The Importance of Multidisciplinary Analytical Strategies to Solve Identification and Characterization Challenges in Gemology: The Example of the “Green Stones”
Abstract
Featured Application
Abstract
1. Introduction
2. Cultural Heritage and Gemstones
3. Analytical Techniques Available
3.1. Standard Gemological Tools and Analyses
3.2. Optical Microscopy (OM) and Imaging Systems
3.3. Spectroscopies
3.4. Complementary Chemical Analyses
4. The Example of the Green Stones
4.1. The Nomenclature of the Green Stones
4.2. Classical Gemological and Mineralogical Properties
4.3. The Raman Investigation
4.4. The Fourier Transform InfraRed Spectroscopy (FTIR) Investigation
4.5. The Chromophores Study and the UV-Vis/NIR Spectroscopy Application
4.6. Fluorescence Imaging
4.7. Chemical Characterization: Elemental Analyses
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wicklein, R.C.; Schell, J.W. Case Studies of Multidisciplinary Approaches to Integrating Mathematics, Science and TechnologyEducation. J. Technol. Educ. 1995, 6, 59–76. [Google Scholar] [CrossRef]
- Taylor, G.; Spencer, S. Social Identities, Multidisciplinary Approaches, 1st ed.; Routledger: Abingdon, UK, 2004; pp. 1–13. [Google Scholar]
- Lucassen, J.; Lucassen, L.; Manning, P. Migration History in World History, Multidisciplinary Approaches; Brill: Leiden, The Netherlands, 2010; pp. vii–viii. [Google Scholar]
- Isurin, L.; Winford, D.; Bot, K. Multidisciplinary Approaches to Code Switching; John Benjamin BV: Amsterdam, The Netherlands, 2009; pp. ix–xviii. [Google Scholar]
- Staller, J.E.; Tykot, R.H.; Benz, B.F. Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize; Left Coast Press Inc.: Walnut Creek, CA, USA, 2009; p. 23. [Google Scholar]
- WordReferences. Available online: https://www.wordreference.com/enit/multidisciplinary (accessed on 18 May 2022).
- Pasquarella, C.; Balocco, C.; Pasquariello, G.; Petrone, G.; Saccani, E.; Manotti, P.; Ugolotti, M.; Palla, F.; Maggi, O.; Albertini, R. A multidisciplinary approach to the study of Cultural Heritage environments: Experience at the Palatina Library in Parma. Sci. Total Environ. 2015, 536, 557–567. [Google Scholar] [CrossRef]
- Cardoso, I.; Macedo, M.F.; Vermeulen, F.; Corsi, C.; Santos Silva, S.; Rosado, L.; Candeias, A.; Mirao, J. A Multidisciplinary Approach to the Study of Archaeological Mortars from the Town of Ammaia in the Roman Province of Lusitania (Portugal). Archaeometry 2014, 56, 1–24. [Google Scholar] [CrossRef]
- Berto, L.; Doria, A.; Faccio, P.; Saetta, A.; Talledo, D. Vulnerability Analysis of Built Cultural Heritage: A Multidisciplinary Approach for Studying the Palladio’s Tempietto Barbaro. Int. J. Archit. Herit. 2017, 1, 773–790. [Google Scholar] [CrossRef]
- Masciotta, M.-G.; Roque, J.C.A.; Ramos, L.F.; Lourenço, P.B. A multidisciplinary approach to assess the health state of heritage structures: The case study of the Church of Monastery of Jerónimos in Lisbon. Constr. Build. Mater. 2016, 116, 169–187. [Google Scholar] [CrossRef]
- Jaillot, V.; Istasse, M.; Servigne, S.; Gesquière, G.; Rautenberg, M.; Lefort, I. Describing, Comparing and Analyzing Digital Urban Heritage Tools: A Methodology Designed with a Multidisciplinary Approach. Digit. Appl. Archaeol. Cult. Herit. 2020, 17, e00135. [Google Scholar] [CrossRef]
- Vacca, G.; Fiorino, D.R.; Pili, D. A Spatial Information System (SIS) for the Architectural and Cultural Heritage of Sardinia (Italy). ISPRS Int. J. Geo-Inf. 2018, 7, 49. [Google Scholar] [CrossRef]
- Jans, M.M.E.; Kars, H.; Nielsen-Marsh, C.M.; Smith, C.I.; Nord, A.G.; Arthur, P.; Earl, N. In Situ preservation of archaeological bone: A histological study within a multidisciplinary approach. Archaeometry 2002, 44, 343–352. [Google Scholar] [CrossRef]
- Maritan, L.; Mazzoli, C.; Melis, E. A multidisciplinary approach to the characterization of Roman gravestones from Aquileia (Udine, Italy). Archaeometry 2003, 45, 363–374. [Google Scholar] [CrossRef]
- Ricca, M.; Paladini, G.; Rovella, N.; Ruffolo, S.A.; Randazzo, L.; Crupi, V.; Fazio, B.; Majolino, B.; Venuti, V.; Galli, G.; et al. Archaeometric Characterisation of Decorated Pottery from the Archaeological Site of Villa dei Quintili (Rome, Italy): Preliminary Study. Geosciences 2019, 9, 172. [Google Scholar] [CrossRef]
- Fluzin, P.; Bauvais, S.; Berranger, M.; Pagès, G.; Dillmann, P. The multidisciplinary approach (archaeology and archaeometry) to bloomsmithing activities in France: Examples of results from the last twenty years. In The Archaeometallurgy of Iron, Recent Developments in Archaeological and Scientic Research; Hosek, J., Cleere, H., Mihok, L., Eds.; Institute of Archaeoology of the ASCR: Prague, Czech Republic, 2011; pp. 223–236, 311–312. [Google Scholar]
- Agapiou, A.; Lysandrou, V.; Alexakis, D.D.; Themistocleous, K.; Cuca, B.; Argyriou, A.; Sarris, A.; Hadjimitsis, D.G. Cultural heritage management and monitoring using remote sensing data and GIS: The case study of Paphos area, Cyprus. Comput. Environ. Urban Syst. 2015, 54, 230–239. [Google Scholar] [CrossRef]
- Belfiore, C.M.; Fichera, G.V.; La Russa, M.F.; Pezzino, A.; Ruffolo, S.A.; Galli, G.; Barca, D. A Multidisciplinary Approach for the Archaeometric Study of Pozzolanic Aggregate in Roman Mortars: The Case of Villa dei Quintili (Rome, Italy). Archaeometry 2015, 57, 269–296. [Google Scholar] [CrossRef]
