Characterization and Evaluation of Commercial Carboxymethyl Cellulose Potential as an Active Ingredient for Cosmetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. CMC Samples
2.2. CMC Solutions Preparation
2.3. CMC Samples Characterization
2.3.1. Structural Characterization
2.3.2. Purity
2.3.3. Degree of Substitution
2.3.4. Average Molecular Weight
2.4. Cell Lines and Culture Conditions
2.5. Cytotoxicity Evaluation
2.6. Pro-Collagen I α I Biosynthesis and Quantification
2.6.1. Pro-Collagen I α I Biosynthesis
2.6.2. Pro-Collagen I α I Quantification
2.7. Inflammatory Response
2.8. Statistical Analysis
3. Results and Discussion
3.1. Carboxymethylcellulose Characterization
3.1.1. Structural Analysis
3.1.2. Physical Characterization
3.1.3. Cytotoxicity Evaluation
3.1.4. Pro-Collagen I α I Production
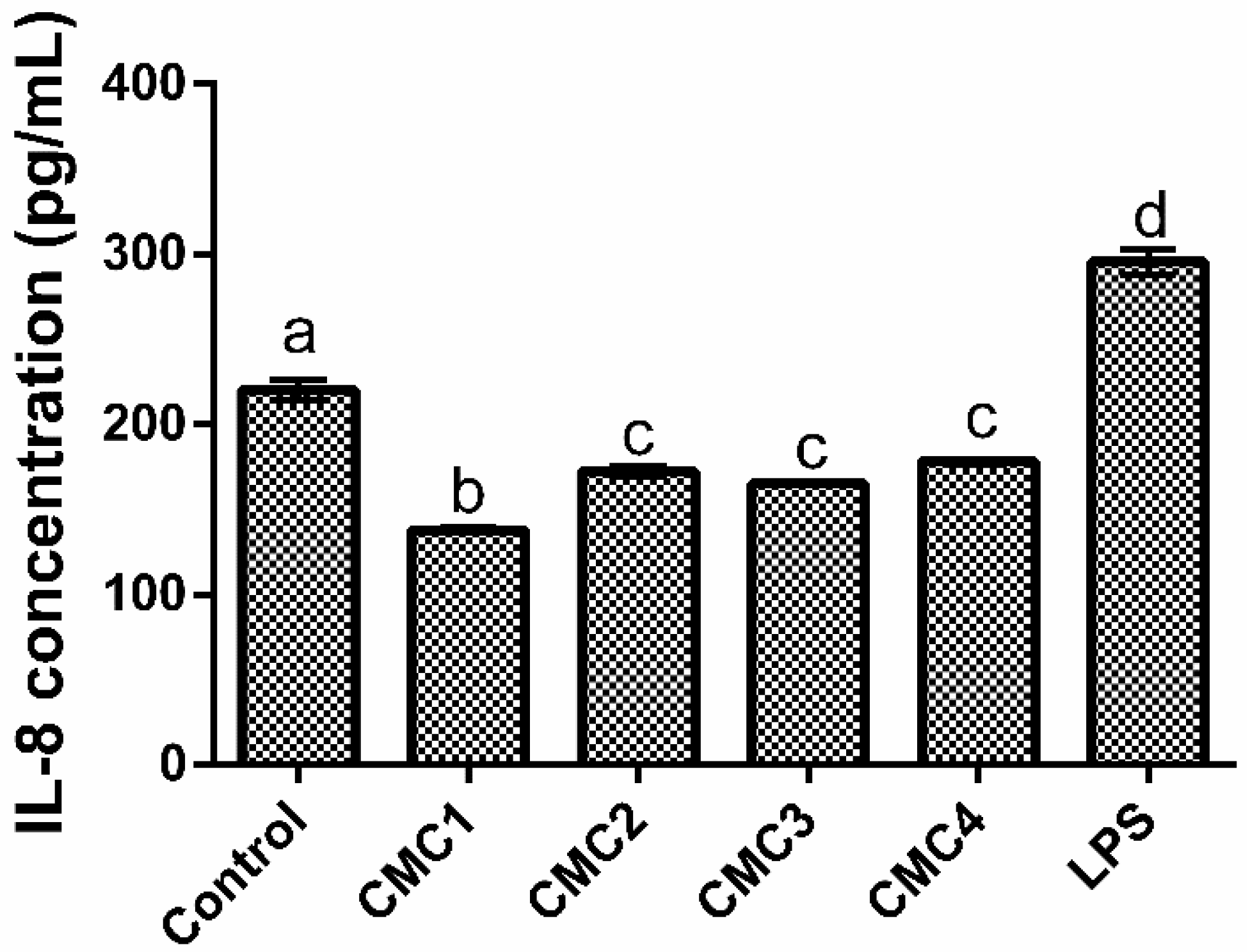
3.1.5. Interleukins Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golbaghi, L.; Khamforoush, M.; Hatami, T. Carboxymethyl cellulose production from sugarcane bagasse with steam explosion pulping: Experimental, modeling, and optimization. Carbohydr. Polym. 2017, 174, 780–788. [Google Scholar] [CrossRef]
- Rahman, M.; Hasan, M.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.; Shiddiky, M.J.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Shui, T.; Feng, S.; Chen, G.; Li, A.; Yuan, Z.; Shui, H.; Kuboki, T.; Xu, C. Synthesis of sodium carboxymethyl cellulose using bleached crude cellulose fractionated from cornstalk. Biomass Bioenergy 2017, 105, 51–58. [Google Scholar] [CrossRef]
- Toğrul, H.; Arslan, N. Production of carboxymethyl cellulose from sugar beet pulp cellulose and rheological behaviour of carboxymethyl cellulose. Carbohydr. Polym. 2003, 54, 73–82. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Trivedi, N.; Reddy, C.R.K. Synthesis and characterization of seaweed cellulose derived carboxymethyl cellulose. Carbohydr. Polym. 2017, 157, 1604–1610. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef]
- Candido, R.; Gonçalves, A. Synthesis of cellulose acetate and carboxymethylcellulose from sugarcane straw. Carbohydr. Polym. 2016, 152, 679–686. [Google Scholar] [CrossRef]
