Quantitative Ultrasound Analysis of Oral Mucosa: An Observational Cross-Sectional Study
Abstract
Featured Application
Abstract
1. Introduction
- -
- obtain multiple, high-definition US images in different regions of the healthy oral cavity.
- -
- perform a quantitative analysis of the high-definition US images obtained based on the analysis of greyscale (GL), echo levels (ELs—dB), and attenuation values (ATT—dB/cm),
2. Materials and Methods
2.1. Ultrasound Investigations
2.2. Study Sample
2.3. Dataset
2.4. Image Processing
2.5. Data Analysis and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A-mode | amplitude mode |
| ATT | attenuation |
| B-mode | brightness mode |
| BM | buccal mucosa |
| CT | computed tomography |
| CL | connective tissue |
| dB | decibels |
| EL | echo level |
| EP | Epithelium |
| GL | greyscale level |
| HFUS | high-frequency ultrasound |
| ICC | intraclass correlation coefficient |
| MRI | magnetic resonance |
| MM | masticatory mucosa |
| ML | muscular layer |
| NBI | narrow-band imaging |
| OCT | optical coherence tomography |
| RCM | reflectance confocal microscopy |
| ROI | region of interest |
| RR | rete ridges |
| TD | target depth |
| TT | tumor thickness |
| US | ultrasonography |
References
- Chan, H.-L.L.; Wang, H.-L.L.; Fowlkes, J.B.; Giannobile, W.V.; Kripfgans, O.D. Non-Ionizing Real-Time Ul-trasonography in Implant and Oral Surgery: A Feasibility Study. Clin. Oral Implant Res. 2017, 28, 341–347. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.D. Ultrasound-Biophysics Mechanisms. Prog. Biophys. Mol. Biol. 2007, 93, 212–255. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Wendtner, M.-H.; Dill-Müller, D. Ultrasound Technology in Dermatology. Semin. Cutan. Med. Surg. 2008, 27, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Serpico, R.; Lucchese, A. In Vivo Imaging of Enamel by Reflectance Confocal Microscopy (RCM): Non-Invasive Analysis of Dental Surface. Odontology 2014, 102, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Pilolli, G.P.; Petruzzi, M.; Crincoli, V.; Scivetti, M.; Favia, G. Analysis of Collagen Distribu-tion in Human Crown Dentin by Confocal Laser Scanning Microscopy. Ultrastruct. Pathol. 2008, 32, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Poh, C.F.; Guillaud, M.; Lucchese, A.; Rullo, R.; Lam, S.; Serpico, R.; Macaulay, C.E.; Lane, P.M. Oral Mucosa Optical Biopsy by a Novel Handheld Fluorescent Confocal Microscope Specifically Developed: Technologic Improvements and Future Prospects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 752–758. [Google Scholar] [CrossRef]
- Romano, A.; di Stasio, D.; Petruzzi, M.; Fiori, F.; Lajolo, C.; Santarelli, A.; Lucchese, A.; Serpico, R.; Contaldo, M. Noninvasive Imaging Methods to Improve the Diagnosis of Oral Carcinoma and Its Precursors: State of the Art and Proposal of a Three-Step Diagnostic Process. Cancers 2021, 13, 2864. [Google Scholar] [CrossRef]
- Gentile, E.; di Stasio, D.; Santoro, R.; Contaldo, M.; Salerno, C.; Serpico, R.; Lucchese, A. In Vivo Microstruc-tural Analysis of Enamel in Permanent and Deciduous Teeth. Ultrastruct. Pathol. 2015, 39, 131–134. [Google Scholar] [CrossRef]
- Lucchese, A.; Scivetti, M.; Pilolli, G.P.; Favia, G. Analysis of Ghost Cells in Calcifying Cystic Odontogenic Tumors by Confocal Laser Scanning Microscopy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, 391–394. [Google Scholar] [CrossRef]
- Contaldo, M.; Lucchese, A.; Gentile, E.; Zulli, C.; Petruzzi, M.; Lauritano, D.; Amato, M.R.; Esposito, P.; Riegler, G.; Serpico, R. Evaluation of the Intraepithelial Papillary Capillary Loops in Benign and Malignant Oral Lesions by In Vivo Virtual Chromoendoscopic Magnification: A Preliminary Study. J. Biol. Regul. Homeost. Agents 2017, 31, 11–22. [Google Scholar]
