Current Advances in the Concept of Quorum Sensing-Based Prevention of Spoilage of Fish Products by Pseudomonads
Abstract
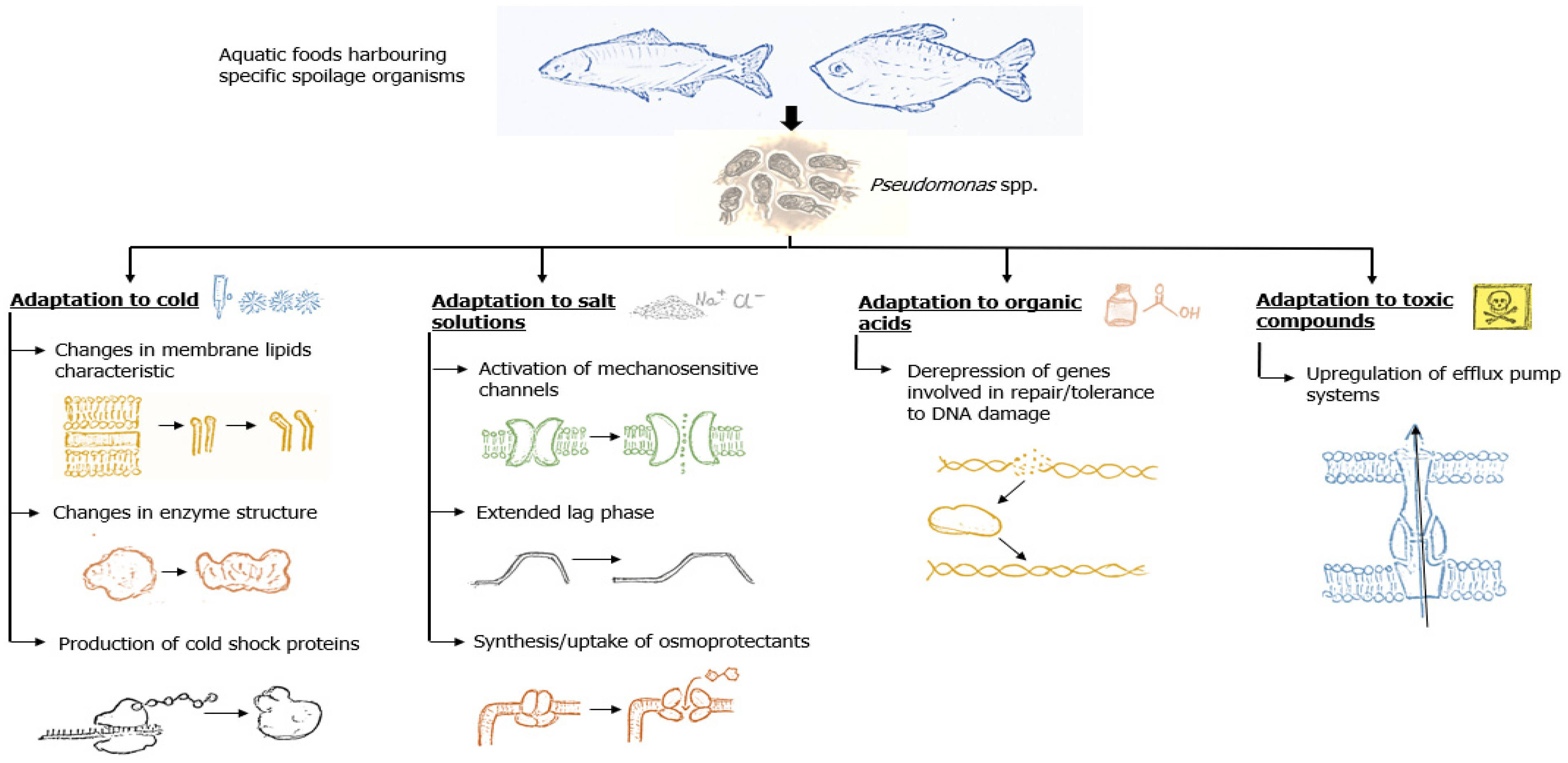
:1. Introduction
2. QS System in Pseudomonads
3. Examples of QS-Based Activities of Pseudomonas spp. Affecting Spoilage of Fish
3.1. QS and Proteolysis
3.2. QS and Lipolysis
3.3. QS and Biofilm Formation
4. QS Inhibition in the Context of Fish Preservation
4.1. QS Inhibiting Agents
4.2. Examples of Use Anti-QS Agents in Fish Preservation
5. Future Work and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture (Sofia)—Meeting the Sustainable Development Goals; FDA: Rome, Italy, 2018; p. pxiii-210. [Google Scholar]
- Comi, G. Spoilage of meat and fish. In The Microbiological Quality of Food; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 179–210. [Google Scholar]
- Raposo, A.; Pérez, E.; de Faria, C.T.; Ferrús, M.A.; Carrascosa, C. Food spoilage by Pseudomonas spp.—An overview. In Foodborne Pathogens and Antibiotic Resistance; Singh, O.V., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 41–71. [Google Scholar]
- Xie, J.; Zhang, Z.; Yang, S.-P.; Cheng, Y.; Qian, Y.-F. Study on the spoilage potential of Pseudomonas fluorescens on salmon stored at different temperatures. J. Food Sci. Technol. 2018, 55, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Myszka, K.; Tomaś, N.; Wolko, Ł.; Szwengiel, A.; Grygier, A.; Nuc, K.; Majcher, M. In situ approaches show the limitation of the spoilage potential of Juniperus phoenicea L. essential oil against cold-tolerant Pseudomonas fluorescens KM24. Appl. Microbiol. Biotechnol. 2021, 105, 4255–4268. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.C.; Chen, C.; Yu, X.; Nielsen, L.; Peterson, K.; Beattle, G.A. Physiological and transcriptional responses to osmotic stress of two Pseudomonas syringae strains that differ in epiphytic fitness and osmotolerance. J. Bacteriol. 2013, 195, 4742–4752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage—Interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish spoilage mechanisms and preservation techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Venturi, V. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol. Rev. 2006, 30, 274–291. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Tribelli, P.M.; López, N.I. Reporting key features in cold-adapted bacteria. Life 2018, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Machado, I.; Silva, L.R.; Giaouris, E.D.; Melo, L.F.; Simões, M. Quorum sensing in food spoilage and natural-based strategies for its inhibition. Food Res. Int. 2020, 127, 108754. [Google Scholar] [CrossRef]
- Bai, A.J.; Rai, V.R. Bacterial quorum sensing and food industry. Compr. Rev. Food Sci. Food Saf. 2011, 10, 183–193. [Google Scholar] [CrossRef]
- Wagner, V.E.; Bushnell, D.; Passador, L.; Brooks, A.I.; Iglewski, B.H. Microarray analysis of Pseudomonas aeruginosa quorum sensing regulons: Effects of growth phase and environment. J. Bacteriol. 2003, 185, 2066–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, M.; Lostroh, C.P.; Ogi, T.; Greenberg, E.P. Identification, timing and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: A transcriptome analysis. J. Bacteriol. 2003, 185, 2066–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diggle, S.P.; Matthijs, S.; Wright, V.J.; Fletcher, M.P.; Chhabra, S.R.; Lamont, I.L.; Kong, X.; Hider, R.C.; Cornelis, P.; Cámara, M.; et al. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem. Biol. 2007, 14, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skandamis, P.N.; Nychas, G.-J.E. Quorum sensing in the context of food microbiology. Appl. Environ. Microbiol. 2012, 78, 5473–5482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Nair, S.K. Quorum sensing: How bacteria can coordinate activity and synchronize their response to external signals? Protein Sci. 2012, 21, 1403–1417. [Google Scholar] [CrossRef]