- Wagner, G.A. Einfuhrung in Die Archaometrie; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007. [Google Scholar]
- Reindel, M.; Wagner, G.A. New Technologies for Archaeology Multidisciplinary Investigations in Palpa and Nasca, Peru; Springer: Berlin/Heidelberg, Germany, 2009; pp. 9–10. [Google Scholar]
- Giannossa, L.C.; Forleo, T.; Mangone, A. The Distinctive Role of Chemical Composition in Archaeometry. The Case of Apulian Red Figure Pottery. Appl. Sci. 2021, 11, 3073. [Google Scholar] [CrossRef]
- Columbu, S.; Sitzia, F.; Verdiani, G. Contribution of petrophysical analysis and 3D digital survey in the archaeometric investigations of the Emperor Hadrian’s Baths (Tivoli, Italy). Rend. Lincei 2015, 26, 455–474. [Google Scholar] [CrossRef]
- Jessup, R.A. Saxon Jewellery. Faber (M0005997SH); Wilson Collection: London, UK, 1950; Volume 124, p. 200349501. [Google Scholar]
- Arrhenius, B. Why the king needed his own goldsmith. Laborativ Arkeol. 1988, 10–11, 109–111. [Google Scholar]
- Collareta, M. Oreficeria e Tecniche Orafe. In Arti e Storia nel Medioevo. II. Del Costruire: Tecniche, Artisti, Artigiani, Committenti; Sergi, G., Castelnuovo, E., Eds.; Giulio Enaudi: Turin, Italy, 2003; pp. 549–560. [Google Scholar]
- Collareta, M. Storia dell’arte medievale, Intervento introduttivo. Reti Mediev. Riv. 2011, 12, 29–41. [Google Scholar]
- Mottana, A. Italian gemology during the Renaissance: A step toward modern mineralogy. In The Origins of Geology in Italy; Vai, G.B., Caldwell, W.G.E., Eds.; Geological Society of America: Boulder, CO, USA, 2006; Special Paper 411; pp. 1–21. [Google Scholar]
- Aimone, M. Nuovi dati sull’oreficeria cloisonne’ in Italia tra V e VI secolo. Ricerche stilistiche, indagini tecniche, questioni cronologiche. Archeol. Mediev. 2011, XXXVIII, 369–418. [Google Scholar]
- Hilgner, A. Oreficeria cloisonne’ nell’alto medioevo: Inquadramento cronologico sulla base degli aspetti tecnologici e dell’origine delle materie prime. In Small Finds e Cronologia (V-IX Sec.) Esempi, Medoti e Risultati; Pinar Gil, J., Ed.; Bradypus: Bologna, Italy, 2017; pp. 9–29. [Google Scholar]
- CIBJO. The Gemstone Book; Coloured Stones Commission 2020–2021; CIBJO: London, UK, 2021. [Google Scholar]
- Panczer, G.; Riondet, G.; Forest, L.; Krzemnicki, M.S.; Carole, D.; Faure, F. The Talisman of Charle Magne: New historical and Gemological discoveries. Gems Gemol. 2019, 55, 30–46. [Google Scholar] [CrossRef]
- Bertelli, C. La Corona Ferrea; Skira: Ginevrea-Milano, Italy, 2017. [Google Scholar]
- Riccardi, M.P.; Prosperi, L.; Tarantino, S.C.; Zema, M. Gemmology in the service of archaeometry. EMU Notes Mineral. 2019, 20, 345–366. [Google Scholar]
- Di Martino, D.; Benati, G.; Alberti, R.; Baroni, S.; Bertelli, C.; Blumer, F.; Caselli, L.; Cattaneo, R.; Cucini, C.; D’Amico, F.; et al. The Chiaravalle Cross: Results of a multidisciplinary study. Heritage 2019, 2, 2555–2572. [Google Scholar] [CrossRef]
- Musa, M. Gulf Institute of Gemology: An archaeometry analytical facility available “on-site”. IASA Bull. 2021, 26, 45–46. [Google Scholar]
- Martin, F.; Merigoux, H.; Zecchini, P. Reflectance InfraRed spectroscopy in Gemology. Gems Gemol. 1989, 25, 226–231. [Google Scholar] [CrossRef]
- Fritsch, E. Gem Characterization: A Forecast of Important Techniques in the Coming Decade. Gems Gemol. 2006, 42, 90. [Google Scholar]
- Raneri, S.; Barone, G.; Mazzoleni, P.; Bersani, D. Non-destructive spectroscopic methods for gem analysis: A short review. In Proceedings of the IMEKO TC-4 International Conference on Metrology for Archaeology and Cultural Heritage Trento, Trento, Italy, 22–24 October 2020. [Google Scholar]
- Humphreys, E.S. How to spot a fake. Mater. Today 2002, 5, 32–37. [Google Scholar] [CrossRef]
- Barboza, D.; Bowley, G.; Cox, A. Forging an Art Market in China. New York Times, 28 October 2013. [Google Scholar]
- Barone, G.; Bersani, D.; Jehlicka, J.; Lottici, P.P.; Mazzoleni, P.; Raneri, S.; Vandenabeele, P.; Di Giacomo, C.; Larinà, G. Nondestructive investigation on the 17–18th centuries Sicilian jewelry collection at the Messina regional museum using mobile Raman equipment. J. Raman Spectrosc. 2015, 46, 989–995. [Google Scholar] [CrossRef]
- Rudoe, J. The faking of gems in the x8th century. In Why Fakes Matter. Essays on the Problems of Authenticity; Jones, M., Ed.; The British Museum Press: London, UK, 1992; pp. 23–28. [Google Scholar]
- Gordon, R. Archaeologies of Magical Gems. In ‘Gems of Heaven’: Recent Research on Engraved Gemstones in Late Antiquity, AD 200-600; Entwistle, C., Adams, N., Eds.; British Museum Research Publications: London, UK, 2011; pp. 39–49. [Google Scholar]
- Buttrey, T.V. Natter on gem collecting. Thomas hollis and some problems in the Museum Britannicum. J. Hist. Collect. 1990, 2, 219–226. [Google Scholar] [CrossRef]
- Rudoe, J. Eighteenth and Nineteenth-Century Engraved Gems in the British Museum; Collectors and Collections from Sir Hans Sloane to Anne Hull Grundy. Z. Kunstgesch. 1996, 59, 198–213. [Google Scholar] [CrossRef]
- Drayman-Weisser, T. From fake to fabulous: Redeeming fakes at the Walters Art Museum. AIC Objects Spec. Group Postprints 2007, 14, 90–109. [Google Scholar]
- McClure, S.F.; Moses, T.M.; Shigley, J.E. The Geographic Origin Dilemma. Gems Gemol. 2019, 37, 457–462. [Google Scholar]
- Gubelin Gem Lab Provenance Proof Project. Available online: https://www.provenanceproof.com/ (accessed on 25 May 2022).
