Biotechnological and Technical Challenges Related to Cultured Meat Production
Abstract
:1. Introduction
Cellular Agriculture and Cultured Meat
2. Cultured Meat Production Process
3. Challenges Related to Cultured Meat Production
3.1. Biotechnological Challenges
3.1.1. Choice of Animal for Cell Harvesting
3.1.2. Choice of Site of Collection
3.1.3. Methods for Cell Harvesting
3.1.4. Fetal Bovine Serum: Ethical Challenges
3.1.5. High Cell Proliferation and Genetic Instability
3.1.6. Nutritional and Functional Properties of Cultured Meat
3.1.7. FBS Alternatives
3.1.8. Food Control System for Cultured Meat
4. Technical Challenges in Cultured Meat Production
4.1. Scaffold Fabrication
4.1.1. Conventional Porous Scaffold Fabrication Technologies
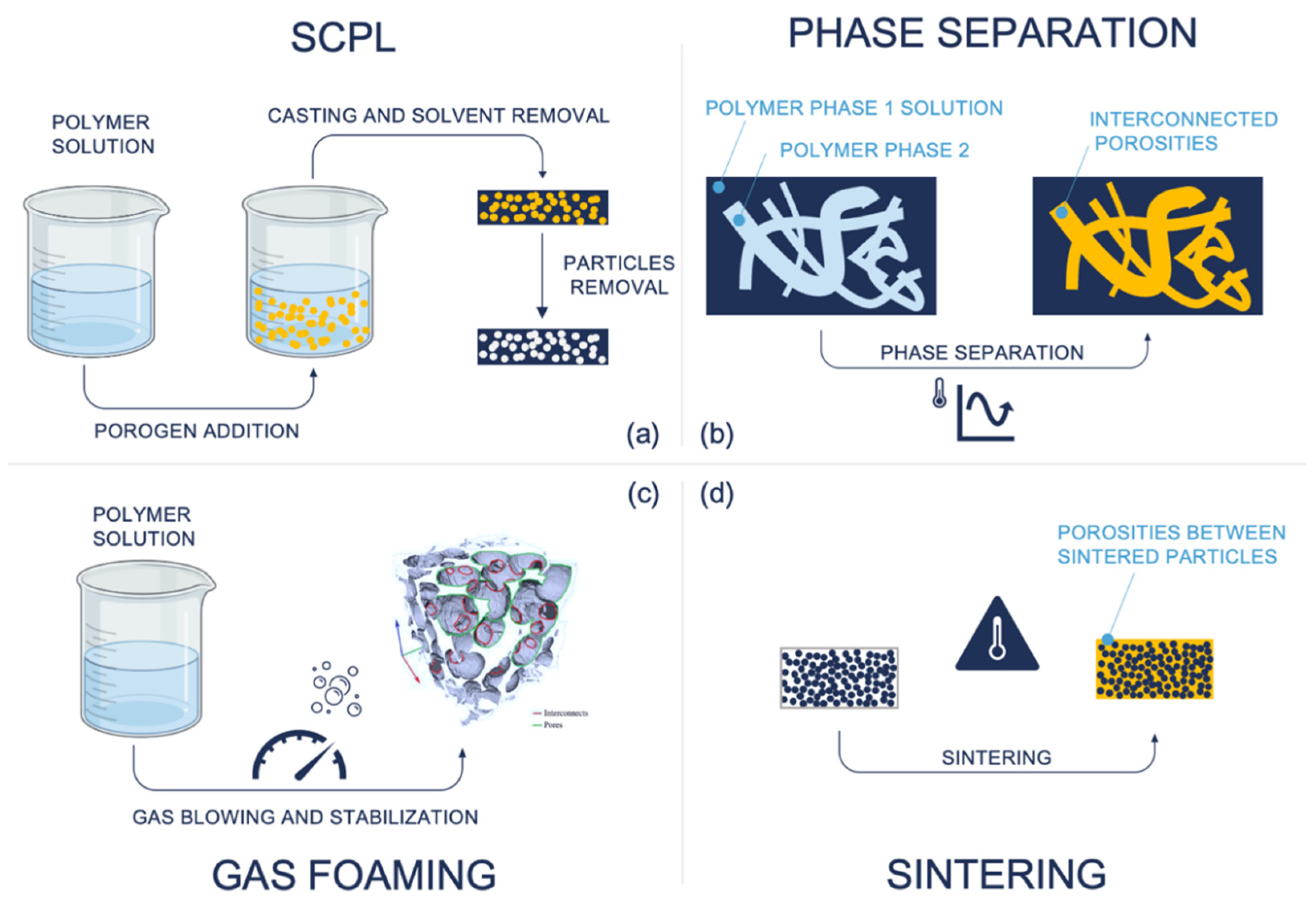
- Solvent casting and porogen leaching (SCPL) [68]: This process involves mixing a polymer solution dissolved in an organic solvent composed of insoluble particulates (porogen). The mixture is then cast into a mold or a membrane, and the solvent is evaporated. Finally, the structure is immersed in an aqueous solution to leach out particulates in the structure. Porosity, in terms of shape, size, and uniformity, depends on the particulate choice of particulates, typically salt particles. The main drawbacks are the lack of control of internal architecture and uniformity, reduced reproducibility, formation of a skin layer due to polymer thickening that can limit access to internal porous, limited thickness (2–3 mm) [69], weak mechanical properties, and possible cytotoxicity due to residual solvent and porogen [70].
- Phase separation [71]: The technique is used to produce a scaffold through the separation of a mixture into two phases: a polymer-rich one and a polymer-poor one. This is achieved under thermodynamically unstable conditions. For example, cooling the solution below the freezing point of the solver induces crystal nucleation inside the solution; after that, the solid material is sublimed, ensuring that the structure is composed of only the polymer-poor region with porosity, because the solvent and the polymer-rich phase are evacuated from the scaffold. This technique leads to highly interconnected porosity which can be used to reproduce channel-like structures by applying a directional temperature gradient. Nevertheless, control and optimization of process parameters (e.g., temperature, polymer concentration, surfactants use, crystal nucleation) are the main problems in managing pore size and distribution [72]. Moreover, the typical pore size achievable is often smaller than the typical dimension in tissue engineering applications (<200 µm).
- Gas foaming [73]: This is a class of techniques for scaffold fabrication exploiting a blowing agent to generate gas inside the material which acts as a porogen agent. The main advantage is the absence of solvents or porogen materials, which can induce cytotoxicity due to possible residuals. The Gas formation can be induced chemically or thermally or by pressure change. The main drawbacks of the technique are low control over pore size and interconnectivity, low reproducibility and structural uniformity, and difficulty in incorporating biological molecules in thermally induced processes [74,75].