- Li, T.; Wang, D.; Ren, L.; Mei, Y.; Ding, T.; Li, Q.; Chen, H.; Li, J. Involvement of exogenous N-Acyl-homoserine lactones in spoilage potential of Pseudomonas fluorescens isolated from refrigerated turbot. Front. Microbiol. 2019, 10, 2716. [Google Scholar] [CrossRef]
- Fuqua, C.; Parsek, M.R.; Greenberg, E.P. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001, 35, 439–468. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory mechanisms and promising application of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef]
- Gallagher, L.A.; McKnight, S.L.; Kuznetsova, M.S.; Pesci, E.C.; Manoil, C. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol. 2002, 184, 6472–6480. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Cheng, J.; Wang, Y.; Shen, X. The Pseudomonas quinolone signal (PQS): Not just for quorum sensing any more. Front. Cell. Infect. Microbiol. 2018, 8, 230. [Google Scholar] [CrossRef]
- Bru, J.L.; Rawson, B.; Trinh, C.; Whiteson, K.; Høyland-Kroghsbo, N.M.; Siryaporn, A. PQS produced by the Pseudomonas aeruginosa stress response repels swarms away from bacteriophage and antibiotics. J. Bacteriol. 2019, 201, e00383-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diggle, S.P.; Winzer, K.; Chhabra, S.R.; Worrall, K.E.; Cámara, M.; Williams, P. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol. 2003, 50, 29–43. [Google Scholar] [CrossRef]
- Lee, J.; Wu, J.; Deng, Y.; Wang, J.; Wang, C.; Wang, J.; Chang, C.; Dong, Y.; Williams, P.; Zhang, L.H. A cell-cell communication signal integrates quorum sensing and stress response. Nat. Chem. Biol. 2013, 9, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Pesci, E.C.; Pearson, J.P.; Seed, P.C.; Iglewski, B.H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1997, 179, 3127–3132. [Google Scholar] [CrossRef] [Green Version]
- Ventre, I.; Ledgham, F.; Prima, V.; Lazdunski, A.; Foglino, M.; Sturgis, J.N. Dimerization of the quorum sensing regulator RhlR: Development of a method using EGFP fluorescence anisotropy. Mol. Microbiol. 2003, 48, 187–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Krishnan, G.; Goumnerov, B.; Tsongalis, J.; Tompkins, R.; Rahme, L.G. A quorum sensing-associated virulence genes of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc. Natl. Acad. Sci. USA 2001, 98, 14613–14618. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Yang, B.; Li, X.; Li, J.; Zhao, G.; Kan, J. Quorum sensing system and influence on food spoilage in Pseudomonas fluorescens from turbot. J. Food Sci. Technol. 2018, 55, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- Meliani, A.; Bensoltane, A. Review of Pseudomonas attachment and biofilm formation in food industry. Poult. Fish. Wildl. Sci. 2015, 3, 2–7. [Google Scholar] [CrossRef]
- Venugopal, V. Extracellular proteases of contaminant bacteria in fish spoilage: A review. J. Food Prot. 1990, 53, 341–350. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. Biomed. Res. Int. 2015, 2015, 759348. [Google Scholar] [CrossRef] [Green Version]