- Karataş, M.; Arslan, N. Flow behaviours of cellulose and carboxymethyl cellulose from grapefruit peel. Food Hydrocoll. 2016, 58, 235–245. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Kirthika, S.; Keerthana, S.; Kumar, N.M.; Jeyanthi, J. Fabrication and characterization of Spinacia oleracea extract incorporated alginate/carboxymethyl cellulose microporous scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 156, 430–437. [Google Scholar] [CrossRef]
- Matinfar, M.; Mesgar, A.S.; Mohammadi, Z. Evaluation of physicochemical, mechanical and biological properties of chitosan/carboxymethyl cellulose reinforced with multiphasic calcium phosphate whisker-like fibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 341–353. [Google Scholar] [CrossRef]
- Wang, X.; Mo, X. High Hygroscopic Wound Dressing and Preparation Method and Use Thereof. C.N. Patent PCT/CN2013/082801, 2 September 2013. [Google Scholar]
- Yan, M.; Chen, T.; Zhang, S.; Lu, T.; Sun, X. A core-shell structured alginate hydrogel beads with tunable thickness of carboxymethyl cellulose coating for pH responsive drug delivery. J. Biomater. Sci. Polym. Ed. 2021, 32, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Doucet, O.; Bernini, D.; Robert, C.; Pujos, M. Cosmetic with Enhanced Collagen i Synthesis. European Patent EP12745428.8A, 14 July 2014. [Google Scholar]
- Gawade, R.P.; Chinke, S.L.; Alegaonkar, P.S. Chapter 17—Polymers in cosmetics. In Polymer Science and Innovative Applications; AlMaadeed, M.A.A., Ponnamma, D., Carignano, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 545–565. [Google Scholar]
- Martins, D.; Rocha, C.; Dourado, F.; Gama, M. Bacterial Cellulose-Carboxymethyl Cellulose (BC:CMC) dry formulation as stabilizer and texturizing agent for surfactant-free cosmetic formulations. Colloids Surf. Physicochem. Eng. Asp. 2021, 617, 126380. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Wongkom, L.; Jimtaisong, A. Novel biocomposite of carboxymethyl chitosan and pineapple peel carboxymethylcellulose as sunscreen carrier. Int. J. Biol. Macromol. 2017, 95, 873–880. [Google Scholar] [CrossRef]
- ASTM D1439-03; Standard Test Methods for Sodium Carboxymethylcellulose. ASTM International: West Conshohocken, PA, USA, 2016; p. 9.