- Sintering [76]: The technique is used to produce cohesive porous scaffolds through bonding of a polymeric phase and ceramic particles or fibers. The usual procedures involve a bed of randomly packed particles bonded through heating up to a temperature above the glass transition temperature of the base material, but lower than its melting point, creating a local fuse area only in the contact surfaces, leading to a porous microstructure. Alternative sintering modes are mild solvent treatment and pressure. Sintered scaffolds are characterized by lower porosity, small pore size with difficulty in precise control and distribution, and higher mechanical properties, and they are mainly used in dental and bone-repairing applications [77].
4.1.2. Non-Conventional Porous Scaffold Fabrication Technologies
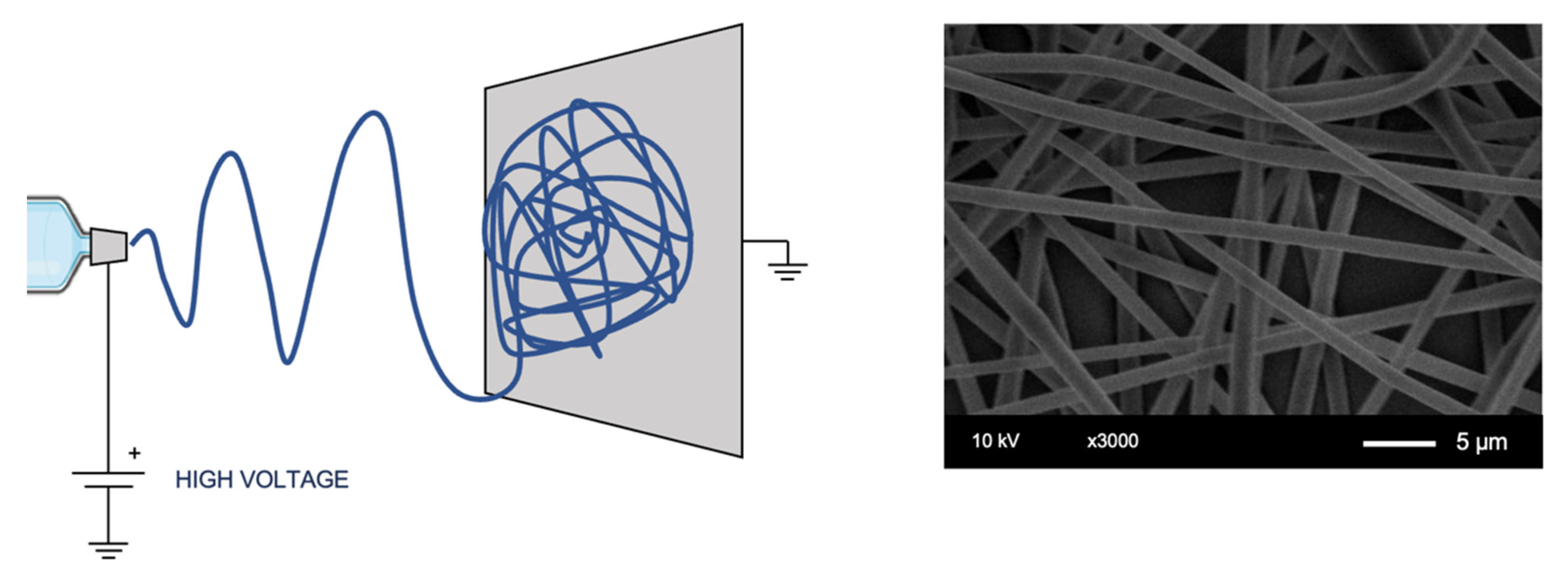
- Electrospinning [78]: The method is based on an electric field generated between a polymer solution delivery system at a controlled flow rate and a collector, drawing the solution into a fiber, an illustrative example is shown in Figure 3. The result is a membrane of non-woven fibers. The textile-based technique has been created to reproduce fiber-based materials, such as those similar to the extracellular matrix (ECM). The resulting porosity is interconnected and the achievable pore size is lower than that by other scaffolding techniques, achieving fibers with diameter up to a few nanometers [78], which can be an advantage for specific applications (e.g., vascular [79]), but tends to limits the cell migration to a point where its applicability in tissue engineering becomes a problem. Several process parameters can be controlled to tune fiber diameter and alignment, adapting textural properties to the specific cell type to be seeded.
- Self-assembly [81]: The technique involves specifically designed amphiphilic peptides with the capacity to spontaneously organize into ordered structures, including nanofibers. The method allows a great process control starting from the building blocks in a bottom-up design approach for tissue engineering application.
- Hybrid scaffolds: With the aim of controlling structural and composition features, mainly porosity, at different length-scales, several approaches with mixed techniques are used, such as SCPL and electrospinning combination [82], multilayer electrospun composites with different parameters [83], and a combination of more than two fabrication techniques [84,85].
- Additive manufacturing (AM): The conventional techniques of porous scaffolds fabrication, as well as other emerging alternatives, are implemented to produce scaffolds to recreate the complex micro and macrostructures of biological tissues. However, all of them have limitations and allow narrow control over important textural parameters such as pore shape, dimensions, and interconnectivity [86]. An emerging family of technologies, based on additive AM techniques, has proved to enable the manufacture and control of complex shapes. AM, popularly called 3D printing, is a generic definition and represents a large group of processes that can be classified in several ways [87]. Within the porous scaffold fabrication context, according to Rey et al., some of the AM technologies could be used to produce scaffold with a high spatial resolution, the structural complexity, and control over the internal pore architecture. The most promising techniques are powder-bed based 3DP, such as selective laser sintering (SLS), and liquid raw-material-based 3DP, such as stereolithography (SLA) [86].