- GRS GemResearch SwissLab—Reference Collection. Available online: https://www.gemresearch.ch/research/reference-collection (accessed on 25 May 2022).
- Krzemnicki, M.S. Origin Determination and Traceability: An Overview for Gemstones. Presented at the GemGeneva, Geneva, Swiss, SSEF Presentation. Available online: https://www.ssef.ch/presentations/ (accessed on 25 May 2022).
- Gem Society. Available online: https://www.gemsociety.org/article/evaluating-color-hue-tone-and-saturation/ (accessed on 18 June 2022).
- Laboratory Manual Harmonisation Committee—LMHC. Information Sheet 0: Guidelines for Gemmological Laboratory Reports; Version 2; LMHC: Lucerne, Switzerland, 2018. [Google Scholar]
- Cibjo, Retailers’ Reference Guide (RRG). Available online: https://www.cibjo.org/the-retailers-reference-guide/ (accessed on 20 June 2022).
- Nickerson, D. History of the Munsell Color System and Its Scientific Application. J. Opt. Soc. Am. 1940, 30, 575–586. [Google Scholar] [CrossRef]
- CIBJO. The Gemmological Laboratory Book; Gemmological Commission 2020–2021; CIBJO: London, UK, 2021. [Google Scholar]
- GIG—Gulf Institute of Gemology. Gemstones Identification Manual; GIG: Muscat, Oman, 2020. [Google Scholar]
- Sturman, D.K. A new approach to the teaching and use of the refractometer. J. Gemmol. 2010, 32, 74–89. [Google Scholar] [CrossRef]
- Burbage, E.J.; Anderson, B.W. An analysis of the movements of shadow-edges on the refractometer in the case of biaxial gemstones. Mineral. Mag. 1942, 26, 246–253. [Google Scholar] [CrossRef]
- Thibault, W.N. A simple dichroscope. Am. Mineral. 1940, 25, 88–90. [Google Scholar]
- He, T. The Applications of Ultraviolet Visible Absorption Spectrum Detection Technology in Gemstone Identification. In Proceedings of the 5th International Conference on Materials Engineering for Advanced Technologies—ICMEAT, Quebec, QC, Canada, 5–6 August 2016. ISBN 978-1-60595-373-1. [Google Scholar]
- Sinkankas, J. Contribution to history of gemology: Specific gravity—Origins and development of the hydrostatic method. Gems Gemol. 1986, 157, 156–165. [Google Scholar] [CrossRef][Green Version]
- Read, P. Gemmology, 2nd ed.; Routledge: London, UK, 1999; Chapter 6. [Google Scholar]
- Koivula, J.I. Photomicrigraphy for gemologists. Gems Gemol. 2003, 39, 4–24. [Google Scholar] [CrossRef]
- Breeding, C.M.; Shen, A.H.; Eaton-Magana, S.; Rossman, G.R.; Shigley, J.E.; Gilbertson, A. Developments in Gemstone Analysis Techniques and Instrumentation during the 2000s. Gems Gemol. 2010, 46, 241–257. [Google Scholar] [CrossRef]
- Dietzek, B.; Cialla, D.; Schmitt, M.; Popp, J. Introduction to the Fundamentals of Raman Spectroscopy. In Confocal Raman Microscopy, 2nd ed.; Toporski, J., Dieing, T., Hollricher, O., Eds.; Springer: Berlin, Germany, 2018; pp. 47–67. [Google Scholar]
- Rinaudo, C.; Croce, A.; Musa, M.; Allegrina, M. La Spettroscopia Raman come mezzo di identificazione e caratterizzazione di materiali gemmologici. Riv. Gemmol. Ital. 2010, 5, 108–112. [Google Scholar]
- Downs, R.T.; Denton, M.B. Report on the Progress of the RRUFF Project: An Integrated Database of Raman Spectra, X-ray Diffraction, and Chemical Data for Minerals. Gems Gemol. 2006, 42, 89–90. [Google Scholar]
- Hollricher, O. Raman Instrumentation for Confocal Raman Microscopy. In Confocal Raman Microscopy, 2nd ed.; Toporski, J., Dieing, T., Hollricher, O., Eds.; Springer: Berlin, Germany, 2018; pp. 69–87. [Google Scholar]
- Frezzotti, M.L. Applicazione della Spettroscopia Raman agli studi di mineralogia e petrologia. Plinius 2001, 26, 62–73. [Google Scholar]
- Fritsch, E.; Stockton, C.M. Infrared spectroscopy in gem identification. Gems Gemol. 1987, 23, 18–26. [Google Scholar] [CrossRef]
- Musa, M.; Kounturaki, E. High-Tech Box: Fourier Transform Infrared Spectroscopy (FTIR). Gulf Gemol. Mag. 2020, 57. Available online: https://gulfgemology.com/gulf-gemology-mag-00-special-issue/ (accessed on 13 July 2022).
