Fatty Acid Content, Lipid Quality Indices, and Mineral Composition of Cow Milk and Yogurts Produced with Different Starter Cultures Enriched with Bifidobacterium bifidum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fat Extraction
2.2.2. Preparation of Fatty Acid Methyl Esters
2.2.3. Analysis of Fatty Acid Composition by GC Method
2.2.4. The Lipid Quality Indices
2.3. Mineral Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition and Lipid Quality Indices
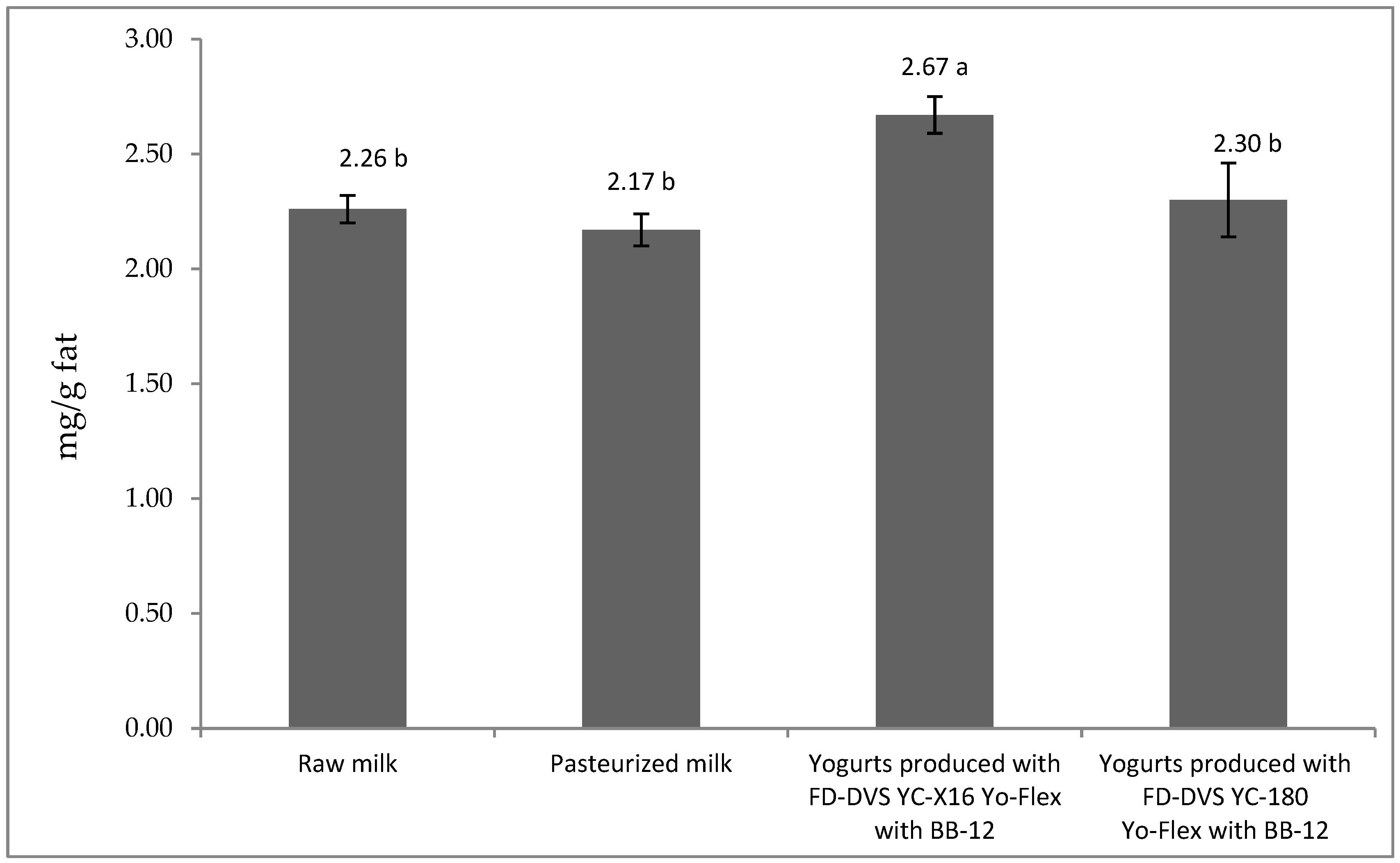
3.2. CLA Content in Milk and Yogurts
3.3. The Mineral Composition of Milk and Yogurts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korhenen, H. Bioactive Components in Bovine Milk. In Bioactive Components in Milk and Dairy Products; Park, Y.W., Ed.; Wiley-Blackwell: Singapore, 2009; pp. 15–42. [Google Scholar]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy fats and cardiovascular disease: Do we really need to be concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrzański, Z.; Kołacz, R.; Górecka, H.; Chojnacka, K.; Bartkowiak, A. The Content of Microelements and Trace Elements in Raw Milk from Cows in the Silesian Region. Pol. J. Environ. Stud. 2005, 14, 685–689. [Google Scholar]
- Brodziak, A.; Litwińczuk, A.; Kędzierska-Matysek, M.; Król, J. Zawartość wybranych makro- i mikroelementów w mleku krów różnych ras i serwatce podpuszczkowej. Ochr. Środ. i Zasob. Nat. 2011, 48, 467–474. [Google Scholar]
- Barłowska, J.; Wolanciuk, A.; Kędzierska-Matysek, M.; Litwińczuk, Z. Eeffect of production season on basic chemical composition and content of macro- and microelements in cow’s and goat’s milk. ŻYWNOŚĆ. Nauka. Technologia. Jakość 2013, 6, 69–78. [Google Scholar]
- Adolphi, B.; Scholz-Ahrens, K.E.; de Vrese, M.; Açil, Y.; Laue, C.; Schrezenmeir, J. Short-term effect of bedtime consumption of fermented milk supplemented with calcium, inulin-type fructans and caseinphosphopeptides on bone metabolism in healthy, postmenopausal women. Eur. J. Nutr. 2009, 48, 45–53. [Google Scholar] [CrossRef]
- Cashman, K. Milk minerals (including trace elements) and bone health. Int. Dairy J. 2006, 16, 1389–1398. [Google Scholar] [CrossRef]
- Hanuš, O.; Samková, E.; Krížová, L.; Hasǒnová, L.; Kala, R. Role of fatty acids in milk fat and the influenceof selected factors on their variability—A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef] [Green Version]
- Albenzio, M.; Santillo, A.; Avando, M.; Nudda, A.; Chesse, S.; Pirisi, A.; Banni, S. Nutritional properties of small ruminant food products and their role on human health. Small Rumin. Res. 2016, 136, 3–12. [Google Scholar] [CrossRef]