- Ammor, M.S.; Michaelidis, C.; Nychas, G.-J.E. Insights into the role of quorum sensing in food spoilage. J. Food Prot. 2008, 71, 1510–1525. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Zhu, J.; Feng, L.; Li, J.; Liu, X. Characterization of LuxI/LuxR and their regulation involved in biofilm formation and stress resistance in fish spoilers Pseudomonas fluorescens. Int. J. Food Microbiol. 2019, 297, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Stepaniak, L.; Fox, P.F.; Daly, C. Isolation and general characterization of a heat-stable proteinase from Pseudomonas fluorescens. Biochim. Biophys. Acta 1982, 717, 376–383. [Google Scholar] [CrossRef]
- Sterniša, M.; Purgatorio, C.; Paparella, A.; Mraz, J.; Smole Možina, S. Combination of rosemary extract and buffered vinegar inhibits Pseudomonas and Shewanella growth in common carp (Cypronus carpio). J. Sci. Food Agric. 2020, 100, 2305–2312. [Google Scholar] [CrossRef]
- Liu, M.; Wang, H.; Griffiths, M.W. Regulation of alkaline metalloprotease promoter by N-acyl homoserine lactone quorum sensing in Pseudomonas fluorescens: Protease promoter regulation by AHLs in Pseudomonas fluorescens. J. Appl. Microbiol. 2007, 103, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Sobieszczańska, N.; Myszka, K.; Szwengiel, A.; Majcher, M.; Grygier, A.; Wolko, Ł. Tarragon essential oil as a source of bioactive compounds with anti-quorum sensing and anti-proteolytic activity against Pseudomonas spp. isolated from fish—In vitro, in silico and in situ approaches. Int. J. Food Microbiol. 2020, 331, 108732. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.B.; Riedel, K.; Eberl, L.; Flodgaard, L.R.; Molin, S.; Gram, L.; Givskov, M. Quorum-sensing-directed protein expression in Serratia proteamaculans B5a. Microbiology 2003, 149, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Beven, C.-A.; Dieckelmann, M.; Beacham, I.R. A strain of Pseudomonas fluorescens with two lipase-encoding genes, one of which possibly encodes cytoplasmic lipolytic activity. J. Appl. Microbiol. 2001, 90, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, R.G.; Burger, M.; Beven, C.-A.; Beachman, I.R. The apX-lipA operon of Pseuodmonas fluorescens B52: A molecular analysis of metalloprotease and lipase production. Microbiology 2001, 147, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Riedel, K.; Hentzer, M.; Geisenberger, O.; Huber, B.; Steidle, A.; Wu, H.; Høiby, N.; Givskov, M.; Molin, S.; Eberl., L. N-Acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Brukholderia cepacia in mixed biofilms. Microbiology 2001, 147, 3249–3262. [Google Scholar] [CrossRef] [Green Version]
- Udine, C.; Brackman, G.; Bazzini, S.; Buroni, S.; Van Acker, H.; Pasca, M.R.; Riccardi, G.; Coenye, T. Phenotypic and genotypic characterization of Brukholderia cenocepacia J2315 mutants affected in homoserine lactone and diffusible signal factor-based quorum sensing system suggests interplay between both types systems. PLoS ONE 2013, 8, e55112. [Google Scholar] [CrossRef] [Green Version]
- Devescovi, G.; Bigirimana, A.; Degrassi, G.; Cabrio, L.; LiPuma, J.J.; Kim, J.; Hwang, I.; Venturi, V. Involvement of a quorum-sensing-regulated lipase secreted by a clinical isolate of Brukholderia glumae in severe disease symptoms in rice. Appl. Environ. Microbiol. 2007, 73, 4950–4958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Daniels, R.; Vanderleyden, J.; Michiels, J. Quorum sensing and swarming migration in bacteria. FEMS Microbiol. Rev. 2004, 28, 261–289. [Google Scholar] [CrossRef]
- Vetrivel, A.; Ramasamy, M.; Vetrivel, P.; Natchimuthu, S.; Arnuachalam, S.; Kim, G.-S.; Murugesan, R. Pseudomonas aeruginosa biofilm formation and its control. Biologics 2021, 1, 312–336. [Google Scholar] [CrossRef]