- Grassia, V.; Gentile, E.; di Stasio, D.; Jamilian, A.; Matarese, G.; D’Apuzzo, F.; Santoro, R.; Perillo, L.; Serpico, R.; Lucchese, A. In Vivo Confocal Microscopy Analysis of Enamel Defects after Orthodontic Treatment: A Preliminary Study. Ultrastruct. Pathol. 2016, 40, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Schulze, R.K.W.; Ćurić, D.; D’Hoedt, B. B-Mode versus A-Mode Ultrasonographic Measurements of Mucosal Thickness In Vivo. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 93, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Gropper, C.A.; Stiller, M.J.; Shupack, J.L.; Driller, J.; Rorke, M.; Lizzi, F. Diagnostic high-resolution ultrasound in dermatology. Int. J. Dermatol. 1993, 32, 243–250. [Google Scholar] [CrossRef]
- Sugawara, C.; Takahashi, A.; Kawano, F.; Kudo, Y.; Ishimaru, N.; Miyamoto, Y. Intraoral Ultrasonography of Tongue Mass Lesions. Dentomaxillofac. Radiol. 2016, 45, 20150362. [Google Scholar] [CrossRef]
- Siva Kalyan, U.; Moturi, K.; Padma Rayalu, K. The Role of Ultrasound in Diagnosis of Temporomandibular Joint Disc Displacement: A Case—Control Study. J. Maxillofac. Oral Surg. 2018, 17, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Yoshihama, Y.; Ueyama, Y.; Terakado, N.; Kamei, S.; Fijimoto, Y.; Hasegawa, Y.; Matsuura, H.; Matsumura, T. The Usefulness of Intraoral Ultrasonography in the Evaluation of Oral Cancer. Int. J. Oral Maxillofac. Surg. 2001, 30, 139–143. [Google Scholar] [CrossRef]
- Salmon, B.; Le Denmat, D. Intraoral Ultrasonography: Development of a Specific High-Frequency Probe and Clinical Pilot Study. Clin. Oral Investig. 2012, 16, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Angelelli, G.; Moschetta, M.; Limongelli, L.; Albergo, A.; Lacalendola, E.; Brindicci, F.; Favia, G.; Maiorano, E. Endocavitary Sonography of Early Oral Cavity Malignant Tumors. Head Neck 2017, 39, 1349–1356. [Google Scholar] [CrossRef]
- di Stasio, D.; Lauritano, D.; Paparella, R.; Franco, R.; Montella, M.; Serpico, R.; Lucchese, A. Ultrasound Imaging of Oral Fibroma: A Case Report. J. Biol. Regul. Homeost. Agents 2017, 31, 23–26. [Google Scholar]
- di Stasio, D.; Montella, M.; Romano, A.; Colella, G.; Serpico, R.; Lucchese, A. High-Definition Ultrasound Characterization of Squamous Carcinoma of the Tongue: A Descriptive Observational Study. Cancers 2022, 14, 564. [Google Scholar] [CrossRef]
- Contaldo, M.; di Stasio, D.; Petruzzi, M.; Serpico, R.; Lucchese, A. In Vivo Reflectance Confocal Microscopy of Oral Lichen Planus. Int. J. Dermatol. 2019, 58, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi-Farahani, A. A Scoping Review of the Efficacy of Diode Lasers Used for Minimally Invasive Exposure of Impacted Teeth or Teeth with Delayed Eruption. Photonics 2022, 9, 265. [Google Scholar] [CrossRef]
- Lam, M.; Chaudhari, A.J.; Sun, Y.; Zhou, F.; Dobbie, A.; Gandour-Edwards, R.F.; Tinling, S.P.; Farwell, D.G.; Monsky, W.L.; Shung, K.K.; et al. Ultrasound Backscatter Microscopy for Imaging of Oral Carcinoma. J. Ultrasound Med. 2013, 32, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Meel-van den Abeelen, A.S.S.; Weijers, G.; van Zelst, J.C.M.; Thijssen, J.M.; Mann, R.M.; de Korte, C.L. 3D Quantitative Breast Ultrasound Analysis for Differentiating Fibroadenomas and Carcinomas Smaller than 1 cm. Eur. J. Radiol. 2017, 88, 141–147. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Al-Buqain-Ortega, A.; Arias-Buría, J.L.; Fernández-de-las-Peñas, C.; Varol, U.; Orte-ga-Santiago, R. Echo-Intensity, Fatty Infiltration, and Morphology Ultrasound Imaging Assessment in Healthy and Whiplash Associated Disorders Populations: An Observational Study. Eur. Spine J. 2021, 30, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Horos Project. Available online: https://horosproject.org/ (accessed on 16 December 2021).