- Claeys, W.L.; Verraes, C.; Cardoen, S.; de Block, J.; Huyghebaer, A.; Raes, K.; Dewettinck, K.; Herman, L. Consumption of raw or heated milk from different species: An evaluation of the nutritional and potential health benefits. Food Control 2014, 42, 188–201. [Google Scholar] [CrossRef]
- Wongtangtintharn, S.; Oku, H.; Iwasaki, H.; Toda, T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J. Nutr. Sci. Vitaminol. 2004, 50, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Adamska, A.; Rutkowska, J. Odd- and branched-chain fatty acids in milk fat—Characteristic and health properties. Postepy Hig. Med. Dosw. 2014, 68, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Lim, J.-N.; Oh, J.-J.; Wang, T.; Lee, J.-S.; Kim, S.-H.; Kim, Y.-H.; Lee, H.-G. trans-11 18:1 vaccenic acid (TVA) has a direct anti-carcinogenic effect on MCF-7 human mammary adenocarcinoma cells. Nutrients 2014, 6, 627–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.M. Dietary fatty acids and human health. Ann. De Zootech. 2000, 49, 165–180. [Google Scholar] [CrossRef] [Green Version]
- Haug, A.; Hostmark, A.T.; Harstad, O.M. Bovine milk in human nutrition: A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnould, V.M.-R.; Soyeurt, H. Genetic variability of milk fatty acids. J. Appl. Genet. 2009, 50, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akalln, A.S.; Tokusoglu, Ö. A potential Anticarcinogenic Agent: Conjugated linoleic acid (CLA). Pak. J. Nutr. 2003, 2, 109–110. [Google Scholar] [CrossRef] [Green Version]
- Aydin, R. Conjugated linoleic acid: Structure, sources and biological properties. Turk. J. Vet. Anim. Sci. 2005, 29, 189–195. [Google Scholar]
- Kee, J.-I.; Ganesan, P.; Kwak, H.-S. Bioactive conjugated linoleic acid (CLA) in milk. Korean J. Food Sci. Ani. Resour. 2010, 30, 879–885. [Google Scholar] [CrossRef] [Green Version]
- Park, Y. Conjugated linoleic acid (CLA): Good or bad trans fat? J. Food Comp. Anal. 2009, 22S, S4–S12. [Google Scholar] [CrossRef]
- Parodi, P.W. Anti-cancer agents in milkfat. Austr. J. Dairy Technol. 2003, 58, 114–118. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Thorning, T.K.; Bertram, H.C.; Bonjour, J.-F.; de Groot, L.; Dupont, D.; Feeney, E.; Ipsen, R.; Lecerf, J.M.; Mackie, A.; McKinley, M.C.; et al. Whole dairy matrix or single nutrients in assessment of health effects: Current evidence and knowledge gaps. Am. J. Clin. Nutr. 2017, 105, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Juárez, M.; Angel de la Fuente, M. Milk fat acids and potential health benefits: An update vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ayaz, M.; Ajmal, M.; Ellahi, M.Y.; Khalique, A. Antioxidant capacity and fatty acids characterization of heat treated cow and buffalo milk. Lipids Health Dis. 2017, 16, 227. [Google Scholar] [CrossRef] [Green Version]