- Pamp, S.J.; Tolker-Nielsen, T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 2531–2539. [Google Scholar] [CrossRef] [Green Version]
- Bai, A.J.; Vittal, R. Quorum sensing regulation and inhibition of exoenzyme production and biofilm formation in the food spoilage bacteria Pseudomonas psychrophila PSPF19. Food Biotechnol. 2014, 28, 293–308. [Google Scholar] [CrossRef]
- Myszka, K.; Sobieszczańska, N.; Olejnik, A.; Majcher, M.; Szwengiel, A.; Wolko, Ł.; Juzwa, W. Studies on the anti-proliferative and anti-quorum sensing potentials of Myrtus communis L. essential oil for the improved microbial stability of salmon-based products. LWT 2020, 127, 109380. [Google Scholar] [CrossRef]
- Guðbjörnsdóttir, B.; Einarsson, H.; Thorkelsson, G. Microbial adhesion to processing lines for fish filets and cooked shrimp: Influence of stainless steel surface finish and presence of gram-negative bacteria on the attachment of Listeria monocytogenes. Food Technol. Biotechnol. 2005, 43, 55–61. [Google Scholar]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin influences quorum sensing in food borne bacteria: In-vitro and in-silico evidence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Cui, F.; Bai, F.; Zhao, G.; Li, J. Involvement of Acylaated Homoserine Lactones (AHLs) of Aeromonas sorbia in spoilage of refrigerated turbot (Scophthalmus maximus L.). Sensors 2016, 16, 1083. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A. New weapons to fight old enemies: Novel strategies for the (bio)control of bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar]
- Utari, P.D.; Vogel, J.; Quax, W.J. Deciphering physiological functions of AHL quorum quenching acylases. Front. Microbiol. 2017, 8, 1123. [Google Scholar] [CrossRef] [Green Version]
- Teiber, J.F.; Horke, S.; Haines, D.C.; Chowdhary, P.K.; Xiao, J.; Kramer, G.L.; Haley, R.W.; Draganov, D.I. Dominant role of paraoxonases in inactivation of the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone. Infect. Immun. 2008, 76, 2512–2519. [Google Scholar] [CrossRef] [Green Version]
- Bergonzi, C.; Schwab, M.; Naik, T.; Elias, M. The structural determinants accounting for the board substrate specificity of the quorum quenching lactonase Gcl. Chem. Bio. Chem. 2019, 20, 1848–1855. [Google Scholar]
- Rémy, B.; Plener, L.; Decloquement, P.; Armstrong, N.; Elias, M.; Daudé, D.; Chabrère, E. Lactonase specificity is key to quorum quenching in Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 762. [Google Scholar] [CrossRef]
- Huang, J.J.; Petersen, A.; Whiteley, M.; Leadbetter, J.R. Identification of QuiP, the product of gene PA1032, as the second acyl-homoserine lactone acylase of Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2006, 72, 1190–1197. [Google Scholar] [CrossRef] [Green Version]
- Wahjudi, M.; Papaioannou, E.; Hendrawati, O.; van Assen, A.H.G.; van Merkerk, R.; Cool, R.H.; Poelarends, G.J.; Quax, W.J. PA0305 of Pseudomonas aeruginosa is a quorum quenching acylhomoserine lactone acylase belonging to the Ntn hydrolase superfamily. Microbiology 2011, 157, 2042–2055. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Gao, Y.; Chen, X.; Yu, Z.; Li, X. Quorum quenching enzymes and their application in degrading signal molecules to block quorum sensing-dependent infection. Int. J. Mol. Sci. 2013, 14, 17477–17500. [Google Scholar] [CrossRef]