4.2. Alternatives to Scaffold Fabrication
4.2.1. Tissue Decellularization
4.2.2. Microcarrier Cultures
4.3. Biofabrication and 3D Bioprinting
4.3.1. 3D Bioprinting Strategies
| Properties | Extrusion | Inkjet | Laser-Assisted | Stereolithography | Two-Photon |
|---|---|---|---|---|---|
| Speed | Slow | Fast | Medium | Fast | Very fast |
| Cost | Moderate | Low | High | Low | Very high |
| Cell viability | 85–95% | 80–95% | <85% | 25–90% | >80% |
| Cell density | High | Low | Medium | Medium | Medium |
| Scalability | High | High | Low | Medium-high | - |
| Resolution | 100–500 µm | 100–500 µm | 20–100 µm | 20–100 µm | 0.1–10 µm |
| Bioink viscosity | 6–30 × 107 mPa⋅s | <10 mPa⋅s | 1–300 mPa⋅s | No limitation | No limitation |
| Advantages | Is Simple, is capable of printing various biomaterials, high cell densities | Has the ability to print low viscosity biomaterials, fast fabrication speed, low cost, and a high resolution | Has a high resolution, is nozzle-free, and can deposit biomaterials in solid or liquid phase | Is nozzle-free, has high complexity, and has a high resolution | Is nozzle-free, has a high complexity, has the highest resolution, and has high cell viability |
| Drawbacks | Only for viscous liquids, resolution | Limited to low viscous fluids, resolution, cell density | High cost, thermal damage due to nanosecond/femtosecond laser irritation, scalability, cost | Lack of printing multi-cells, cell damage during photo-curing | Lack of printing multi-cells, cost |
| Applications | Tissue models for cell research, drug testing and regenerative medicine, meat-analogue constructs [101] | Supplementary to other technologies | Precise cells deposition | Scaffolds and complex structures with channels | Vascularized and high precision models |
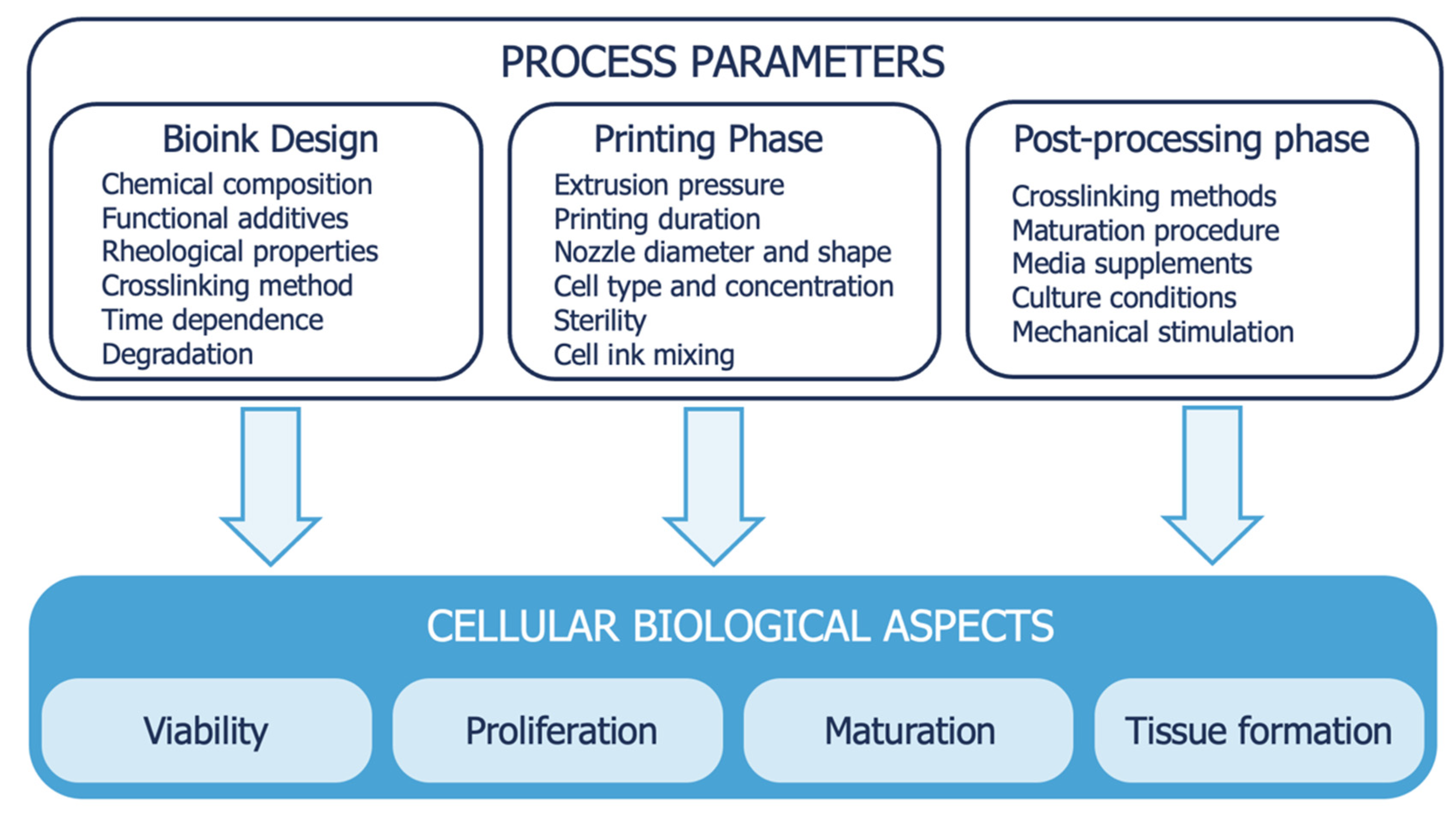
4.3.2. Bioink Formulation
- There is a circular relationship between cells and bioink rheology: the firsts impact the rheology, and thus process parameters, and vice versa. For example, Billiet et al. found a twofold lowering in the viscosity of a GelMA-based bioink when prepared with 0.5 and 1.5 million cells/mL and a fourfold lowering when prepared with 2.5 million cells/mL [107]. Hence, there is the need to predict or test the rheological properties of ink with cells inside.
- Mechanical stresses must be minimized, reducing printing pressure and increasing nozzle diameter because cells are mechanical sensing and suffer higher mechanical stresses.
- The modulus of the gel-phase highly impacts cell viability, and probably also molecular weight and polydispersity. The mechanical properties of the material surrounding the cells is a crucial aspect that is poorly understood.
- Post-printing crosslinking can affect cell viability as well. Frequently, bioprinted constructs are UV-crosslinked and the amount irradiation cells can tolerate is not clear, probably between 30 s and a few minutes. Moreover, the final degree of cross-linking can interfere with cell projections and network formation, an important mechanism to ensure tissue formation. This can become important when polymer concentration is increased to increase printability. According to the author, the concentration of the polymer must be between 5 and 10%, but it is obviously a polymer-dependent quantity.
4.4. Bioreactors
- Static culture systems: They are the simplest and provide the required nutrient in a static fluid environment. Thus, the media must be changed often, and it perfuses by passive fluid diffusion [25]. These systems can be easily coupled with load-bearing mechanisms, for example, to provide a compressive load to the engineered tissues [110,111].