- Ochiai, S. Diffuse-Reflection Measurements. In Introduction to Experimental Infrared Spectroscopy; Tasumi, M., Sakamoto, A., Eds.; Wiley: Oxford, UK, 2015; pp. 169–178. [Google Scholar]
- Griffiths, P.R.; De Haseth, J.A. microscpetroscopy and imaging. In Fourier Infrared Spectrometry, 2nd ed.; Wiley: Oxford, UK, 2007; pp. 303–320. [Google Scholar]
- Zhou, D.; Lu, T.; Zhang, J. Study on the correlation between trace elements and colorimetric parameters of natural blue sapphire. Color 2022, 47, 691–696. [Google Scholar] [CrossRef]
- Karampelas, S.; Fritsch, E.; Gauthier, J.-P.; Hainschwang, T. UV-Vis-NIR reflectance spectroscopy of natural-color saltwater cultured pearls from Pinctada margaritifera. Gems Gemol. 2011, 47, 31–35. [Google Scholar] [CrossRef]
- Bernini, D.; Caucia, F.; Boiocchi, M. Application of the Vis-NIR Avaspec-2048 portable automatic spectrometer to distinguish GEM quality materials. N. Jb. Miner. Abh. 2009, 185, 281–288. [Google Scholar] [CrossRef]
- Muhlmeister, S.; Fritsch, E.; Shigley, J.E.; Devouard, B.; Laurs, B.M. Separating natural and synthetic Rubies on the basis of trace element Chemistry. Gems Gemol. 1998, 34, 80–101. [Google Scholar] [CrossRef]
- Brouwer, P. What is XRF. In Theory of XRF; PANalytical, B.V.: Almelo, The Netherlands, 2010; Chapter 2; pp. 8–9. [Google Scholar]
- Shackley, M.S. An Introduction to X-Ray Fluorescence (XRF) Analysis in Archaeology. In X-ray Fluorescence Spectrometry (XRF) in Geoarchaeology; Shackley, M.S., Ed.; Springer: New York, NY, USA, 2011; pp. 12–13. [Google Scholar]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Variable Pressure Scanning Electron Microscopy (VPSEM). In Scanning Electron Microscopy and X-ray Microanalyses, 4th ed.; Springer: New York, NY, USA, 2018; Chapter 12; pp. 173–186. [Google Scholar]
- Abduriyim, A.; Kitawaki, H. Application of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to gemology. Gems Gemol. 2006, 42, 98–118. [Google Scholar] [CrossRef]
- Sylvestor, P. Laser Ablation ICP-MS in the earth sciences: Current practices and outstanding issues. Min. Ass. Can. Short Course 2008, 40, 356. [Google Scholar]
- Harlow, E.; Murphy, A.R.; Hozan, D.; De Mille, C.N.; Levinson, A.A. Pre-Columbian Jadeite axes from Antigua, West Indies: Description and possible sources. Can. Mineral. 2006, 44, 305–321. [Google Scholar] [CrossRef]
- Laboratory Manual Harmonisation Committee—LMHC. Information Sheet 11: Jade and Related Minerals; Version 4; LMHC: Lucerne, Switzerland, 2011. [Google Scholar]
- Karampelas, S.; Kiefert, L.; Bersani, D.; Vandenabeele, P. Gems and Gemmology: An Introduction for Archaeologists, Art-Historians and Conservators, 1st ed.; Springer: Cham, Switzerland, 2020; pp. 18–83. [Google Scholar]
- Salviati, F. Jade, the radiance from within. In The Asian Art Legacy; Oljeda, A., Ed.; Casa Asia: Madrid, Spain, 2010; pp. 141–147. [Google Scholar]
- Krzemnicki, M.S. Jade, stone of “Gods”: Terms, concepts & certification. Facette 2012, 8–11. Available online: https://www.ssef.ch/ssef-facette/ (accessed on 13 July 2022).
- Middleton, A.; Freestone, I. The mineralogy and occurrence of Jade. In Chinese Jade from the Neolithic to the Qing; Rawson, J., Ed.; British Museum Press: London, UK, 1995; p. 413. [Google Scholar]
- Salviati, F. Alla ricerca della pietra verde. La giada nel mondo antico. Archeo 1998, 6, 59. [Google Scholar]
- Al Kindi, M.; Charpentier, V.; Maiorano, M.P.; Musa, M.; Pavan, A.; Heward, A.; Vosges, J.; Marchand, G.; Pickford, M. Neolithic long-distance exchanges in Southern Arabia: A supposed road for the ‘Jade’ axes. J. Archaeol. Sci. Rep. 2021, 39, 103116. [Google Scholar] [CrossRef]
- Harlow, G.E.; Tsujimori, T.; Sorensen, S.S. Jadeites and Plate Tectonics. Annu. Rev. Earth Planet. Sci. 2014, 43, 105–138. [Google Scholar] [CrossRef]
- Bakamska, A.; Abrashev, M.; Kostov, R.I. Omphacite-bearing axes from the Early Neolithic site Galabnik (Western Bulgaria): Mineral identification by Raman spectroscopy. Rev. Bulg. Geolog. Soc. 2018, 79, 51–57. [Google Scholar]
- Bertorino, G.; Franceschelli, M.; Marchi, M.; Luglié, C.; Columbu, S. Petrographic characterization of polished stone axes from Neolithic Sardinia, archaeological implications. Per. Miner. 2002, 71, 87–100. [Google Scholar]
- Pétrequin, P.; Errera, M.; Pétrequin, A.-M.; Allard, P. The neolithic quarries of Mont Viso, Piedmont, Italy: Initial radiocarbon dates. Eur. J. Archaeol. 2006, 9, 7–30. [Google Scholar] [CrossRef]
- Domínguez-Bella, S.; Cassen, S.; Pétrequin, P.; Přichystal, A.; Martínez, J.; Ramos, J.; Medina, N. Aroche (huelva, andalucía): A new neolithic axehead of alpine jade in the southwest of the iberian Peninsula. Archaeol. Anthropol. Sci. 2016, 8, 205–222. [Google Scholar] [CrossRef]
- Franz, L.; Sun, T.T.; Hanni, H.A.; De Capitani, C.; Thanasuthipitak, T.; Atichat, W. A Comparative Study of Jadeite, Omphacite and Kosmochlor Jades from Myanmar, and Suggestions for a Practical Nomenclature. J. Gemmol. 2014, 34, 210–229. [Google Scholar] [CrossRef]
- HKSM/FCT-2016; Standard Methods for Testing Fei Cui for Hong Kong. The Gemmological Association of Hong Kong (Limited): Hongkong, China, 2016.