- Bijtenhoorn, P.; Mayerhofe, H.; Müller-Dieckman, J.; Utpatel, C.; Schippe, C.; Hornung, C.; Szesny, M.; Grond, S.; Thürmer, A.; Brzuszkiewicz, E.; et al. A novel metagenomics short-chain dehydrogenase/reductase attenuates Pseudomonas aeruginosa biofilm formation and virulence on Caenorhabditis elegans. PLoS ONE 2011, 6, e26278. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Amado, S.; Alcalde-Rico, M.; Gil-Gil, T.; Valverde, J.R.; Martinez, J.I. Naringenin inhibition of the Pseudomonas aeruginosa quorum sensing response is based on its time-dependent competition with N-(3-oxo-dodecanoyl)-L-homoserine lactone for LasR binding. Front. Mol. Biosci. 2020, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Tomaś, N.; Myszka, K.; Wolko, Ł.; Nuc, K.; Szwengiel, A.; Grygier, A.; Majcher, M. Effect of black pepper essential oil on quorum sensing and efflux pump systems in the fish-borne spoiler Pseudomonas psychrophila KM02 identified by RNA-seq, RT-qPCR and molecular docking analyses. Food Cont. 2021, 130, 108284. [Google Scholar] [CrossRef]
- Klebe, G. Drug Design: Methodology, Concepts, and Mode-of-Action; Springer: Berlin/Heidelberg, Germany, 2003; p. 71. [Google Scholar]
- Kumar, L.; Chhibber, S.; Kumar, R.; Kumar, M.; Harjai, K. Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia 2015, 102, 84–95. [Google Scholar] [CrossRef]
- Sepahi, E.; Tarighi, S.; Ahmadi, F.S.; Bagheri, A. Inhibition of quorum sensing in Pseudomonas aeruginosa by two herbal essential oils from Apiaceae family. J. Microbiol. 2015, 53, 176–180. [Google Scholar] [CrossRef]
- Kerekes, E.-B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related microorganisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef]
- Li, T.; Wang, D.; Liu, N.; Ma, Y.; Ding, T.; Mei, Y.; Li, J. Inhibition of quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas fluorescens by cinnamaldehyde. Int. J. Food Microbiol. 2018, 269, 98–106. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, M.; D’Morris, S.; Paul, V.; Warrier, S.; Vasudevan, A.K.; Vanuopadath, M.; Nair, S.S.; Paul-Prasanth, B.; Mohan, C.G.; Biwas, R. Mechanistic understanding of phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2017, 101, 8223–8236. [Google Scholar] [CrossRef] [PubMed]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum sensing receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Zhang, Y.; Deng, J.; Jiang, H.; Zhuang, L.; Ye, W.; Ma, J.; Jiang, J.; Feng, L. Diketopiperazines synthesis gene in Shewanella baltica and roles of diketopiperazines and resveratrol in quorum sensing. J. Agric. Food Chem. 2019, 67, 12013–12025. [Google Scholar] [CrossRef] [PubMed]
- Myszka, K.; Olejnik, A.; Majcher, M.; Sobieszczańska, N.; Grygier, A.; Powierska-Czarny, J.; Rudzińska, M. Green pepper essential oil as a biopreservative agent for fish-based products: Antimicrobial and antivirulence activities against Pseudomonas aeruginosa KM01. LWT 2019, 108, 6–13. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Van der Meeren, P.; Sampers, I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control 2016, 68, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Eskandari, S.; Hosseini, H.; Gholamzadeh, M.; Mousavi Khaneghah, A.; Hosseini, E. The effects of black cumin, black caraway extracts and their combination on shelf life extension of silver carp (Hypophthalmichthys molitrix) during refrigerated storage. J. Food Saf. 2014, 35, 154–160. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Gutierrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Rodriguez-Garcia, I.; Gonzalez-Aguilar, G.A.; Ponce, A.; Moreira, M.; del Roura, S.I.; Ayala-Zavala, J.F. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Front. Microbiol. 