- Condezo-Hoyos, L.; Pérez-López, E.; Rupérez, P. Improved evaporative light scattering detection for carbohydrate analysis. Food Chem. 2015, 180, 265–271. [Google Scholar] [CrossRef]
- Baik, Y.-S.; Cheong, W.-J. Determination of molecular weight distribution and average molecular weights of oligosaccharides by HPLC with a common C18 phase and a mobile phase with high water content. Bull. Korean Chem. Soc. 2007, 28, 847–850. [Google Scholar]
- Melander, M.; Vuorinen, T. Determination of the degree of polymerisation of carboxymethyl cellulose by size exclusion chromatography. Carbohydr. Polym. 2001, 46, 227–233. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Veiga, M.; Baptista, P.; Tavaria, F.K.; Pintado, M.E. Textile dyes loaded chitosan nanoparticles: Characterization, biocompatibility and staining capacity. Carbohydr. Polym. 2021, 251, 117120. [Google Scholar] [CrossRef]
- Chua, K.Y.; Azzahari, A.D.; Abouloula, C.N.; Sonsudin, F.; Shahabudin, N.; Yahya, R. Cellulose-based polymer electrolyte derived from waste coconut husk: Residual lignin as a natural plasticizer. J. Polym. Res. 2020, 27, 115. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Doh, S.J.; Lee, J.Y.; Lim, D.Y.; Im, J.N. Manufacturing and analyses of wet-laid nonwoven consisting of carboxymethyl cellulose fibers. Fibers Polym. 2013, 14, 2176–2184. [Google Scholar] [CrossRef]
- Shlieout, G.; Arnold, K.; Müller, G. Powder and mechanical properties of microcrystalline cellulose with different degrees of polymerization. AAPS PharmSciTech 2002, 3, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Romeo, M.-C.; Cavallaro, A.A.; Chan, H.-K. Protective effect of sodium stearate on the moisture-induced deterioration of hygroscopic spray-dried powders. Int. J. Pharm. 2018, 541, 11–18. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices, in Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009; p. 34.
- Moharamzadeh, K.; Van Noort, R.; Brook, I.M.; Scutt, A.M. Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytes. Dent. Mater. 2007, 23, 40–44. [Google Scholar] [CrossRef]
- Damour, O.; Hua, S.Z.; Lasne, F.; Villain, M.; Rousselle, P.; Collombel, C. Cytotoxicity evaluation of antiseptics and antibiotics on cultured human fibroblasts and keratinocytes. Burns 1992, 18, 479–485. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.-C. Evaluation of biocompatibility and cytotoxicity using keratinocyte and fibroblast cultures. Ski. Pharmacol. Physiol. 2009, 22, 74–82. [Google Scholar] [CrossRef]
- Shah, R.; Saha, N.; Kuceková, Z.; Humpolicek, P.; Saha, P. Properties of biomineralized (CaCO3) PVP-CMC hydrogel with reference to its cytotoxicity. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 619–628. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Humpolicek, P.; Saha, P. Permeability and biocompatibility of novel medicated hydrogel wound dressings. Soft Mater. 2010, 8, 338–357. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; Mansur, H.S.; de Jesus, A.C.; Carvalho, S.M.; Chagas, P.; de Oliveira, L.C. Eco-friendly and biocompatible cross-linked carboxymethylcellulose hydrogels as adsorbents for the removal of organic dye pollutants for environmental applications. Environ. Technol. 2018, 39, 2856–2872. [Google Scholar] [CrossRef] [PubMed]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Vilela, C.; Costa, E.; Veiga, M.; Antunes, F.; Pintado, M.; Sèbe, G.; Coma, V. Bacterial nanocellulose membranes loaded with vitamin B-based ionic liquids for dermal care applications. J. Mol. Liq. 2020, 302, 112547. [Google Scholar] [CrossRef]
