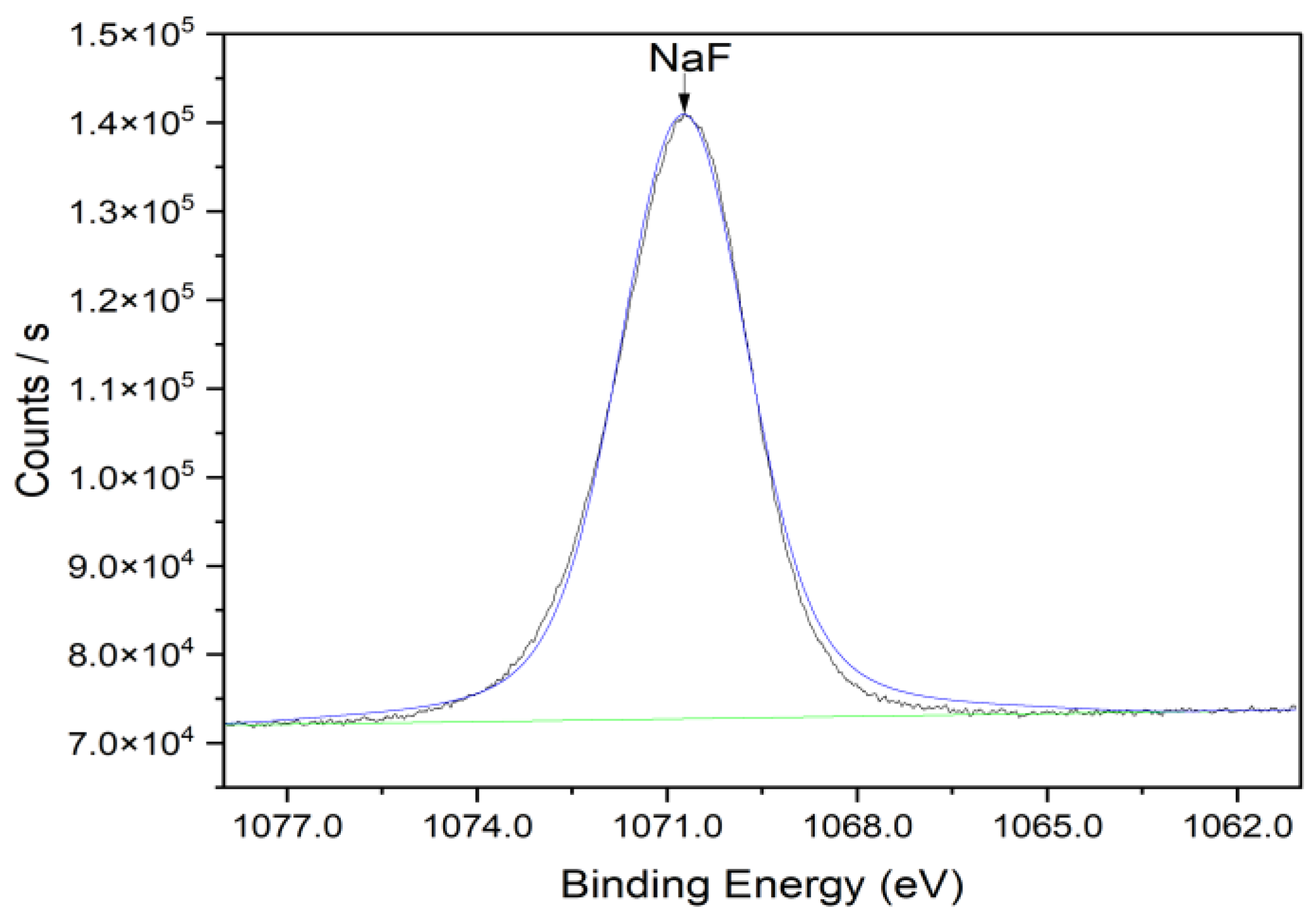
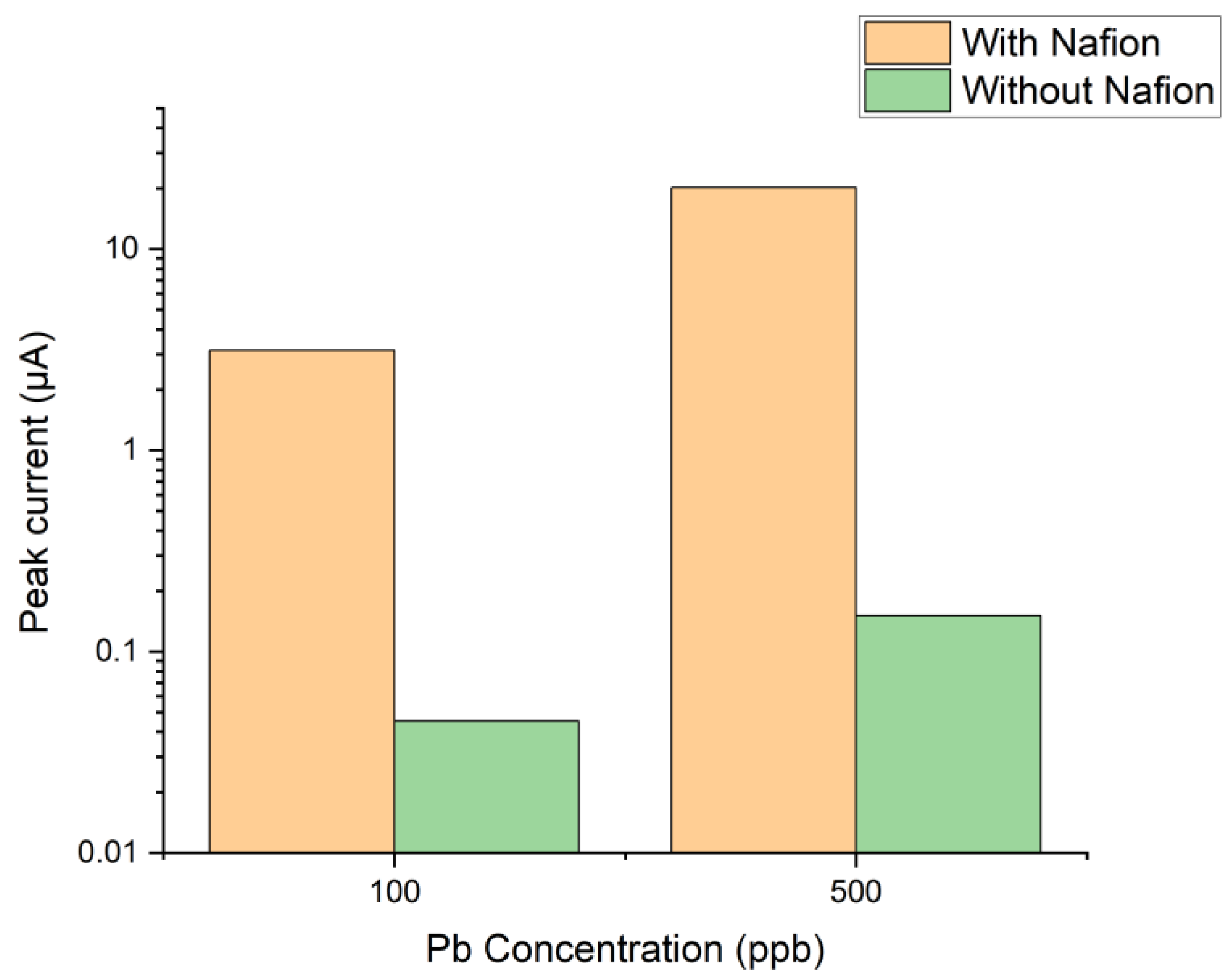
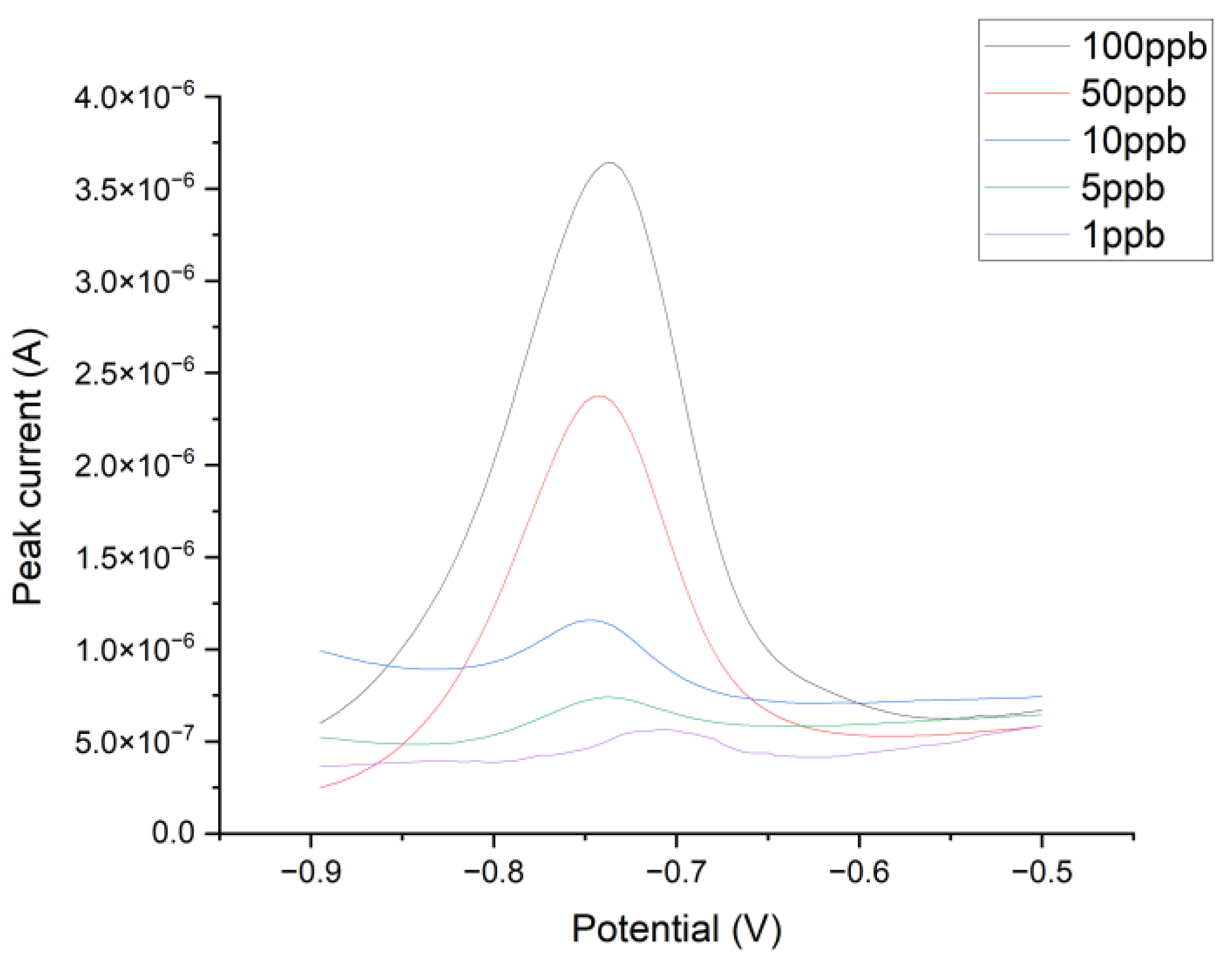

| Sample | S% | C% | O% | F% | Na% |
|---|---|---|---|---|---|
| Theoretical [29] | 1.50 | 30.80 | 7.70 | 60.00 | 0.00 |
| Commercial Nafion | 0.90 | 32.29 | 6.09 | 60.73 | 0.00 |
| Modified Nafion | 1.63 | 30.19 | 8.94 | 57.27 | 1.96 |
| Sample | Avg Height (µm) | Roughness (Sa) (µm) |
|---|---|---|
| Nafion Film 1 | 16.028 | 3.710 |
| Nafion Film 2 | 15.777 | 2.906 |