- Pestana, J.M.; Gennari, A.; Monteiro, B.W.; Lehn, D.N.; Souza, C.F.V. Effects of Pasteurization and Ultra-High Temperature Processes on Proximate Composition and Fatty Acid Profile in Bovine Milk. Am. J. Food Technol. 2015, 10, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Ajmal, M.; Nadeem, M.; Imran, M.; Junaid, M. Lipid compositional changes and oxidation status of ultra-high temperature treated milk. Lipids Health Dis. 2018, 17, 227. [Google Scholar] [CrossRef] [Green Version]
- Simionato, J.I.; Hiroki, A.P.; Katsuda, M.S.; Pedrão, M.R.; Dias, L.F.; Evelazio de Souza, N. Effects of Seasonality on the Proximate Composition and Fatty Acid Profile in Cow Milk. Int. J. Food Sci. Nutr. Eng. 2012, 2, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Laučienė, L.; Andrulevičiūtė, V.; Sinkevičienė, I.; Sederevičius, A.; Musayeva, K.; Šernienė, L. Analysis of fatty acid composition and healthy lipids indices in raw and processed milk. J. Food Nutr. Res. 2019, 7, 386–390. [Google Scholar] [CrossRef]
- Gassem, M.; Osman, M.; Mohamed Ahmed, I.; Abdel Rahman, I.; Fadol, M.; Al-Maiman, S. Effect of fermentation by selected lactic acid bacteria on the chemical composition and fatty acids of camel milk. J. Camel. Pract. Res. 2016, 23, 277. [Google Scholar] [CrossRef]
- Santos Júnior, O.O.; Pedrao, M.R.; Dias, L.F.; Paula, L.N.; Coro, F.A.G.; De Souza, N.E. Fatty Acid Content of Bovine Milkfat From Raw Milk to Yoghurt. Am. J. Appl. Sci. 2012, 9, 1300–1306. [Google Scholar] [CrossRef]
- Serafeimidou, A.; Zlatanos, S.; Kritikos, G.; Tourianis, A. Change of fatty acid profile, including conjugated linoleic acid (CLA) content, during refrigerated storage of yogurt made of cow and sheep milk. J. Food Compos. Anal. 2013, 31, 24–30. [Google Scholar] [CrossRef]
- Paszczyk, B.; Brandt, W.; Łuczyńska, J. Content of conjugated linoleic acid (CLA) and trans isomers of C18:1 and C18:2 acids in fresh and stored fermented milks produced with selected starter cultures. Czech. J. Food Sc. 2016, 34, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Czarnowska-Kujawska, M.; Paszczyk, B. Changes in the folate content and fatty acid profile in fermented milk produced with different starter cultures during storage. Molecules 2021, 26, 6063. [Google Scholar] [CrossRef] [PubMed]
- Domagała, J.; Sady, M.; Najgebauer-Lejko, D.; Czernicka, M.; Witeska, I. The content of conjugated linoleic acid (CLA) in cream fermented using different starter cultures. Biotechnol. Anim. Husb. 2009, 25, 745–751. [Google Scholar]