- Gubelin, E. Maw-sit-sit: A new decorative gemstone from Burma. Gems Gemol. 1965, 11, 227–238. [Google Scholar]
- Gubelin, E. Maw-sit-sit proves to be jade-albite. Gems Gemol. 1965, 11, 302–308. [Google Scholar] [CrossRef]
- Manson, D.V. Recent activities in GIA’s research department. Gems Gemol. 1979, 16, 217–219. [Google Scholar]
- Hanni, H.A.; Meyer, J. Maw-sit-sit (kosmochlor-jade): A metamorphic rock with complex composition from Myanmar (Burma). In Proceedings of the 26th International Gemmological Conference, Idar-Oberstein, Germany, 27 September–3 October 1997; pp. 22–24. [Google Scholar]
- Colombo, R.; Rinaudo, C.; Trossarelli, C. The mineralogical composition of maw-sit-sit from Myanmar. J. Gemm. 2000, 27, 87–92. [Google Scholar] [CrossRef]
- Barrie, C. Electron backscatter diffraction investigation of length-fast chalcedony in agate: Implications for agate genesi. Geofluids 2012, 13, 32–44. [Google Scholar] [CrossRef]
- Chauviré, B.; Rondeau, B.; Mangold, N. Near infrared signature of opal and chalcedony as a proxy for their structure and formation conditions. Eur. J. Mineral. 2017, 29, 409–421. [Google Scholar] [CrossRef]
- Lule-Whip, C. Chromium Chalcedony from Turkey and Its Possible Archeological Connections. Gems Gemol. 2006, 42, 115. [Google Scholar]
- Shigley, J.E.; Laurs, B.M.; Renfro, N.D. Chrysoprase and prase Opal from Haneti, central Tanzania. Gems Gemol. 2009, 45, 271–279. [Google Scholar] [CrossRef]
- World Health Organization (WHO). 6.2 Asbestos. In Air Quality Guidelines for Europe, 2nd ed.; WHO Regional Publications, European Series, No. 91; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2000; Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/74732/E71922.pdf (accessed on 11 March 2014).
- Tremain, C.G. Pre-Columbian “Jade”: Towards an improved identification of green colored stone in Mesoamerica. Lithic Technol. 2014, 39, 137–150. [Google Scholar] [CrossRef]
- Gunther, B. Tables of Gemstones identification—Bestimmungstabellen fur Edelstein, Synthesen, Imitationen, 3rd ed.; Verlagsbuchhandlung Birgit Günther: Idar-Oberstein, Germany, 2008; p. 256. [Google Scholar]
- Anderson, B.W. Gem Testing, 10th ed.; Jobbins, E.A., Ed.; Butterworth Co., Ltd.: Oxford, UK, 1990; ISBN 0-408-02320-1. [Google Scholar]
- Liddicoat, R.T. Handbook of Gem Identification, 12th ed.; Gemmological Institute of America: Carlsbad, CA, USA, 1993; ISBN 0-87311-021-8. [Google Scholar]
- Webster, R. Gemmologists’ Compendium, 7th ed.; N.A.G. Press: London, UK, 1998. [Google Scholar]
- Shi, G.; Wang, X.; Chu, B.; Cui, W. Jadeite Jade from Myanmar: Its texture and gemmological implications. J. Gemmol. 2009, 31, 5–8. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; Longman Group UK Limited: London, UK, 1992. [Google Scholar]
- Groat, L.A.; Giuliani, G.; Stone-Sundberg, J.; Sun, Z.; Renfro, N.D.; Palke, A.C. A Review of analytical methods used in geographic origin determination of gemstones. Gems Gemol. 2019, 55, 512–535. [Google Scholar] [CrossRef]
- Prockor, S.A. The genesis of Nephrite and emplacement of the Nephrite bearing ultramafic complexes of east sayan. Int. Geol. Rev. 1991, 33, 290–300. [Google Scholar] [CrossRef]
- Heaney, P.J. A proposed mechanism for the growth of chalcedony. Contrib. Mineral. Petrol. 1993, 115, 66–74. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Larsen, R.A. Gemstone identification using Raman Spectroscopy. Spectrosc 2004, 19, 20–25. [Google Scholar]
- Kiefert, L.; Karampelas, S. Use of the Raman spectrometer in gemmological laboratories: Review. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 80, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Schubnel, H.J.; Pinet, M.; Smith, D.C.; Lasnier, B. (IV) Spectres Raman des principales gemmes. In La Microsonde Raman en Gemmologie; Ruskoné, E., Ed.; Association Francaise de Gemmologie: Paris, France, 1992; pp. 18–54. [Google Scholar]
- Rinaudo, C.; Belluso, E.; Gastaldi, D. Assessment of the use of Raman Spectroscopy for the determination of amphibole asbestos. Mineral. Mag. 2004, 68, 455–465. [Google Scholar] [CrossRef]
- Manrique-Ortega, M.D.; Mitrani, A.; Casanova-Gonzalez, E.; Perez-Ireta, G.; Garcìa-Bucio, M.A.; Rangel-Chàvez, I.; Aguilar-Melo, V.; De Lucio, O.G.; Ruvalcaba-Sil, J.L.; Sugiyama, N.; et al. Material study of green stone artifacts from a Teotihuacan complex. Mater. Manuf. 2020, 35, 1431–1445. [Google Scholar] [CrossRef]
- Hernandez-Murillo, C.; Garcìa-Pedra, S.; Alfaro-Còrdoba, M.; Fernandez-Esquivel, P.; Ménager, M.; Montero, M.L. Influence of surface roughness on the spectroscopic characterization of jadeite and greenstones archaeological artifacts: The axe-god pendants case study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120508. [Google Scholar] [CrossRef]
- Rinaudo, C.; Allegrina, M.; Fornero, E.; Musa, M.; Croce, A.; Bellis, D. Micro-Raman spectroscopy and VP-SEM/EDS applied to identification of mineral particles and fibres in histological sections. J. Raman Spectrosc. 2010, 41, 27–32. [Google Scholar] [CrossRef]
- Rinaudo, C.; Gastaldi, D.; Belluso, E. Characterization of Chrysotile, Antigorite and Lizardite by FT-Raman Spectroscopy. Can. Mineral. 2003, 41, 883–890. [Google Scholar] [CrossRef]
- Liu, S.I.; Ouyang, C.M.; Ng, F.Y. The application of VPSEM-Raman coupled system in studying Fei Cui. In Proceedings of the 34th International Gemmological Conference IGC, Vilnius, Lithuania, 26–30 August 2015. [Google Scholar]
- RRUFF Kosmochlor Reference N. R120015. Available online: https://rruff.info/kosmochlor/display=default (accessed on 13 June 2022).