- Zhou, W.; Long, Z.; Tradup, D.J.; Stekel, S.F.; Browne, J.E.; Brown, D.L.; Hangiandreou, N.J. Ultrasound Gray-scale Image Quality Comparison between a 2D Intracavitary Transducer and a 3D Intracavitary Transducer Used in 2D Mode: A Phantom Study. J. Appl. Clin. Med. Phys. 2019, 20, 134–140. [Google Scholar] [CrossRef]
- Yesuratnam, A.; Wiesenfeld, D.; Tsui, A.; Iseli, T.A.; Hoorn, S.V.; Ang, M.T.; Guiney, A.; Phal, P.M. Preoperative Evaluation of Oral Tongue Squamous Cell Carcinoma with Intraoral Ultrasound and Magnetic Resonance Imaging—Comparison with Histopathological Tumour Thickness and Accuracy in Guiding Patient Management. Int. J. Oral Maxillofac. Surg. 2014, 43, 787–794. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; Noorlag, R.; van Cann, E.M.; Pameijer, F.A.; Willems, S.M.; Yesuratnam, A.; Rosenberg, A.J.W.P.; de Bree, R.; van Es, R.J.J. Intraoral Ultrasonography to Measure Tumor Thickness of Oral Cancer: A Systematic Review and Meta-Analysis. Oral Oncol. 2018, 77, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tarabichi, O.; Bulbul, M.G.; Kanumuri, V.V.; Faquin, W.C.; Juliano, A.F.; Cunnane, M.E.; Varvares, M.A. Utility of Intraoral Ultrasound in Managing Oral Tongue Squamous Cell Carcinoma: Systematic Review. Laryn-Goscope 2019, 129, 662–670. [Google Scholar] [CrossRef]
- Izzetti, R.; Vitali, S.; Aringhieri, G.; Caramella, D.; Nisi, M.; Oranges, T.; Dini, V.; Graziani, F.; Gabriele, M. The Efficacy of Ultra-High Frequency Ultrasonography in the Diagnosis of Intraoral Lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 401–410. [Google Scholar] [CrossRef]