- Spinner flasks: Spinner-flask-based systems are used to apply fluid-induced shear stresses to constructs submerged within a re-circulating and nutrient-rich medium solution [25]. Although this system provides a better environment to construct with respect to the static culture, spinner flasks may not be optimal due to turbulent flow and the related higher shear stress generated [112].
- Perfusion systems: The poor diffusion condition of static culture can be improved by perfusion bioreactors, especially in the internal parts of porous scaffolds [113]. These systems are characterized by a culture encasing bioreactor, vessels for the medium (nutrient-rich and oxygenated), and a pump generating the flow [27]. Moreover, perfusion systems allow for automatic media circulation, waste removal, and provide shear stress due to the flow, which is beneficial in specific tissue cultures such as for dermis and cartilaginous tissues [23,112].
- Rotating wall vessel: An alternative approach for reducing diffusional limitations of nutrients and waste with limited shear stress is the use of rotating wall vessel bioreactors [24]. Although shear stress is important for cell maturation, an excessive force will lead to damages or to the formation of undesired capsules surrounding the tissue [112]. This method uses a dynamic laminar flow induced by the rotating fluid inside the bioreactor, and it has been proved to be effective for cell cultures, especially chondrocytes and cardiac cells [24]. The main drawback is the non-uniform tissue growth, due to the force field. Moreover, the centrifugal force can cause collision between scaffolds and the walls of the bioreactor [112].
- Pulsatile flow: For cardiovascular cell cultures that require a pulsatile stimulation, bioreactors exploiting pulsatile flow are used to mimic in vivo conditions. Typically, vascular cells are cultured into tubular scaffolds [112].
4.5. Industrial Process Scaling-Up
5. Consumer Acceptance
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Röös, E.; Bajžel, B.; Smith, P.; Patel, M.; Little, D.; Garnett, T. Greedy or needy? Land use and climate impacts of food in 2050 under different livestock futures. Glob. Environ. Chang. 2017, 47, 1–12. [Google Scholar] [CrossRef]
- Bellet, C.; Rushton, J. World food security, globalisation and animal farming: Unlocking dominant paradigms of animal health science. Rev. Sci. Tech. 2019, 38, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Rischer, H.; Szilvay, G.R.; Oksman-Caldentey, K.M. Cellular agriculture—Industrial biotechnology for food and materials. Curr. Opin. Biotechnol. 2020, 61, 128–134. [Google Scholar] [CrossRef]
- Rubio, N.R.; Xiang, N.; Kaplan, D.L. Plant-based and cell-based approaches to meat production. Nat. Commun. 2020, 11, 6276. [Google Scholar] [CrossRef]
- Guerci, M.; Bava, L.; Zucali, M.; Sandrucci, A.; Penati, C.; Tamburini, A. Effect of farming strategies on environmental impact of intensive dairy farms in Italy. J. Dairy Res. 2013, 80, 300–308. [Google Scholar] [CrossRef] [PubMed]
- FAO. News Article: Major Cuts of Green House Gas Emission from Livestock within Reach; FAO: Rome, Italy, 2022; Available online: http://www.fao.org/news/story/en/item/197608/icode (accessed on 29 June 2022).
- Chriki, S.; Hocquette, J.F. The Myth of Cultured Meat: A Review. Front. Nutr. 2020, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.V.; Pierrehumbert, T.R. Climate impacts of cultured meat and beef cattle. Front. Sustain. Food Syst. 2019, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Stehfest, E.; Bouwman, L.; van Vuuren, D.P.; den Elzen, M.G.J.; Eickhout, B.; Kabat, P. Climate benefits of changing diet. Clim. Chang. 2009, 95, 83–102. [Google Scholar] [CrossRef]
- Rizvi, S.; Pagnutti, C.; Fraser, E.; Bauch, C.T.; Anand, M. Global land use implications of dietary trends. PLoS ONE 2018, 13, e0200781. [Google Scholar] [CrossRef]
- Eibl, R.; Senn, Y.; Gubser, G.; Jossen, V.; van den Bos, C.; Eibl, D. Cellular Agriculture: Opportunities and Challenges. Annu. Rev. Food. Sci. Technol 2021, 12, 51–73. [Google Scholar] [CrossRef]
- Warner, R.D. Review: Analysis of the process and drivers for cellular meat production. Animal 2019, 13, 3041–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djisalov, M.; Knežić, T.; Podunavac, I.; Živojević, K.; Radonic, V.; Knežević, N.Ž.; Bobrinetskiy, I.; Gadjanski, I. Cultivating Multidisciplinarity: Manufacturing and Sensing Challenges in Cultured Meat Production. Biology 2021, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Robertson, S.; Suzuki, M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. Int. J. Mol. Sci. 2021, 22, 7513. [Google Scholar] [CrossRef] [PubMed]
- Soice, E.; Johnston, J. Immortalizing Cells for Human Consumption. Int. J. Mol. Sci. 2021, 22, 11660. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Yoon, J.W.; Kim, M.; Lee, H.J.; Jeong, J.; Ryu, M.; Jo, C.; Lee, C.K. Muscle stem cell isolation and in vitro culture for meat production: A methodological review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 429–457. [Google Scholar] [CrossRef]
- Moritz, M.; Verbruggen, S.; Post, M. Alternatives for large-scale production of cultured beef: A review. J. Integr. Agric. 2015, 14, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.J.; Johnston, J.; Datar, I.; Sewalt, V.; Holmes, D.; Shatkin, J.A. Food safety considerations and research priorities for the cultured meat and seafood industry. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5421–5448. [Google Scholar] [CrossRef]
- O’Brien, F. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Seah, J.; Singh, S.; Tan, L.; Choudhury, D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2021, 42, 311–323. [Google Scholar] [CrossRef]
- Moroni, L.; Burdick, J.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef]