- Turky, G.; Moussa, M.A.; Hasanin, M.; El-Sayed, N.S.; Kamel, S. Carboxymethyl Cellulose-Based Hydrogel: Dielectric Study, Antimicrobial Activity and Biocompatibility. Arab. J. Sci. Eng. 2021, 46, 17–30. [Google Scholar] [CrossRef]
- Kundu, J.; Mohapatra, R.; Kundu, S. Silk fibroin/sodium carboxymethylcellulose blended films for biotechnological applications. J. Biomater. Sci. Polym. Ed. 2011, 22, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Namkaew, J.; Sawaddee, N.; Yodmuang, S. Polyvinyl Alcohol-Carboxymethyl Cellulose Scaffolds for Cartilage Tissue Formation. In Proceedings of the 2019 12th Biomedical Engineering International Conference (BMEiCON), Ubon Ratchathani, Thailand and Pakse, Laos, 19–22 November 2019. [Google Scholar]
- Priya, G.; Madhan, B.; Narendrakumar, U.; Suresh Kumar, R.V.; Manjubala, I. In Vitro and In Vivo Evaluation of Carboxymethyl Cellulose Scaffolds for Bone Tissue Engineering Applications. ACS Omega 2021, 6, 1246–1253. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; Carvalho, S.M.; Mansur, L.L.; Ramos, C.P.; Lage, A.P.; Mansur, H.S. Physicochemical properties and antimicrobial activity of biocompatible carboxymethylcellulose-silver nanoparticle hybrids for wound dressing and epidermal repair. J. Appl. Polym. Sci. 2018, 135, 45812. [Google Scholar] [CrossRef]
- Nordli, H.R.; Chinga-Carrasco, G.; Rokstad, A.M.; Pukstad, B. Producing ultrapure wood cellulose nanofibrils and evaluating the cytotoxicity using human skin cells. Carbohydr. Polym. 2016, 150, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Fattahi, A.; Petrini, P.; Munarin, F.; Shokoohinia, Y.; Golozar, M.A.; Varshosaz, J.; Tanzi, M.C. Polysaccharides derived from tragacanth as biocompatible polymers and gels. J. Appl. Polym. Sci. 2013, 129, 2092–2102. [Google Scholar] [CrossRef]
- Summa, M.; Russo, D.; Penna, I.; Margaroli, N.; Bayer, I.S.; Bandiera, T.; Athanassiou, A.; Bertorelli, R. A biocompatible sodium alginate/povidone iodine film enhances wound healing. Eur. J. Pharm. Biopharm. 2018, 122, 17–24. [Google Scholar] [CrossRef]
- Pereira, R.F.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Ogen-Shtern, N.; Chumin, K.; Cohen, G.; Borkow, G. Increased pro-collagen 1, elastin, and TGF-β1 expression by copper ions in an ex-vivo human skin model. J. Cosmet. Dermatol. 2020, 19, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Kono, H. Characterization and properties of carboxymethyl cellulose hydrogels crosslinked by polyethylene glycol. Carbohydr. Polym. 2014, 106, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Areias, A.; Ribeiro, C.; Sencadas, V.; García-Giralt, N.; Diez-Perez, A.; Ribelles, J.G.; Lanceros-Méndez, S. Influence of crystallinity and fiber orientation on hydrophobicity and biological response of poly (l-lactide) electrospun mats. Soft Matter 2012, 8, 5818–5825. [Google Scholar] [CrossRef] [Green Version]