- Chammas, M.C.; MacEdo, T.A.A.; Moyses, R.A.; Gerhard, R.; Durazzo, M.D.; Cernea, C.R.; Cerri, G.G. Rela-tionship between the Appearance of Tongue Carcinoma on Intraoral Ultrasonography and Neck Metastasis. Oral Radiol. 2011, 27, 1–7. [Google Scholar] [CrossRef]
- Marchi, F.; Filauro, M.; Iandelli, A.; Carobbio, A.L.C.; Mazzola, F.; Santori, G.; Parrinello, G.; Canevari, F.R.M.; Piazza, C.; Peretti, G. Magnetic Resonance vs. Intraoral Ultrasonography in the Preoperative Assessment of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, J.; Starke, A.; Weijers, G.; Haudum, A.; Herzog, K.; Wohlsein, P.; Rehage, J.; de Korte, C. Computer-Aided B-Mode Ultrasound Diagnosis of Hepatic Steatosis: A Feasibility Study. IEEE Trans Ultrason. Ferroe-Lectr. Freq. Control 2008, 55, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Tadayyon, H.; Sannachi, L.; Gangeh, M.; Sadeghi-Naini, A.; Tran, W.; Trudeau, M.E.; Pritchard, K.; Ghandi, S.; Verma, S.; Czarnota, G.J. Quantitative Ultrasound Assessment of Breast Tumor Response to Chemotherapy Using a Multi-Parameter Approach. Oncotarget 2016, 7, 45094. [Google Scholar] [CrossRef]
- Izzetti, R.; Vitali, S.; Aringhieri, G.; Oranges, T.; Dini, V.; Nisi, M.; Graziani, F.; Gabriele, M.; Caramella, D. Discovering a New Anatomy: Exploration of Oral Mucosa with Ultra-High Frequency Ultrasound. Den-Tomaxillofac. Radiol. 2020, 49, 20190318. [Google Scholar] [CrossRef]