- Handral, H.; Tay, S.; Chan, W.W.; Choudhury, D. 3D Printing of cultured meat products. Crit. Rev. Food Sci. Nutr. 2020, 62, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Rosser, J.; Thomas-Vazquez, D. Bioreactor processes for maturation of 3D bioprinted tissue. In 3D Bioprinting for Reconstructive Surgery, 1st ed.; Whitaker, T.J., Ed.; Elsevier Wordmark: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Zidarič, T.; Milojević, M.; Vajda, J.; Vihar, B.; Maver, U. Cultured Meat: Meat Industry Hand in Hand with Biomedical Production Methods. Food Eng. Rev. 2020, 12, 498–519. [Google Scholar] [CrossRef]
- Post, M.; Levenberg, S.; Kaplan, D.; Genovese, N.; Fu, J.; Bryant, C.; Negowetti, N.; Verzijden, K.; Moutsatsou, P. Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food. 2020, 1, 403–415. [Google Scholar] [CrossRef]
- Specht, E.; Welch, D.; Clayton, E.; Lagally, C. Opportunities for Applying Biomedical Production and Manufacturing Methods to the Development of the Clean Meat Industry. Biochem. Eng. J. 2018, 132, 161–168. [Google Scholar] [CrossRef]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surf. B 2017, 159, 62–77. [Google Scholar] [CrossRef]
- Nienow, A.W. A potentially scalable method for the harvesting of hMSCs from microcarriers. Biochem. Eng. J. 2014, 85, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Melzener, L.; Verzijden, K.E.; Buijs, A.J.; Post, M.J.; Flack, J.E. Cultured beef: From small biopsy to substantial quantity. J. Sci. Food Agric. 2021, 101, 7–14. [Google Scholar] [CrossRef]
- Mulvaney, D.R.; Marple, D.N.; Merkel, R.A. proliferation of skeletal muscle satellite cells after castration and administration of testosterone propionate. Proc. Soc. Exp. Biol. Med. 1988, 188, 40–45. [Google Scholar] [CrossRef]
- Vestergaard, M.; Oksbjerg, N.; Henckel, P. Influence of feeding intensity, grazing and finishing feeding on muscle fibre characteristics and meat colour of semitendinosus, longissimus dorsi and supraspinatus muscles of young bulls. Meat Sci. 2000, 54, 177–185. [Google Scholar] [CrossRef]
- Brunner, D.; Frank, J.; Appl, H.; Schöffl, H.; Pfaller, W.; Gstraunthaler, G. Serum-free cell culture: The serum-free media interactive online database. ALTEX 2010, 27, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Subbiahanadar Chelladurai, K.; Selvan Christyraj, J.D.; Rajagopalan, K.; Yesudhason, B.V.; Venkatachalam, S.; Mohan, M.; Chellathurai Vasantha, N.; Selvan Christyraj, J.R.S. Alternative to FBS in animal cell culture—An overview and future perspective. Heliyon 2021, 7, e07686. [Google Scholar] [CrossRef] [PubMed]
- Jochems, C.E.; van der Valk, J.B.; Stafleu, F.R.; Baumans, V. The use of fetal bovine serum: Ethical or scientific problem? Altern. Lab. Anim. 2002, 30, 219–227. [Google Scholar] [CrossRef]
- Gstraunthaler, G. Alternatives to the use of fetal bovine serum: Serum-free cell culture. ALTEX 2003, 20.4, 275–281. [Google Scholar] [CrossRef]
- Kolkmann, A.M.; Post, M.J.; Rutjens, M.A.M.; van Essen, A.L.M.; Moutsatsou, P. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology 2020, 72, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Honn, K.V.; Singley, J.A.; Chavin, W. Fetal bovine serum: A multivariate standard. Proc. Soc. Exp. Biol. Med. 1975, 149, 344–347. [Google Scholar] [CrossRef]
- Anderson, I. Fetal calf serum drought hits cell culture laboratories. Nature 1980, 285, 63. [Google Scholar] [CrossRef] [Green Version]
- Hocquette, J.F. Is in vitro meat the solution for the future? Meat Sci. 2016, 120, 167–176. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Liu, W.; Pushparaj, K.; Park, S. The Epic of In Vitro Meat Production-A Fiction into Reality. Foods 2021, 10, 1395. [Google Scholar] [CrossRef]
- Rubio, N.R.; Fish, K.D.; Trimmer, B.A.; Kaplan, D.L. In vitro insect muscle for tissue engineering applications. ACS Biomater. Sci. Eng. 2019, 5, 1071–1082. [Google Scholar] [CrossRef]
- Calder, P.C. Conference on the future of animal products in the human diet: Health and environmental concerns Plenary Lecture 3 n-3 PUFA and health: Fact, fiction and the future Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2019, 7, 52–72. [Google Scholar]
- Paranjape, S. Goat serum: An alternative to fetal bovine serum in biomedical research. Indian J. Exp. Biol. 2004, 42, 26–35. [Google Scholar] [PubMed]
- Guiotto, M.; Raffoul, W.; Hart, A.M.; Riehle, M.O.; di Summa, P.G. Human platelet lysate to substitute fetal bovine serum in hMSC expansion for translational applications: A systematic review. J. Transl. Med. 2020, 18, 351. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.Y.; Lu, H.K.; Lim, Z.F.S.; Lim, H.W.; Ho, Y.S.; Ng, S.K. Applications and analysis of hydrolysates in animal cell culture. Bioresour. Bioprocess. 2021, 8, 93. [Google Scholar] [CrossRef]
- Burteau, C.C.; Verhoeye, F.R.; Mols, J.F.; Ballez, J.S.; Agathos, S.N.; Schneider, Y.J. Fortification of a protein-free cell culture medium with plant peptones improves cultivation and productivity of an interferon-gamma-producing CHO cell line. Vitr. Cell Dev. Biol. Anim. 2003, 39, 291–296. [Google Scholar] [CrossRef]
- Spearman, M.; Lodewyks, C.; Richmond, M.; Butler, M. The bioactivity and fractionation of peptide hydrolysates in cultures of CHO cells. Biotechnol. Prog. 2014, 30, 584–593. [Google Scholar] [CrossRef]
- Sung, Y.H.; Lim, S.W.; Chung, J.Y.; Lee, G.M. Yeast hydrolysate as a low-cost additive to serum-free medium for the production of human thrombopoietin in suspension cultures of Chinese hamster ovary cells. Appl. Microbiol. Biotechnol. 2004, 63, 527–536. [Google Scholar] [CrossRef]
- Mols, J.; Peeters-Joris, C.; Agathos, S.N.; Schneider, Y.J. Origin of rice protein hydrolysates added to protein-free media alters secretion and extracellular proteolysis of recombinant interferon-γ as well as CHO-320 cell growth. Biotechnol. Lett. 2004, 26, 1043–1046. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, B.; Zhang, X.; Liao, Z.; Gu, L.; Liu, Z.; Zhong, Q.; Wei, H.; Fang, X. The effect of green tea polyphenols on gut microbial diversity and fat deposition in C57BL/6J HFA mice. Food Funct. 2016, 7, 4956–4966. [Google Scholar] [CrossRef]