- Shendi, D.; Marzi, J.; Linthicum, W.; Rickards, A.; Dolivo, D.; Keller, S.; Kauss, M.; Wen, Q.; McDevitt, T.; Dominko, T. Hyaluronic acid as a macromolecular crowding agent for production of cell-derived matrices. Acta Biomater. 2019, 100, 292–305. [Google Scholar] [CrossRef]
- Smith, A.M.; Hunt, N.C.; Shelton, R.M.; Birdi, G.; Grover, L.M. Alginate hydrogel has a negative impact on in vitro collagen 1 deposition by fibroblasts. Biomacromolecules 2012, 13, 4032–4038. [Google Scholar] [CrossRef] [Green Version]
- Mesquida, P.; Kohl, D.; Andriotis, O.G.; Thurner, P.J.; Duer, M.; Bansode, S.; Schitter, G. Evaluation of surface charge shift of collagen fibrils exposed to glutaraldehyde. Sci. Rep. 2018, 8, 10126. [Google Scholar] [CrossRef]
- Wu, J.; Liu, F.; Yu, Z.; Ma, Y.; Goff, H.D.; Ma, J.; Zhong, F. Facile preparation of collagen fiber–glycerol-carboxymethyl cellulose composite film by immersing method. Carbohydr. Polym. 2020, 229, 115429. [Google Scholar] [CrossRef] [PubMed]
- Dalir Abdolahinia, E.; Jafari, B.; Parvizpour, S.; Barar, J.; Nadri, S.; Omidi, Y. Role of cellulose family in fibril organization of collagen for forming 3D cancer spheroids: In vitro and in silico approach. BioImpacts BI 2021, 11, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Hwang, Y.; Sahu, A.; Min, K.; Sung, D.; Tae, G.; Chang, J.H. An injectable and physical levan-based hydrogel as a dermal filler for soft tissue augmentation. Biomater. Sci. 2018, 6, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Sathish, P.B.; Gayathri, S.; Priyanka, J.; Muthusamy, S.; Narmadha, R.; Krishnakumar, G.S.; Selvakumar, R. Tricomposite gelatin-carboxymethylcellulose-alginate bioink for direct and indirect 3D printing of human knee meniscal scaffold. Int. J. Biol. Macromol. 2022, 195, 179–189. [Google Scholar]
- Brackmann, C.; Zaborowska, M.; Sundberg, J.; Gatenholm, P.; Enejder, A. In situ imaging of collagen synthesis by osteoprogenitor cells in microporous bacterial cellulose scaffolds. Tissue Eng. Methods 2012, 18, 227–234. [Google Scholar] [CrossRef]
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Mohd Amin, M.C.I. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2019, 9, 444–452. [Google Scholar] [CrossRef]
- Feng, X.; Ullah, N.; Wang, X.; Sun, X.; Li, C.; Bai, Y.; Chen, L.; Li, Z. Characterization of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917. J. Food Sci. 2015, 80, E2217–E2227. [Google Scholar] [CrossRef]
- Hayakawa, D.; Nishiyama, Y.; Mazeau, K.; Ueda, K. Evaluation of hydrogen bond networks in cellulose Iβ and II crystals using density functional theory and Car–Parrinello molecular dynamics. Carbohydr. Res. 2017, 449, 103–113. [Google Scholar] [CrossRef]
- Casaburi, A.; Rojo, Ú.M.; Cerrutti, P.; Vázquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocoll. 2018, 75, 147–156. [Google Scholar] [CrossRef]
- Tang, S.-C.; Liao, P.-Y.; Hung, S.-J.; Ge, J.-S.; Chen, S.-M.; Lai, J.-C.; Hsiao, Y.-P.; Yang, J.-H. Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-κB signaling pathway in keratinocytes and mice skin. J. Dermatol. Sci. 2017, 86, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Esquivel-García, R.; Ayiania, M.; Abu-Lail, N.; López-Meza, J.E.; Rosa, E.; García-Pérez, M.; Ochoa-Zarzosa, A.; García-Pérez, M.-E. Pyrolytic oils from Amphipterygium adstringens bark inhibit IL-8 production of IL-17-stimulated HaCaT keratinocytes. J. Anal. Appl. Pyrolysis 2020, 145, 104749. [Google Scholar] [CrossRef]