| Nafion Film 3 | 15.397 | 3.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Patel, R.; Berry, J.; Keyes, C.; Satterfield, C.; Simmons, C.; Neeson, A.; Cao, X.; Wu, Q. Development of Screen-Printable Nafion Dispersion for Electrochemical Sensor. Appl. Sci. 2022, 12, 6533. https://doi.org/10.3390/app12136533
Chen Z, Patel R, Berry J, Keyes C, Satterfield C, Simmons C, Neeson A, Cao X, Wu Q. Development of Screen-Printable Nafion Dispersion for Electrochemical Sensor. Applied Sciences. 2022; 12(13):6533. https://doi.org/10.3390/app12136533
Chicago/Turabian StyleChen, Zun, Rishi Patel, Jacob Berry, Chloe Keyes, Camron Satterfield, Cody Simmons, Alec Neeson, Xi Cao, and Qihua Wu. 2022. "Development of Screen-Printable Nafion Dispersion for Electrochemical Sensor" Applied Sciences 12, no. 13: 6533. https://doi.org/10.3390/app12136533
APA StyleChen, Z., Patel, R., Berry, J., Keyes, C., Satterfield, C., Simmons, C., Neeson, A., Cao, X., & Wu, Q. (2022). Development of Screen-Printable Nafion Dispersion for Electrochemical Sensor. Applied Sciences, 12(13), 6533. https://doi.org/10.3390/app12136533