- Goetsch, A.L.; Zeng, S.S.; Gipson, T.A. Factors affecting goat milk production and quality. Small Rumin. Res. 2011, 101, 55–63. [Google Scholar] [CrossRef]
- El-Abbadi, N.H.; Dao, M.C.; Meydani, S.N. Yogurt: Role in healthy and active aging. Am. J. Clin. Nutr. 2014, 99, 1263S–1270S. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, H.; Sasaki, Y. Effect of oxygen on symbiosis between Lactobacillus bulgaricus and Streptococcus thermophilus. J. Dairy Sci. 2012, 95, 2904–2909. [Google Scholar] [CrossRef]
- Coakley, R.P.; Ross, R.P.; Nordgren, M.; Fitzgerald, G.; Devery, R.; Stanton, C. Conjugated linoleic acid biosynthesis by human-derived Bifidobacterium species. J. Appl. Microbiol. 2003, 94, 138–145. [Google Scholar] [CrossRef]
- Lin, T.Y. Conjugated linoleic acid concentration as affected by lactic cultures and additives. Food Chem. 2000, 69, 27–31. [Google Scholar] [CrossRef]
- Sieber, R.; Collomb, M.; Aeschlimann, A.; Jelen, P.; Eyer, H. Impact of microbial cultures on conjugated linoleic acid in dairy products—A review. Int. Dairy J. 2004, 14, 1–15. [Google Scholar] [CrossRef]
- Xu, S.; Boylston, T.D.; Glatz, B.A. Effect of lipid source on probiotic bacteria and conjugated linoleic acid formation in milk model systems. J. Am. Oil. Chem. Soc. 2004, 81, 589–595. [Google Scholar] [CrossRef]
- Gorissen, L.; Raes, K.; Weckx, S.; Dannenberger, D.; Leroy, F.; De Vuyst, L.; De Smet, S. Production of conjugated linoleic acid and conjugated linolenic acid isomers by Bifidobacterium species. Appl. Microbiol. Biotechnol. 2010, 87, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Rosberg-Cody, E.; Ross, R.P.; Hussey, S.; Ryan, C.A.; Murphy, B.P.; Fitzgerald, G.F.; Devery, R.; Stanton, C. Mining the microbiota of the neonatal gastrointestinal tract for conjugated linoleic acid-producing. Bifidobact. Appl. Environ. Microbiol. 2004, 70, 4635–4641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PN-EN ISO 1211:2011; Milk-Determination of Fat Content—Gravimetric Method (Reference Method). ISO: Geneva, Switzerland, 2011.
- Christie, W.W. (Ed.) The isolation of lipids from tissues. Recommended Procedures. Chloroform-methanol (2:1, v/v) extraction and “Folch” wash. In Lipid Analysis. Isolation, Separation, Identification and Structural Analysis of Lipids; Pergamon Press: Oxford, UK; New York, NY, USA; Toronto, ON, Canada; Tokyo, Japan; Sydney, Australia; Braunschweig, Germany, 1973; pp. 39–40. [Google Scholar]
- ISO 15884:2002 (IDF 182:2002); Milkfat: Preparation of Fatty Acid Methyl Esters. Available online: https://www.iso.org/obp/ui/#iso:std:iso:15884:ed-1:v1:en (accessed on 12 July 2003).