- RRUFF Chromite Reference N. R110060. Available online: https://rruff.info/chromite/R110060 (accessed on 19 June 2022).
- RRUFF Eckermannite Reference N. R060320. Available online: https://rruff.info/eckermannite/display=default (accessed on 13 June 2022).
- Goryainov, S.V.; Smirnov, M.B. Raman spectra and lattice-dynamical calculations of natrolite. Eur. J. Mineral. 2001, 13, 507–519. [Google Scholar] [CrossRef]
- Perraki, M.; Karipi, S.; Rigopoulos, I.; Tsikouras, B.; Pomonis, P.; Hatzipanagiotou, K. Grossular/Hydrogrossular in rodingite from Othrys Ophiolite, (central Greece): Raman spectroscopy as a tool to distinguish it from Vesuvianite. In Proceedings of the XIX CBGA Congress, Thessaloniki, Greece, 23–26 September 2010; Scientific Annals, School of Geology, Aristotle University of Thessaloniki Special Volume. Volume 99, pp. 317–322. [Google Scholar]
- Butek, J.; Spisiak, J.; Milovska, S. Garnet-Vesuvianite equilibrium in Rodingite from Dobsina (Western Carpathians). Minerals 2021, 11, 189. [Google Scholar] [CrossRef]
- Huang, E.; Chen, C.H.; Huang, T.; Lin, E.H.; Xu, J.A. Raman spectroscopic characteristics of Mg-Fe-Ca pyroxenes. Am. Miner. 2000, 85, 473–479. [Google Scholar] [CrossRef]
- Katerinopoulou, A.; Musso, M.; Amthauer, G. A Raman spectroscopic study of the phase transition in omphacite. Vibr. Spectr. 2008, 48, 163–167. [Google Scholar] [CrossRef]
- RRUFF Pumpellyite Reference N. R120172. Available online: https://rruff.info/pumpellyite/display=default/R120172 (accessed on 13 June 2022).
- Schmidt, P.; Bellot-Gurlet, L.; Lea, V.; Sciau, P. Moganite detection in silica rocks using Raman and infrared spectroscopy. Eur. J. Mineral. 2013, 25, 797–805. [Google Scholar] [CrossRef]
- Gotze, J.; Nasdala, L.; Kleeberg, R.; Wenzel, M. Occurrence and distribution of “Moganite” in agate/chalcedony: A combined micro-Raman, Rietveld, and cathodoluminescence study. Contrib. Mineral. Petrol. 1998, 133, 96–105. [Google Scholar] [CrossRef]
- Rossetti, P.; Ferrero, S. The Zn-Pb deposits of Casario (Ligurian Alps, NW Italy): Late Palaeozoic sedimentary-exhalative bodies affected by the alpine metamorphism. Geodin. Acta 2008, 21, 117–137. [Google Scholar] [CrossRef][Green Version]
- Gendron, F.; Smith, D.C.; Masson, P.; Rodríguez Martínezd, M.C.; Ortiz Ceballose, P. Portable Raman verification and quantification of jade in Olmec ceremonial axes from El Manatí, Veracruz, Mexico. J. Raman Spectrosc. 2017, 48, 1618–1632. [Google Scholar] [CrossRef]
- Zhao, H.X.; Li, Q.H.; Liu, S.; Hub, Y.Q.; Gana, F.X. Nondestructive analysis of jade artifacts from the Cemetery of the Ying State in Henan Province, China using confocal Raman microspectroscopy and portable X-ray fluorescence spectroscopy. J. Raman Spectrosc. 2014, 45, 173–178. [Google Scholar] [CrossRef]
- Shurvella, H.F.; Rintoul, L.; Fredericks, P.M. Infrared and Raman spectra of jade and jade minerals. Int. J. Veh. Saf. 2001, 5. Available online: http://www.irdg.org/the-infrared-and-raman-discussion-group/ijvs/ijvs-volume-5-edition-5/infrared-and-raman-spectra-of-jade-and-jade-minerals/ (accessed on 13 July 2022).
- Coccato, A.; Karampelas, S.; Worle, M.; Van Willingen, S.; Petrequin, P. Gem Quality and Archeological Green “Jadeite Jade” vs “Omphacite Jade”: A Multi-Method Study. J. Raman Spectrosc. 2014, 45, 1260–1265. [Google Scholar] [CrossRef]
- RRUFF Jadeite Reference N. R050220. Available online: https://rruff.info/jadeite/display=default/R050220 (accessed on 13 June 2022).
- RRUFF Actinolite Reference N. R040063. Available online: https://rruff.info/actinolite/display=default/R040063 (accessed on 13 June 2022).
- RRUFF Tremolite Reference N. R050498. Available online: https://rruff.info/tremolite/display=default/R050498 (accessed on 19 June 2022).
- Fritsch, E.; Balan, E.; Petit, S.; Juillot, F. Structural, textural, and chemical controls on the OH stretching vibrations in serpentine-group minerals. Eur. J. Mineral. 2021, 33, 447–462. [Google Scholar] [CrossRef]
- RRUFF Antigorite Reference N. R070228. Available online: https://rruff.info/antigorite/display=default (accessed on 13 June 2022).