- Davami, F.; Eghbalpour, F.; Nematollahi, L.; Barkhordari, F.; Mahboudi, F. Effects of Peptone Supplementation in Different Culture Media on Growth, Metabolic Pathway and Productivity of CHO DG44 Cells; a New Insight into Amino Acid Profiles. Iran. Biomed. J. 2015, 19, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Nian, R.; Woen, S.; Chng, J.; Zhang, P.; Yang, Y. Impact of hydrolysates on monoclonal antibody productivity, purification and quality in Chinese hamster ovary cells. J. Biosci. Bioeng. 2016, 122, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Choi, W.Y.; Park, A.; Lee, Y.J.; Lee, Y.; Park, G.H.; Lee, S.J.; Lee, W.K.; Ryu, Y.K.; Kang, D.H. Marine cyanobacterium Spirulina maxima as an alternate to the animal cell culture medium supplement. Sci. Rep. 2021, 11, 4906. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.Y.; Chua, M.L.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Chlorella vulgaris extract as a serum replacement that enhances mammalian cell growth and protein expression. Front. Bioeng. Biotechnol. 2020, 8, 564667. [Google Scholar] [CrossRef]
- Chabanon, G.; Chevalot, I.; Farges, B.; Harscoat, C.; Chenu, S.; Goergen, J.L.; Marc, A.; Marc, I. Influence of Rapeseed protein Hydrolysys Conditions on Animal Cell Growth in Serum-free Media Supplemented with Hydrolysates. In Cell Technology for Cell Products; Springer: Dordrecht, The Netherlands, 2007; pp. 667–669. [Google Scholar]
- Zhang, C.; Li, H.; Li, C.; Li, Z. Fe-Loaded MOF-545(Fe): Peroxidase-Like Activity for Dye Degradation Dyes and High Adsorption for the Removal of Dyes from Wastewater. Molecules 2019, 25, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradkar, P.H.; Vaidya, A.D.; Talwalkar, S.C.; Mishra, L.S.; Agashe, S.V.; Vaidya, R.A. Bovine whey protein and other biological fluids as alternative to fetal bovine serum in supplementing cell culture media. Indian J. Exp. Biol. 2019, 57, 123–130. [Google Scholar]
- Suryawanshi, R.; Kanoujia, J.; Parashar, P.; Saraf, S.A. Sericin: A Versatile Protein Biopolymer with Therapeutic Significance. Curr. Pharm. Des. 2020, 26, 5414–5429. [Google Scholar] [CrossRef]
- Thongsook, T.; Tiyaboonchai, W. Inhibitory effect of sericin on polyphenol oxidase and its application as edible coating. Int. J. Food Sci. Technol 2011, 46, 2052–2061. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Kerasioti, E.; Skaperda, Z.; Papapostolou, P.A.; Nepka, C.; Spandidos, D.A.; Asprodini, E.; Taitzoglou, I.; Kouretas, D. Whey protein boosts the antioxidant profile of rats by enhancing the activities of crucial antioxidant enzymes in a tissue-specific manner. Food Chem. Toxicol. 2020, 142, 111508. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—from ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Kerasioti, E.; Kiskini, A.; Veskoukis, A.; Jamurtas, A.; Tsitsimpikou, C.; Tsatsakis, A.M.; Koutedakis, Y.; Stagos, D.; Kouretas, D.; Karathanos, V. Effect of a special carbohydrate-protein cake on oxidative stress markers after exhaustive cycling in humans. Food Chem. Toxicol. 2012, 50, 2805–2810. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.; Dunsford, I.; Silvio, L.; Ellis, D.; Glencross, A.; Sexton, A. Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in Cellular Agriculture. Trends Food Sci. Technol. 2018, 78, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Post, M. Cultured beef: Medical technology to produce food. J. Sci. Food Agric. 2014, 94, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Post, M. An alternative animal protein source: Cultured beef. N. Y. Acad. Sci. 2014, 1328, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Mikos, A.G.; Thorsen, A.J.; Czerwonka, L.A.; Bao, Y.; Langer, R.; Winslow, D.N. Preparation and characterization of poly(l-lactic acid) foams. Polymer 1994, 35, 1068–1077. [Google Scholar] [CrossRef]
- Prasad, A.; Sankar, M.R.; Katiyar, V. State of art on solvent casting particulate leaching method for orthopedic scaffolds fabrication. Mater. Today Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Mikos, A.G.; Sarakinos, G.; Leite, S.M.; Vacanti, J.P.; Langer, R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials 1992, 14, 323–330. [Google Scholar] [CrossRef]
- Martínez-Pérez, C.A.; Olivas-Armendariz, I.; Castro-Carmona, J.S.; García-Casillas, P.E. Scaffolds for tissue engineering via thermally induced phase separation. In Advances in Regenerative Medicine; Wislet-Gendebien, S., Ed.; InTechOpen: London, UK, 2011; pp. 275–294. [Google Scholar]
- Akbarzadeh, R.; Yousefi, A.M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1304–1315. [Google Scholar] [CrossRef]
- Mooney, D.J.; Baldwin, D.F.; Suh, N.P.; Vacanti, J.P.; Langer, R. Novel approach to fabricate porous sponges of poly(d,l-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 1996, 17, 1417–1422. [Google Scholar] [CrossRef]
- Costantini, M.; Barbetta, A. Gas foaming technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Deng, Y., Kuiper, J., Eds.; Elsevier Woodhead Publishing: Duxford, UK, 2018; pp. 127–149. [Google Scholar]
- Dehghani, F.; Annabi, N. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Pilliar, R.M.; Filiaggi, M.J.; Wells, J.D.; Grynpas, M.D.; Kandel, R.A. Porous calcium polyphosphate scaffolds for bone substitute applications—In vitro characterization. Biomaterials 2001, 22, 963–972. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Steffens, D.; Pranke, D. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef]
- Hobson, C.M.; Amoroso, N.J.; Amini, R.; Ungchusri, E.; Hong, Y.; D’Amore, A. Fabrication of elastomeric scaffolds with curvilinear fibrous structures for heart valve leaflet engineering. J. Biomed. Mater. Res. 2015, 103, 3101–3106. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. 2018, 96, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. Proc. Natl. Acad. Sci. USA 2002, 99, 5133–5138. [Google Scholar] [CrossRef] [Green Version]