- Roach, J.A.G.; Mossoba, M.M.; Yurawecz, M.P.; Kramer, J.K.G. Chromatographic separation and identification of conjugated linoleic acid isomers. Anal. Chim. Acta 2002, 465, 207–226. [Google Scholar] [CrossRef]
- Kramer, J.K.G.; Cruz-Hermantez, C.; Deng, Z.; Zhou, J.; Jahreis, G.; Dugan, M.E.R. Analysis of conjugated linoleic acid and trans 18:1 isomers in syntetic and animal products. Am. J. Clin. Nutr. 2004, 79, 1137S–1145S. [Google Scholar] [CrossRef] [Green Version]
- LeDoux, M.; Chardigny, J.-M.; Darbois, M.; Soustre, Y.; Sébédio, J.-L.; Laloux, L. Fatty acid composition of French butters, with special emphasis on conjugated linoleic acid (CLA) isomers. J. Food Compos. Anal. 2005, 18, 409–425. [Google Scholar] [CrossRef]
- Medeiros, E.; Queiroga, R.; Oliveira, M.; Medeiros, A.; Sabedot, M.; Bomfim, M.; Madruga, M. Fatty Acid Profile of Cheese from Dairy Goats Fed a Diet Enriched with Castor, Sesame and Faveleira Vegetable Oils. Molecules 2014, 19, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Osmari, E.K.; Cecato, U.; Macedo, F.A.F.; Souza, N.E. Nutritional quality indices of milk fat from goats on diets supplemented with different roughages. Small Rumin. Res. 2011, 98, 128–132. [Google Scholar] [CrossRef]
- Ivanova, A.; Hadzhinikolova, L. Evaluation of nutritional quality of common carp (Cyprinus carpio L.) lipids through fatty acid ratios and lipid indices. Bulg. J. Agric. Sci. 2015, 21, 180–185. [Google Scholar]
- Statistica, Version 13.3; StatSoft: Kraków, Poland, 2007.
- Grummer, R.R. Effect of Feed on the Composition of Milk Fat. J. Dairy Sci. 1991, 74, 3244–3257. [Google Scholar] [CrossRef]
- Jensen, R.G. The Composition of Bovine Milk Lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Ghaeni, M.; Ghahfarokhi, K.N.; Zaheri, L. Fatty acids profile, atherogenic (IA) and thrombogenic (IT) health lipid indices in Leiognathusbindus and Upeneussulphureus. J. Marine Sci. Res. Dev. 2013, 3, 1000138. [Google Scholar] [CrossRef] [Green Version]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Jiang, J.; Björck, L.; Fondn, R. Production of conjugated linoleic acid by dairy starter cultures. J. Appl. Microbiol. 1998, 85, 95–102. [Google Scholar] [CrossRef]
- Kelly, M.L.; Berry, J.R.; Dwyer, D.A.; Griinari, J.M.; Chouinard, P.Y.; Van Amburgh, M.E.; Bauman, D.E. Dietary fatty acid sources affect conjugated linoleic acid concentrations in milk from lactating dairy cows. J. Nutr. Sci. 1998, 128, 881–885. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Mansbridge, R.M.; Doreau, M. Ruminant Milk Fat Plasticity: Nutritional Control of Saturated, Polyunsaturated, Trans and Conjugated Fatty Acids. Anim. Res. 2000, 49, 181–205. [Google Scholar] [CrossRef] [Green Version]
- Kelsey, J.; Corl, B.; Collier, R.; Bauman, D. The Effect of Breed, Parity, and Stage of Lactation on Conjugated Linoleic Acid (CLA) in Milk Fat from Dairy Cows. J. Dairy Sci. 2003, 86, 2588–2597. [Google Scholar] [CrossRef] [Green Version]
- Żegarska, Z.; Paszczyk, B.; Rafałowski, R.; Borejszo, Z. Annual Changes in the Content of Unsaturated Fatty Acids with 18 Carbon Atoms, Including Cis9trans11 C18:2 (CLA) Acid, in Milk Fat. Pol. J. Food Nutr. Sci. 2006, 56, 409–414. [Google Scholar]
- Collomb, M.; Schmid, A.; Sieber, R.; Wechsler, D.; Ryhänen, E.-L. Conjugated Linoleic Acids in Milk Fat: Variation and Physiological Effects. Int. Dairy J. 2006, 16, 1347–1361. [Google Scholar] [CrossRef]