- Grulkowski, I.; Nowak, J.K.; Karnowski, K.; Zebryk, P.; Puszczewicz, M.; Walkowiak, J.; Wojtkowski, M. Quantitative Assessment of Oral Mucosa and Labial Minor Salivary Glands in Patients with Sjögren’s Syndrome Using Swept Source OCT. Biomed. Opt. Express 2014, 5, 259–271. [Google Scholar] [CrossRef][Green Version]
- Stasio, D.D.; Lauritano, D.; Loffredo, F.; Gentile, E.; Vella, F.D.; Petruzzi, M.; Lucchese, A. Optical Coherence Tomography Imaging of Oral Mucosa Bullous Diseases: A Preliminary Study. Dentomaxillofac. Radiol. 2020, 49, 20190071. [Google Scholar] [CrossRef]
- di Stasio, D.; Lauritano, D.; Romano, A.; Salerno, C.; Minervini, G.; Minervini, G.; Gentile, E.; Serpico, R.; Lucchese, A. In vivo characterization of oral pemphigus vulgaris by optical coherence tomography. J. Biol. Regul. Homeost. Agents 2015, 29, 39–41. [Google Scholar]
- Grani, G.; D’Alessandri, M.; Carbotta, G.; Nesca, A.; del Sordo, M.; Alessandrini, S.; Coccaro, C.; Rendina, R.; Bianchini, M.; Prinzi, N.; et al. Grey-Scale Analysis Improves the Ultrasonographic Evaluation of Thyroid Nodules. Medicine 2015, 94, e1129. [Google Scholar] [CrossRef]
- Uppal, T. Tissue Harmonic Imaging. Australas. J. Ultrasound Med. 2010, 13, 29–31. [Google Scholar] [CrossRef]
- Abu-Zidan, F.M.; Hefny, A.F.; Corr, P. Clinical Ultrasound Physics. J. Emergencies Trauma Shock 2011, 4, 501–503. [Google Scholar] [CrossRef]
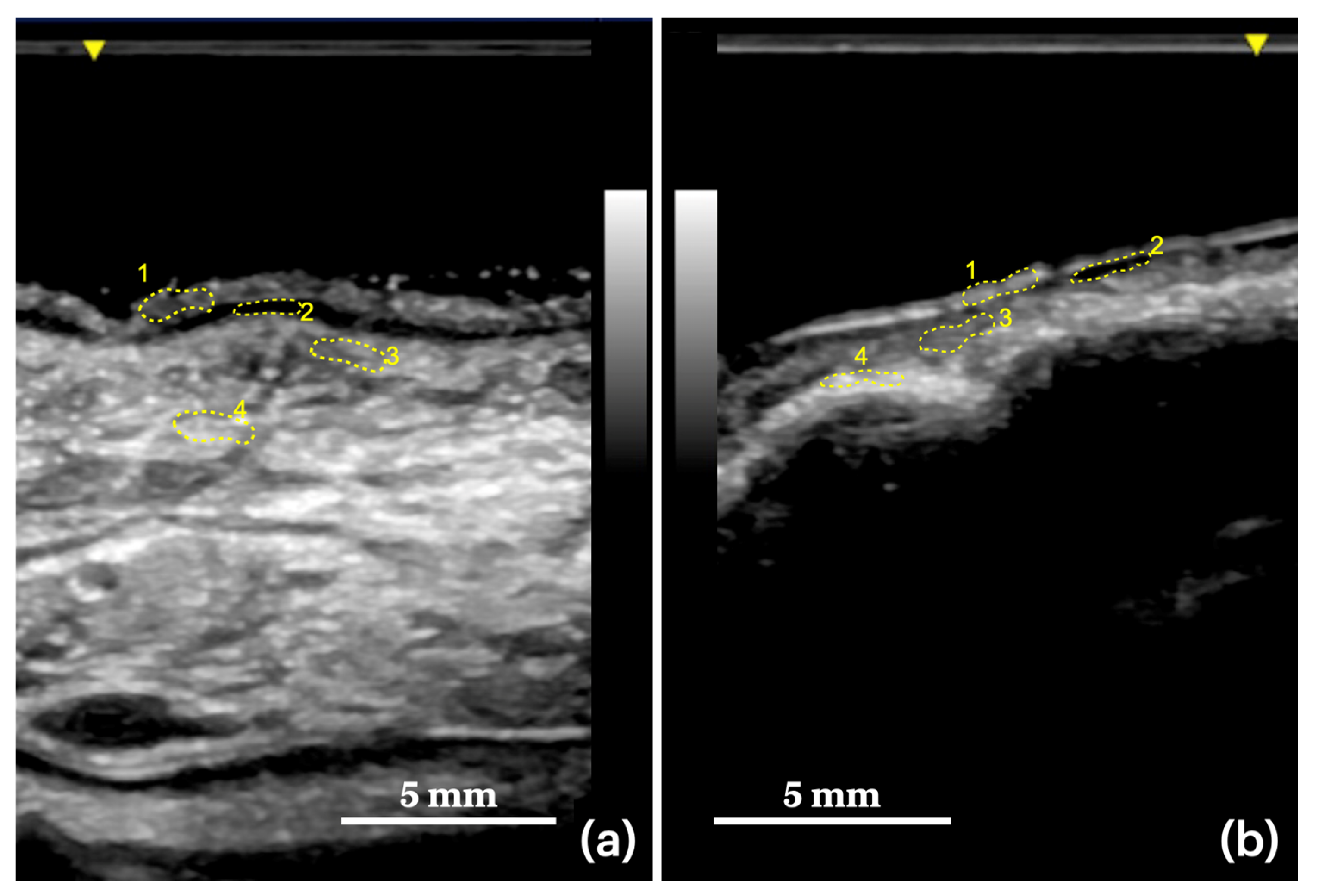
| Masticatory mucosa | upper gingiva (vestibular side) |
| hard palate (palatine crests) | |
| maxillary alveolar process | |
| lower gingiva (buccal and lingual side) | |