- Steele, J.A.M.; McCullen, S.D.; Callanan, A.; Autefage, H.; Accardi, M.A.; Dini, D. Combinatorial scaffold morphologies for zonal articular cartilage engineering. Acta Biomater. 2014, 10, 2065–2075. [Google Scholar] [CrossRef] [Green Version]
- McCullen, S.D.; Autefage, H.; Callanan, A.; Gentleman, E.; Stevens, M.M. Anisotropic fibrous scaffolds for articular cartilage regeneration. Tissue Eng. 2012, 18, 2073–2083. [Google Scholar] [CrossRef] [Green Version]
- Vaquette, C.; Cooper-White, J. A simple method for fabricating 3-D multilayered composite scaffolds. Acta Biomater. 2013, 9, 4599–4608. [Google Scholar] [CrossRef]
- Salerno, A. Processing/structure/property relationship of multi-scaled PCL and PCL-HA composite scaffolds prepared via gas foaming and NaCl reverse templating. Biotechnol. Bioeng. 2011, 108, 963–976. [Google Scholar] [CrossRef]
- Rey, D.F.V.; St-Pierre, J.P. 6—Fabrication techniques of tissue engineering scaffolds. In Woodhead Publishing Series in Biomaterials, Handbook of Tissue Engineering Scaffolds; Woodhead Publishing: Cambridge, UK, 2019; Volume 1, pp. 109–125. ISBN 9780081025635. [Google Scholar] [CrossRef]
- Badylak, S.F.; Tullius, R.; Kokini, K.; Shelbourne, K.D.; Klootwyk, T.; Voytik, S.L. The use of xenogeneic small intestinal submucosa as a biomaterial for Achille’s tendon repair in a dog model. J. Biomed. Mater. Res 1995, 29, 977–985. [Google Scholar] [CrossRef]
- Verbruggen, S.; Luining, D.; van Essen, A.; Post, M. Bovine myoblast cell production in a microcarriers-based system. Cytotechnology 2018, 70, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Bodiou, V.; Moutsatsou, P.; Post, M. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Tenenhaus, M.; Rennekampff, H.O.; Mulder, G. Living cell products as wound healing biomaterials. In Wound Healing Biomaterials, 1st ed.; Agren, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Murphy, S.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 369–392. [Google Scholar] [CrossRef]
- Sachlos, E.; Czernuszka, J. Making Tissue Engineering Scaffolds Work. Review on The Application of Solid Freeform Fabrication Technology to The Production of Tissue Engineering Scaffolds. Eur. Cells Mater. 2003, 5, 29–39. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Vijayavenkataraman, S.; Wei-Cheng, Y.; Wen, L.; Chi-Hwa, W.; Fuh, J. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Santoni, S.; Gugliandolo, S.; Sponchioni, M.; Moscatelli, D.; Colosimo, B. 3D bioprinting: Current status and trends—A guide to the literature and industrial practice. Bio-Des. Manuf. 2021, 5, 14–42. [Google Scholar] [CrossRef]
- Kang, D.H.; Louis, F.; Liu, H.; Shimoda, H.; Nishiyama, Y.; Nozawa, H.; Kakitani, M.; Takagi, D.; Kasa, D.; Nagamori, E.; et al. Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting. Nat. Commun. 2021, 12, 5059. [Google Scholar] [CrossRef]
- Chang, R.; Nam, J.; Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication–based direct cell writing. Tissue Eng. A 2008, 14, 41–48. [Google Scholar] [CrossRef]
- Bejoy, A.; Makkithaya, K.; Hunakunti, B.; Hegde, A.; Krishnamurthy, K.; Sarkar, A.; Lobo, C.; Keshav, D.V.S.; Dharshini, G.; Mascarenhas, S.; et al. An insight on advances and applications of 3d bioprinting: A review. Bioprinting 2021, 24, e00176. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, R.H.; Yang, D.Y.; Park, S.H. Advances in 3D nano/microfabrication using two-photon initiated polymerization. Prog. Polym. Sci. 2008, 33, 631–681. [Google Scholar] [CrossRef]
- Barron, J.A.; Ringeisen, B.R.; Kim, H.; Spargo, B.J.; Chrisey, D.B. Application of laser printing to mammalian cells. Thin Solid Films 2004, 453, 383–387. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2002, 65, 18–23. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.; Weaver, J.; Huebsch, N.; Lee, H.; Lippens, E.; Duda, G.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2015, 15, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Humphries, J.; Byron, A.; Humphries, M.J. Integrin ligand at a glance. J. Cell Sci. 2007, 119, 3901–3903. [Google Scholar] [CrossRef] [Green Version]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef]
- Rutz, A.; Lewis, P.; Shah, R. Toward next-generation bioinks: Tuning material properties pre- and post-printing to optimize cell viability. MRS Bull. 2017, 42, 563–570. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, R.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2013, 35, 49–62. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to Assess Printability of Bioinks for Extrusion-Based Bioprinting and Evaluation of Rheological Properties Governing Bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Waldman, S.D.; Couto, D.C.; Grynpas, M.D.; Pilliar, R.M.; Kandel, R.A. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthr. Cartil. 2006, 14, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, V.; Panadero, J.A.; Ribeiro, C.; Sencadas, V.; Rocha, J.G.; Gomez Ribelles, J.L. Design and validation of a biomechanical bioreactor for cartilage tissue culture. Biomech. Model. Mechanobiol. 2015, 8, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Hu, Y.C. Bioreactors for tissue engineering. Biotechnol. Lett. 2006, 28, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Martin, I. Modulation of the mechanical properties of tissue-engineered cartilage. Biorheology 2000, 37, 141–147. [Google Scholar]
- Tandon, N.; Cimetta, E.; Bhumiratana, S.; Godier-Furnémont, A.; Maidhof, R.; Vunjak-Novakovic, G. Champer II.6.6—Bioreactors for Tissue Engineering. In Biomaterials Science, Third Edition: An Introduction to Materials in Medicine, 3rd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Cambridge, MA, USA, 2013. [Google Scholar] [CrossRef]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.; Wong, D.D.; Chao, P.H. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Grayson, W.L.; Fröhlich, M.; Yeager, K.; Bhumiratana, S.; Chan, M.E. Regenerative medicine special feature: Engineering anatomically shaped human bone grafts. Proc. Natl. Acad. Sci. USA 2009, 107, 3299–3304. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, W.H.; Schneiderbanger, K.; Schubert, P.; Didié, M.; Münzel, F. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 2002, 90, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, W.; Melnychenko, I.; Wasmeier, G.; Didié, M.; Naito, H. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006, 12, 452–458. [Google Scholar] [CrossRef]