- Frelich, J.; Šlachta, M.; Hanuš, O.; Špička, J.; Samková, E.; Weglarz, A.; Zapletal, P. Seasonal Variation in Fatty Acid Composition of Cow Milk in Relation to the Feeding System. Anim. Sci. Pap. Rep. 2012, 30, 219–229. [Google Scholar]
- Hanuš, O.; Krížová, L.; Samková, E.; Špička, J.; Kučera, J.; Klimešová, M.; Roubal, P.; Jedelská, R. The Effect of Cattle Breed, Season and Type of Diet on the Fatty Acid Profile of Raw Milk. Arch. Anim. Breed. 2016, 59, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.Y. Influence of lactic cultures, linoleic acid and fructo-oligosaccharides on conjugated linoleic acid concentration in non-fat set yoghurt. Aust. J. Dairy Technol. 2003, 58, 11–14. [Google Scholar]
- Shantha, N.C.; Decker, E.A.; Ustunol, Z. Conjugated Linoleic Acid Concentration in Processed Cheese. J. Am. Oil Chem. Soc. 1992, 69, 425–428. [Google Scholar] [CrossRef]
- Bisig, W.; Eberhard, P.; Collomb, M.; Rehberger, B. Influence of Processing on the Fatty Acid Composition and the Content of Conjugated Linoleic Acid in Organic and Conventional Dairy Products—A Review. Le Lait. 2007, 87, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Salamon, R.V.; Lóki, K.; Varga-Visi, E.; Mándoki, Z.; Csapó, J. Increase of Conjugated Linoleic Acid Content of Dairy Products by Adding Sunflower Oil. Krmiva 2009, 51, 99–103. [Google Scholar]
- Seçkin, A.K.; Gursoy, O.; Kinik, O.; Akbulut, N. Conjugated Linoleic Acid (CLA) Concentration, Fatty Acid Composition and Cholesterol Content of Some Turkish Dairy Products. LWT 2005, 38, 909–915. [Google Scholar] [CrossRef]
- Prandini, A.; Sigolo, S.; Tansini, G.; Brogna, N.; Piva, G. Different Level of Conjugated Linoleic Acid (CLA) in Dairy Products from Italy. J. Food Compos. Anal. 2007, 20, 472–479. [Google Scholar] [CrossRef]
- Alonso, L.; Cuesta, E.P.; Gilliland, S.E. Production of free conjugated linoleic acid by Lactobacillus acidophilus and Lactobacillus casei of human intestinal origin. J. Dairy Sci. 2003, 86, 1941–1946. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Liu, R. Increase of Conjugated Linoleic Acid Content in Milk by Fermentation with Lactic Acid Bacteria. J. Food Sci. 2002, 67, 1731–1737. [Google Scholar] [CrossRef]
- Hennessy, A.; Ross, R.; Devery, R.; Stanton, C. Optimization of a Reconstituted Skim Milk Based Medium for Enhanced CLA Production by Bifidobacteria. J. Appl. Microbiol. 2009, 106, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, J.; Kishino, S.; Ando, A.; Sugimoto, S.; Mihara, K.; Shimizu, S. Production of Conjugated Fatty Acids by Lactic Acid Bacteria. J. Biosci. Bioeng. 2005, 100, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Boylston, T.D.; Chang, M.J.; Luedecke, L.D.; Shultz, T.D. Survey of the CLA contents of dairy products. J. Dairy Sci. 1995, 78, 2358–2365. [Google Scholar] [CrossRef]
- Zamberlin, Š.; Neven, A.; Havranek, J.; Samaržija, D. Mineral elements in milk and dairy products. Mljekarstvo 2012, 62, 2, 111–125. [Google Scholar]
- Paszczyk, B.; Tońska, E.; Łuczyńska, J. Health-promoting values of cow, sheep and goat milk and yogurts. Mljekarstvo 2019, 69, 182–192. [Google Scholar] [CrossRef]