| Buccal mucosa | cheeks mucosa |
| upper and lower lips (mucous side) | |
| Tongue | dorsal tongue |
| lateral margins | |
| ventral tongue | |
| floor of the mouth |
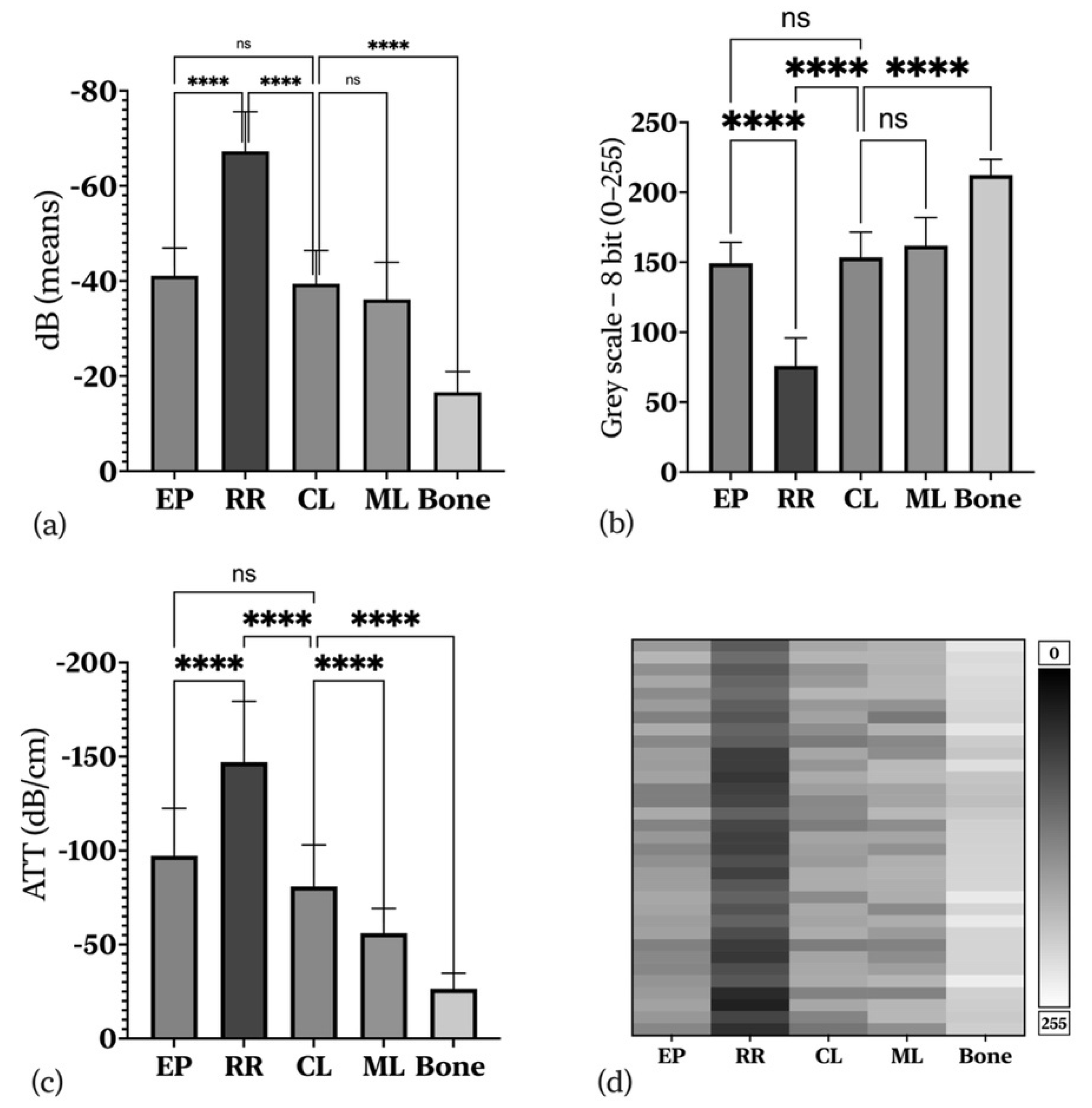
| Tissue Layers | US Parameters | EL (dB) | GL (8-bit) | ATT (dB/cm) | TD (cm) | ICC (Sig.) |
|---|---|---|---|---|---|---|
| EP | Mean | −41.073 | 149.205 | −97.268 | 0.438 | 0.871 (p = 0.001) ** |
| SD | 5.84 | 15.04 | 25.21 | 0.09 | ||
| Minimum | −50.44 | 125.08 | −143.20 | 0.27 | ||
| Maximum | −29.11 | 180.01 | −45.78 | 0.64 | ||
| EP-RR | Mean difference (SE) | 26.18 (±1.77) | 73.21 (±4.34) | 49.73 (±7.14) | ||
| Sig.(p-value) | 0.001 * | 0.001 * | 0.001 * | |||
| RR | Mean | −67.259 | 75.994 | −147.003 | 0.470 | 0.880 (p = 0.001) ** |
| SD | 8.33 | 19.87 | 32.34 | 0.09 | ||
| Minimum | −82.77 | 31.00 | −203.38 | 0.29 | ||
| Maximum | −45.40 | 108.98 | −84.99 | 0.67 | ||
| RR-CL | Mean difference (SE) | −27.85 (±1.89) | −77.51 (±4.34) | −66.08 (±6.81) | ||
| Sig. (p-value) | 0.001 * | 0.001 * | 0.001 * | |||
| CL | Mean | −39.406 | 143.500 | −80.9206 | 0.501 | 0.882 (p = 0.001) ** |
| SD | 7.00 | 18.05 | 22.04 | 0.09 | ||
| Minimum | −53.94 | 116.07 | −119.76 | 0.33 | ||
| Maximum | −29.09 | 180.06 | −42.88 | 0.68 | ||
| CL-ML | Mean difference (SE) | −3.27(±1.89) | −8.44(±4.69) | −24.85(±4.46) | ||
| Sig. (p-value) | 0.383 | 0.383 | 0.001 * | |||
| ML | Mean | −36.130 | 161.939 | −56.071 | 0.644 | 0.906 (p = 0.001) ** |
| SD | 7.77 | 20.01 | 13.02 | 0.13 | ||
| Minimum | −52.49 | 119.80 | −76.59 | 0.39 | ||