- Lembo, S.; Balato, A.; Di Caprio, R.; Cirillo, T.; Giannini, V.; Gasparri, F.; Monfrecola, G. The modulatory effect of ellagic acid and rosmarinic acid on ultraviolet-B-induced cytokine/chemokine gene expression in skin keratinocyte (HaCaT) cells. BioMed Res. Int. 2014, 2014, 346793. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Kundu, S. Sericin–carboxymethyl cellulose porous matrices as cellular wound dressing material. J. Biomed. Mater. Res. Part A 2014, 102, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Kollar, P.; Závalová, V.; Hošek, J.; Havelka, P.; Sopuch, T.; Karpíšek, M.; Třetinová, D.; Suchý Jr, P. Cytotoxicity and effects on inflammatory response of modified types of cellulose in macrophage-like THP-1 cells. Int. Immunopharmacol. 2011, 11, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, W.; Han, B.; Chang, J.; Li, M.; Zhi, X. Effects of carboxymethyl-chitosan on wound healing in vivo and in vitro. J. Ocean Univ. China 2011, 10, 369–378. [Google Scholar] [CrossRef]
- Jeon, B.; Kim, M.O.; Kim, Y.-S.; Han, H.-Y.; Yun, J.-H.; Kim, J.; Huang, Y.; Choi, Y.; Cho, C.-H.; Kang, B.-C. Optimization and validation of a method to identify skin sensitization hazards using IL-1 α and IL-6 secretion from HaCaT. Toxicol. Vitr. 2019, 61, 104589. [Google Scholar] [CrossRef]
- OECD. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins; OECD Publishing: Paris, France, 2014. [Google Scholar]
- Kleinstreuer, N.C.; Hoffmann, S.; Alépée, N.; Allen, D.; Ashikaga, T.; Casey, W.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N. Non-animal methods to predict skin sensitization (II): An assessment of defined approaches. Crit. Rev. Toxicol. 2018, 48, 359–374. [Google Scholar] [CrossRef]






| Compound | Moisture (%) | Purity (%) | DS | Molar Mass Distribution | ||
|---|---|---|---|---|---|---|
| Mw (kDa) | Mn (kDa) | PI | ||||
| CMC1 | 3.67 ± 0.19 a | 99.61 ± 0.21 a | 0.71 ± 0.03 a | 370.1 ± 7.2 a | 313.0 ± 6.3 | 1.2 ± 0.0 |
| CMC2 | 11.74 ± 0.04 b | 97.23 ± 0.55 a | 0.80 ± 0.01 b | 471.7 ± 1.0 b | 382.6 ± 6.0 | 1.2 ± 0.0 |
| CMC3 | 5.09 ± 0.12 c | 99.55 ± 0.33 a | 0.61 ± 0.01 c | 407.0 ± 2.3 c | 316.4 ± 3.7 | 1.3 ± 0.0 |
| CMC4 | 5.97 ± 0.24 c | 99.57 ± 0.25 a | 0.71 ± 0.00 a | 322.5 ± 9.0 d | 288.9 ± 8.5 | 1.1 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, E.M.; Pereira, C.F.; Ribeiro, A.A.; Casanova, F.; Freixo, R.; Pintado, M.; Ramos, O.L. Characterization and Evaluation of Commercial Carboxymethyl Cellulose Potential as an Active Ingredient for Cosmetics. Appl. Sci. 2022, 12, 6560. https://doi.org/10.3390/app12136560
Costa EM, Pereira CF, Ribeiro AA, Casanova F, Freixo R, Pintado M, Ramos OL. Characterization and Evaluation of Commercial Carboxymethyl Cellulose Potential as an Active Ingredient for Cosmetics. Applied Sciences. 2022; 12(13):6560. https://doi.org/10.3390/app12136560
Chicago/Turabian StyleCosta, Eduardo M., Carla F. Pereira, Alessandra A. Ribeiro, Francisca Casanova, Ricardo Freixo, Manuela Pintado, and Oscar L. Ramos. 2022. "Characterization and Evaluation of Commercial Carboxymethyl Cellulose Potential as an Active Ingredient for Cosmetics" Applied Sciences 12, no. 13: 6560. https://doi.org/10.3390/app12136560