- Bosch-Reig, F.; Gimeno-Adelantado, J.V.; Bosch-Mossi, F.; Doménech-Carbó, A. Quantification of minerals from ATR-FTIR spectra with spectral interferences using the MRC method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 181, 7–12. [Google Scholar] [CrossRef]
- Ucbas, Y.; Bozkurt, V.; Bilir, K.; Ipek, H. Concentration of chromite by means of magnetic carrier using sodium oleate and other reagents. Physicochem. Probl. Miner. Process. 2014, 50, 767–782. [Google Scholar]
- Kobayashi, S.; Shoji, T. Infrared analysis of the grossular-hydrogrossular series. Mineral. J. 1983, 11, 331–343. [Google Scholar] [CrossRef]
- RRUFF Vesuvianite Reference N. R050056. Available online: https://rruff.info/vesuvianite/display=default/R050056 (accessed on 13 June 2022).
- RRUFF Diopside Reference N. R040009. Available online: https://rruff.info/diopside/display=default/R040009 (accessed on 13 June 2022).
- RRUFF Pumpellyite Reference N. R070130. Available online: https://rruff.info/tags=101/R070130 (accessed on 13 June 2022).
- RRUFF Quartz Reference N. R040031. Available online: https://rruff.info/quartz/display=default/R040031 (accessed on 13 June 2022).
- Quek, P.L.; Tan, T.L. Identification of B Jade by diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy. J. Gemmol. 1997, 25, 417–427. [Google Scholar] [CrossRef]
- Fritsch, E.; Wu, S.-T.T.; Moses, T.; McClure, S.F.; Moon, M. Identification of bleached and polymer-impregnated Jadeite. Gems Gemol. 1992, 28, 176–187. [Google Scholar] [CrossRef][Green Version]
- Bersani, D.; Lottici, P.P. Applications of Raman spectroscopy to gemology. Anal. Bioanal. Chem. 2010, 397, 2631–2646. [Google Scholar] [CrossRef]
- Hand, D. Dyed and Natural Green Jadeite. Gems Gemol. 2015, 51, 316–317. [Google Scholar]
- Scarani, A.; Åström, M. Gemological applications of UV-Vis-NIR spectroscopy. Riv. Ital. Gemmol. 2017, 7, 1–9. [Google Scholar]
- Tai-An Lai, L.; Tai-An, L. Dyed green marble imitating jadeite. Gems Gemol. 2014, 50, 310–311. [Google Scholar]
- Zhang, J.; Lu, T.; Chen, H. Gemological characteristics of coated Jadeite Jade. Gems Gemol. 2013, 49, 246–251. [Google Scholar] [CrossRef]
- Zhao, J.; Li, W.; Luo, H.; Miao, J. Research on protection of the architectural glazed ceramics in the Palace Museum, Beijing. J. Cult. Herit. 2010, 11, 279–287. [Google Scholar] [CrossRef]
- Re, G.; Croce, A.; D’Angelo, D.; Marchese, L.; Rinaudo, C.; Gatti, G. Application of nano-coating technology for the protection of natural lapideous materials. Surf. Coat. Technol. 2022, 441, 128507. [Google Scholar] [CrossRef]
- Tai-An Lai, L. Polymer-Coated Serpentine. Gems Gemol. 2016, 52, 165. [Google Scholar]