| Maximum | −26.80 | 185.97 | −31.83 | 0.84 | ||
| Bone | Mean | −16.571 | 212.315 | −26.482 | 0.659 | 0.709 (p = 0.005) ** |
| SD | 4.35 | 11.20 | 8.25 | 0.11 | ||
| Minimum | −25.55 | 189.19 | −40.01 | 0.44 | ||
| Maximum | −7.02 | 236.92 | −10.43 | 0.85 | ||
| Bone-CL | Mean difference (SE) | 22.83 (±1.43) | 58.82 (±3.70) | 54.44 (±4.10) | ||
| Sig. (p-value) | 0.001 * | 0.001 * | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stasio, D.; Romano, A.; Montella, M.; Contaldo, M.; Petruzzi, M.; Hasan, I.; Serpico, R.; Lucchese, A. Quantitative Ultrasound Analysis of Oral Mucosa: An Observational Cross-Sectional Study. Appl. Sci. 2022, 12, 6829. https://doi.org/10.3390/app12146829
Di Stasio D, Romano A, Montella M, Contaldo M, Petruzzi M, Hasan I, Serpico R, Lucchese A. Quantitative Ultrasound Analysis of Oral Mucosa: An Observational Cross-Sectional Study. Applied Sciences. 2022; 12(14):6829. https://doi.org/10.3390/app12146829
Chicago/Turabian StyleDi Stasio, Dario, Antonio Romano, Marco Montella, Maria Contaldo, Massimo Petruzzi, Iquebal Hasan, Rosario Serpico, and Alberta Lucchese. 2022. "Quantitative Ultrasound Analysis of Oral Mucosa: An Observational Cross-Sectional Study" Applied Sciences 12, no. 14: 6829. https://doi.org/10.3390/app12146829
APA StyleDi Stasio, D., Romano, A., Montella, M., Contaldo, M., Petruzzi, M., Hasan, I., Serpico, R., & Lucchese, A. (2022). Quantitative Ultrasound Analysis of Oral Mucosa: An Observational Cross-Sectional Study. Applied Sciences, 12(14), 6829. https://doi.org/10.3390/app12146829