- Radisic, M.; Marsano, A.; Maidhof, R.; Wang, Y.; Vunjak-Novakovic, G. Cardiac tissue engineering using perfusion bioreactor systems. Nat. Protoc. 2008, 3, 719–738. [Google Scholar] [CrossRef] [Green Version]
- Tandon, N.; Cannizzaro, C.; Chao, P.H.; Maidhof, R.; Marsano, A. Electrical stimulation systems for cardiac tis- sue engineering. Nat. Protoc. 2009, 4, 155–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maidhof, R.; Tandon, N.; Lee, E.J.; Luo, J.; Duan, Y.; Yeager, K.; Vunjak-Novakovic, G. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J. Tissue Eng. Regen. Med. 2011, 6, e12–e23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, X.; Lei, Q.; Yan, Q.; Li, X.; Zhou, J.; Du, G.; Chen, J. Trends and ideas in technology, regulation and public acceptance of cultured meat. Future Food J. Food Agric. Soc. 2021, 3, 100032. [Google Scholar] [CrossRef]
- Bryant, C.; Barnett, J. Consumer acceptance of cultured meat: A systematic review. Meat Sci. 2018, 143, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Wilks, M.; Phillips, C.J. Attitudes to in vitro meat: A survey of potential consumers in the United States. PLoS ONE 2017, 12, e0171904. [Google Scholar] [CrossRef] [Green Version]
- Hocquette, A.; Lambert, C.; Sinquin, C.; Peterolff, L.; Wagner, Z.; Bonny, S.P.F.; Hocquette, J.F. Educated consumers don’t believe artificial meat is the solution to the problems with the meat industry. J. Integr. Agric. 2015, 14, 273–284. [Google Scholar] [CrossRef]
- Hocquette, É.; Liu, J.; Ellies-Oury, M.P.; Chriki, S.; Hocquette, J.F. Does the future of meat in France depend on cultured muscle cells? Answers from different consumer segments. Meat Sci. 2022, 188, 108776. [Google Scholar] [CrossRef]


| Biotechnological Challenges | Technical Challenges |
|---|---|
| Choice of animal for cell harvesting Choice of site of collection Methods for cell harvesting FBS: ethical challenges High cell proliferation and genetic instability Nutritional and functional properties of cultured meat FBS alternatives Food control system for cultured meat | Scaffold fabrication Alternatives to scaffold fabrication Biofabrication and 3D bioprinting Bioreactors Industrial process scale-up |
| Matrices | Cell Type | Effects | Refs. |
|---|---|---|---|
| Plant peptones | CHO-320 (CHO K1 clone) | Improved cultivation and productivity of Human interferon gamma | [48] |
| Yeast hydrolysate | CHO rCHO (recombinant CHO) | Higher productivity of Human beta interferon Higher cell growth | [49,50] |
| Rice protein hydrolysate | CHO-320 Human HepG cells | Protection against oxidation stress from hydrogen peroxide | [51,52] |
| Soy peptones | CHO DG44 | Improved cell production | [53] |
| Wheat hydrolysates | CHO | Improved cell viability | [54] |
| Marine cyanobacterium Spirulina maxima | Human Lung Carcinoma | Improved cell viability and proliferation | [55] |
| Chlorella vulgaris extract | CHO-K1 and MSC | Promoted cell growth | [56] |
| Rapeseed caked | CHO-C5 | Promoted cell growth | [57] |
| Silk sericin hydrolysate | CHO and Hela cells | Improved cell growth and proliferation | [58] |
| Whey protein | CHO K1 JURKAT E6.1 | Improved cell viability and proliferation | [59] |
| Natural Polymers | Synthetic Polymers | Combination of Natural and Synthetic Polymers |
|---|---|---|
| Anionic polymers: HA, alginic acid, pectin, carrageenan, chondroitin sulfate, dextran sulfate | Polyesters: PEG-PLA-PEG, PEG-PLGA-PEG, PEG-PCL-PEG, PLA-PEG-PLA, PHB, P(PF-co-EG) ± acrylates, P(PEG/PBO terephthalate) | P(PEG-co-peptides), alginate-g-(PEO-PPO-PEO), P(PLGA-co-serine), collagen-acrylate, alginate-acrylate, P(HPMA-g-peptide), P(HEMA/Matrigel®), HA-g-NIPAAm, GelMA |
| Cationic polymers: chitosan, polylysine | Other polymers: PEG-bis-(PLA-acrylate), PEG ± CDs, PEG-g-P(AAm-co-Vamine), PAAm, P(NIPAAm-co-AAc), P(NIPAAm-co-EMA), PVAc/PVA, PNVP, P(MMA-co-HEMA), P(AN-co-allyl sulfo- nate), P(biscarboxy-phenoxy-phosphazene), P(GEMA-sulfate) | |
| Amphipathic polymers: collagen (and gelatin), carboxymethyl chitin, fibrin | ||
| Neutral polymers: dextran, agarose, pullulan |
| Bioink | Cells |
|---|---|
| Printing pressure ↑ | ↓ Viability |
| Nozzle diameter ↓ | ↓ Viability |
| Printing time ↑ | ↓ Viability |
| Degree of crosslinking ↓ | ↑ Density in bioink |
| Viscosity ↓ | ↑ Density in bioink |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzoni, D.; Bracco, F.; Cheli, F.; Colosimo, B.M.; Moscatelli, D.; Baldi, A.; Rebucci, R.; Giromini, C. Biotechnological and Technical Challenges Related to Cultured Meat Production. Appl. Sci. 2022, 12, 6771. https://doi.org/10.3390/app12136771
Lanzoni D, Bracco F, Cheli F, Colosimo BM, Moscatelli D, Baldi A, Rebucci R, Giromini C. Biotechnological and Technical Challenges Related to Cultured Meat Production. Applied Sciences. 2022; 12(13):6771. https://doi.org/10.3390/app12136771
Chicago/Turabian StyleLanzoni, Davide, Filippo Bracco, Federica Cheli, Bianca Maria Colosimo, Davide Moscatelli, Antonella Baldi, Raffaella Rebucci, and Carlotta Giromini. 2022. "Biotechnological and Technical Challenges Related to Cultured Meat Production" Applied Sciences 12, no. 13: 6771. https://doi.org/10.3390/app12136771
APA StyleLanzoni, D., Bracco, F., Cheli, F., Colosimo, B. M., Moscatelli, D., Baldi, A., Rebucci, R., & Giromini, C. (2022). Biotechnological and Technical Challenges Related to Cultured Meat Production. Applied Sciences, 12(13), 6771. https://doi.org/10.3390/app12136771