2014, 5, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farsanipour, A.; Khodanazary, A.; Hosseini, S.M. Effect of chitosan-whey protein isolated coatings incorporated with tarragon Artemisia dracunculus essential oil on the quality of Scomberoides commersonnianus fillets at refrigerated condition. Int. J. Biol. Macromol. 2020, 155, 766–771. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.; Zhao, H.; Zhao, J.; Zhang, J.; Li, J. Effects of gelatin combined with essential oils coating on storage quality of turbot (Psetta maxima) fillets. In Proceedings of the 2017 6th International Conference on Measurement, Instrumentation and Automation (ICMIA 2017), Zhuhai, China, 29–30 June 2017. [Google Scholar]
- Gui, M.; Zhang, Y.; Gao, L.; Li, P. Effect of AHL-lactonase and nisin on microbiological, chemical and sensory quality of vacuum packaged sturgeon storage at 4 °C. Int. J. Food Prop. 2021, 24, 222–232. [Google Scholar] [CrossRef]
| Microorganism | AHL | Phenotypes Regulated by QS | Reference |
|---|---|---|---|
| P. aeruginosa | 3-oxo-C12-HSL | Pyoverdine production | [33] |
| P. psychrophila | C4-HSL | Exoenzyme production | [34] |
| P. fluorescens, P. putida | 3-oxo-C6-HSL, C6-HSL, C8-HSL, C12-HSL | Proteolytic activity | [34] |
| Pseudomonas spp. | C4-HSL, 3-oxo-C6-HSL, C6-HSL, C8-HSL, C12-HSL | Slime formation | [13] |
| P. fluorescens, P. putida | 3-oxo-C6-HSL, C6-HSL, C8-HSL, C12-HSL | Proteolytic activity | [13] |
| P. fluorescens | C4-HSL | Biofilm formation, EPS production | [35] |
| P. fluorescens | C4-HSL | Biofilm formation | [19] |
| P. fluorescens | 3-oxo-C14-HSL, 3-oxo-C6-HSL, C4-HSL | Lipolytic activity | [5] |
| QS Inhibiting Agent | Target Microorganism | Impact on Bacterial QS-Controlled Processes | Reference |
|---|---|---|---|
| Piper nigrum L. EO | P. psychrophila | reduction of proteolytic and lipolytic activities | [66] |
| Ferula asafoetida EO | P. aeruginosa | Reduction of pyocyanin and elastase production; prevention of biofilm formation | [69] |
| Myrtus communis L. EO | P. fluorescens, P. orientalis | Reduction in the EPS production | [52] |
| Origanum majorana EO | P. putida | Prevention of biofilm formation | [70] |
| Juniperus phoenicea EO | P. fluorescens | Reduction of proteolytic and lipolytic activities | [5] |
| Cinnamaldehyde | P. fluorescens | Reduction of proteolytic activity; prevention of biofilm formation | [71] |
| Quercetin | P. aeruginosa | Reduction the EPS production and bacterial motility, prevention of biofilm formation | [54] |
| Garlic extract | P. aeruginosa | Reduction of rhamnolipid production | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaś, N.; Myszka, K. Current Advances in the Concept of Quorum Sensing-Based Prevention of Spoilage of Fish Products by Pseudomonads. Appl. Sci. 2022, 12, 6719. https://doi.org/10.3390/app12136719
Tomaś N, Myszka K. Current Advances in the Concept of Quorum Sensing-Based Prevention of Spoilage of Fish Products by Pseudomonads. Applied Sciences. 2022; 12(13):6719. https://doi.org/10.3390/app12136719
Chicago/Turabian StyleTomaś, Natalia, and Kamila Myszka. 2022. "Current Advances in the Concept of Quorum Sensing-Based Prevention of Spoilage of Fish Products by Pseudomonads" Applied Sciences 12, no. 13: 6719. https://doi.org/10.3390/app12136719
APA StyleTomaś, N., & Myszka, K. (2022). Current Advances in the Concept of Quorum Sensing-Based Prevention of Spoilage of Fish Products by Pseudomonads. Applied Sciences, 12(13), 6719. https://doi.org/10.3390/app12136719