| Raw Milk | Pasteurized Milk | Yogurts Produced Using FD-DVS YC-X16 Yo-Flex with BB-12 | Yogurts Produced Using FD-DVS YC-180 Yo-Flex with BB-12 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | |
| n | 4 | 4 | 8 | 8 | ||||
| ΣSCFA 1 | 63.46 | 4.26 b | 75.69 | 1.18 a | 62.18 | 2.37 b | 62.55 | 4.26 b |
| ΣBCFA 2 | 7.11 | 0.34 a | 9.16 | 0.28 a | 8.84 | 2.21 a | 7.13 | 0.45 a |
| ΣOCFA 3 | 11.76 | 0.40 c | 15.38 | 0.49 a | 13.21 | 0.57 b | 11.78 | 0.77 c |
| ΣSFA 4 | 368.60 | 14.26 c | 466.90 | 9.43 a | 417.04 | 23.50 b | 369.96 | 24.06 c |
| ΣMUFA 5 | 140.38 | 5.33 b | 178.99 | 5.40 a | 162.57 | 12.11 a | 141.88 | 9.36 b |
| ΣPUFA 6 | 16.22 | 0.43 b | 20.19 | 0.45 a | 17.97 | 1.19 b | 15.94 | 1.12 b |
| (n − 3) | 1.38 | 0.08 b | 1.79 | 0.07 a | 1.52 | 0.08 b | 1.41 | 0.11 b |
| (n − 6) | 10.04 | 0.28 c | 12.98 | 0.42 a | 11.56 | 0.60 b | 10.40 | 0.62 b,c |
| (n − 6)/(n − 3) | 7.31 | 0.23 a | 7.24 | 0.04 a | 7.62 | 0.66 a | 7.39 | 0.19 a |
| UFA 7 | 432.06 | 18.53 b | 542.59 | 10.62 a | 479.22 | 24.18 b | 432.51 | 27.88 b |
| DFA 8 | 482.32 | 20.57 b | 606.17 | 12.69 a | 536.76 | 26.86 b | 483.11 | 30.86 b |
| OFA 9 | 318.34 | 12.22 c | 403.32 | 7.36 a | 359.50 | 20.81 b | 319.36 | 21.07 c |
| AI 10 | 1.97 | 0.00 a | 1.95 | 0.03 a | 1.92 | 0.05 a | 1.95 | 0.06 a |
| TI 11 | 3.76 | 0.00 a | 3.72 | 0.05 a | 3.67 | 0.09 a | 3.73 | 0.11 a |
| H/H 12 | 0.40 | 0.00 a | 0.41 | 0.01 a | 0.42 | 0.02 | 0.41 | 0.01 a |
| Raw Milk | Pasteurized Milk | Yogurts Produced Using FD-DVS YC-X16 Yo-Flex with BB-12 | Yogurts Produced Using FD-DVS YC-180 Yo-Flex with BB-12 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | |
| n | 4 | 4 | 8 | 8 | ||||
| Cu | 11.3 | 0.1 b | 12.4 | 0.3 b | 14.2 | 1.5 a | 14.5 | 0.8 a |
| Mn | 2.50 | 0.3 b | 2.8 | 0.3 b | 3.90 | 0.3 a | 3.50 | 0.4 a |
| Fe | 14.9 | 2.3 b | 19.3 | 1.0 b | 19.6 | 1.1 b | 26.9 | 7.3 a |
| Zn | 435.0 | 20.8 b | 555.1 | 32.0 a | 552.6 | 18.4 a | 545.6 | 19.1 a |
| Raw Milk | Pasteurized Milk | Yogurts Produced Using FD-DVS YC-X16 Yo-Flex with BB-12 | Yogurts Produced Using FD-DVS YC-180 Yo-Flex with BB-12 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | |
| n | 4 | 4 | 8 | 8 | ||||
| Mg | 7.6 | 0.8 b | 9.0 | 0.5 a | 9.70 | 0.2 a | 10.0 | 0.2 a |
| Ca | 128.3 | 8.1 b | 154.7 | 8.1 a | 158.7 | 3.9 a | 161.1 | 7.1 a |
| Na | 39.4 | 2.2 b | 46.7 | 2.2 a | 47.1 | 1.0 a | 50.5 | 4.4 a |
| K | 158.1 | 9.6 b | 188.9 | 9.6 a | 188.7 | 5.5 a | 196.4 | 9.0 a |
| P | 94.6 | 5.4 b | 115.4 | 5.4 a | 118.2 | 1.4 a | 121.7 | 8.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszczyk, B.; Tońska, E. Fatty Acid Content, Lipid Quality Indices, and Mineral Composition of Cow Milk and Yogurts Produced with Different Starter Cultures Enriched with Bifidobacterium bifidum. Appl. Sci. 2022, 12, 6558. https://doi.org/10.3390/app12136558
Paszczyk B, Tońska E. Fatty Acid Content, Lipid Quality Indices, and Mineral Composition of Cow Milk and Yogurts Produced with Different Starter Cultures Enriched with Bifidobacterium bifidum. Applied Sciences. 2022; 12(13):6558. https://doi.org/10.3390/app12136558
Chicago/Turabian StylePaszczyk, Beata, and Elżbieta Tońska. 2022. "Fatty Acid Content, Lipid Quality Indices, and Mineral Composition of Cow Milk and Yogurts Produced with Different Starter Cultures Enriched with Bifidobacterium bifidum" Applied Sciences 12, no. 13: 6558. https://doi.org/10.3390/app12136558