- O’Bannon, E.; Williams, Q. Coated lawsonite pseudomorphs presented as chromian lawsonite. Gems Gemol. 2014, 50, 307–309. [Google Scholar]
- Delgado Robles, A.A.; Ruvalcaba Sil, J.L.; Claes, P.; Manrique Ortega, M.D.; Casanova González, E.; Maynez Rojas, M.A.; Cuevas García, M.; García Castillo, S. Non-destructive in situ spectroscopic analysis of greenstone objects from royal burial offerings of the Mayan site of Palenque, Mexico. Herit. Sci. 2015, 3, 20. [Google Scholar] [CrossRef]
- Diella, V.; Bocchio, R.; Caucia, F.; Marinoni, N.; Langone, A.; Possenti, E. New Insights for Gem-Quality Mn-Bearing Diopside-Omphacite, Violane Variety, from Saint Marcel (Val D’Aosta, Italy): Its Trace Elements and Spectroscopic Characterization. Minerals 2021, 11, 171. [Google Scholar] [CrossRef]
- Zhang, B.S.; Wu, X.T.; Sun, Y.F.; Ritchey, M.; Fan, A.C.; Zhang, Y.Y.; Yu, G.; Song, Y.B. Complex raw materials and the supply system mineralogical and geochemical study of the jade artefact of the Longshan culture (2400–2000 BCE) from Sujiacun site in coastal Shandong, China. Archaeometry 2021, 63, 1–18. [Google Scholar] [CrossRef]
- Manrique-Ortega, M.D.; Mitrani, A.; Casanova-González, E.; Jiménez-Galindo, L.A.; Ruvalcaba-Sil, J.L. Methodology for the non–destructive characterization of jadeite-jade for archaeological studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 217, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Mereuta, P.; Costantinescu, B.; Cristea-Stan, D.; Serbanescu, D. SEM-EDS as investigation tool for archaeological artifacts—The case of nephrite adornments. Rom. Rep. Phys. 2019, 71, 802. [Google Scholar]
- Golitko, M. Introduction to Solid Sampling Strategies. In Recent Advances in Laser Ablation ICP-MS for Archaeology; Dussubieux, L., Golitko, M., Gratuze, B., Eds.; Springer: New York, NY, USA, 2016; pp. 23–90. [Google Scholar]
- Martin, C.; Ponzevera, E.; Harlow, G. In situ lithium and boron isotope determinations in mica, pyroxene, and serpentine by LA-MC-ICP-MS. Chem. Geol. 2015, 412, 107–116. [Google Scholar] [CrossRef]
- Barca, D.; De Francesco, A.M.; Crisci, G.M.; Tozzi, C. Provenance of obsidian artifacts from site of Colle Cera, Italy, by LA-ICP-MS method. Period. Mineral. 2008, 77, 41–52. [Google Scholar]
- Wang, H.A.O.; Krzemnicki, M.S.; Chalain, J.P.; Lefévre, P.; Zhou, W.; Cartier, L.E. Simoultaneous high sensitivity trace elements and isotopic analysis of gemstones using laser ablation inductively coupled plasma time-of-flight mass spectrometry. J. Gemmol. 2016, 35, 212–223. [Google Scholar] [CrossRef]


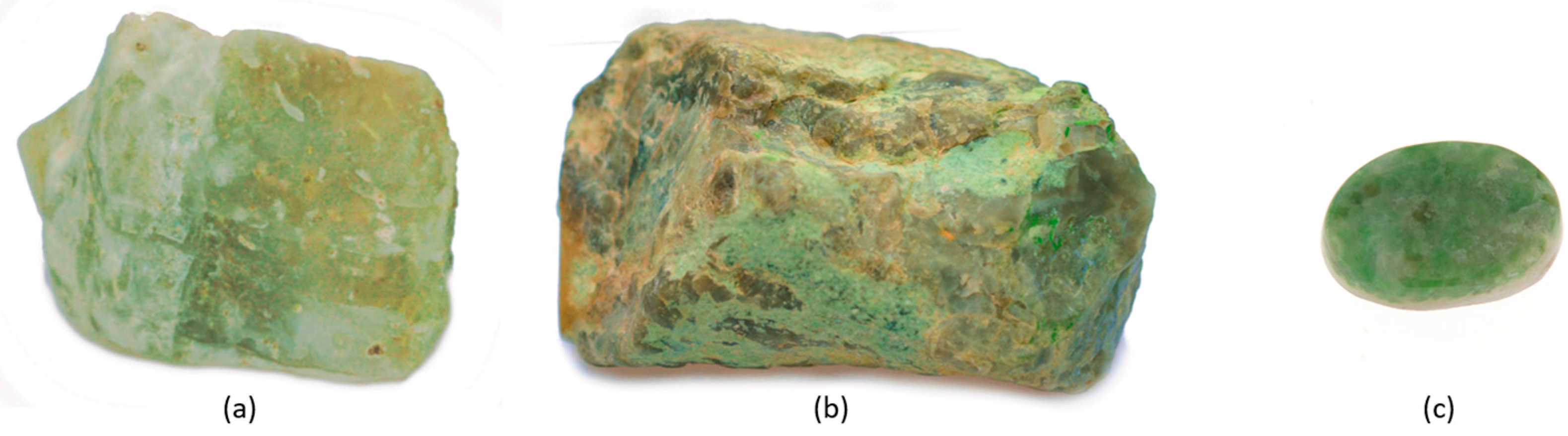



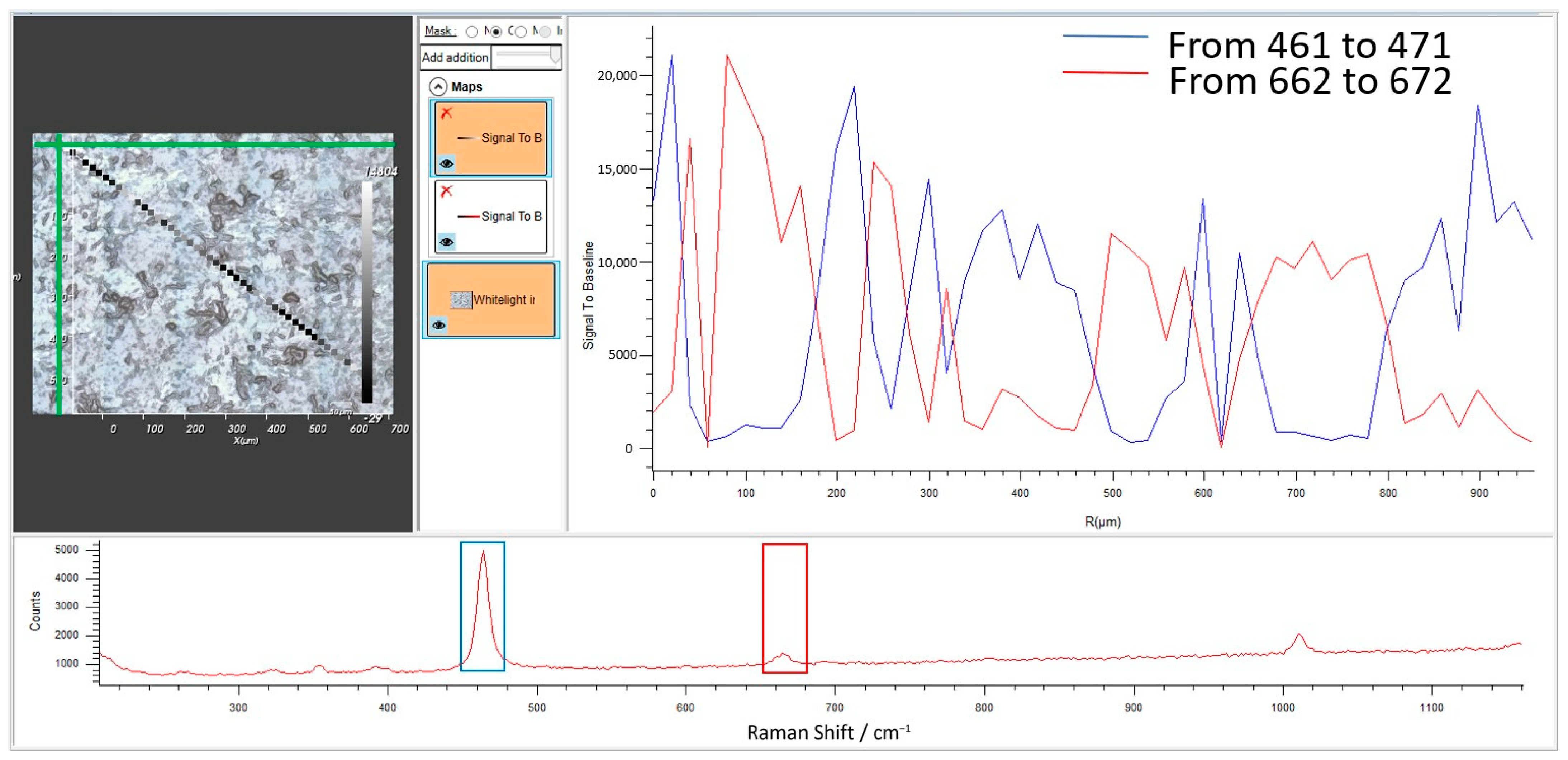

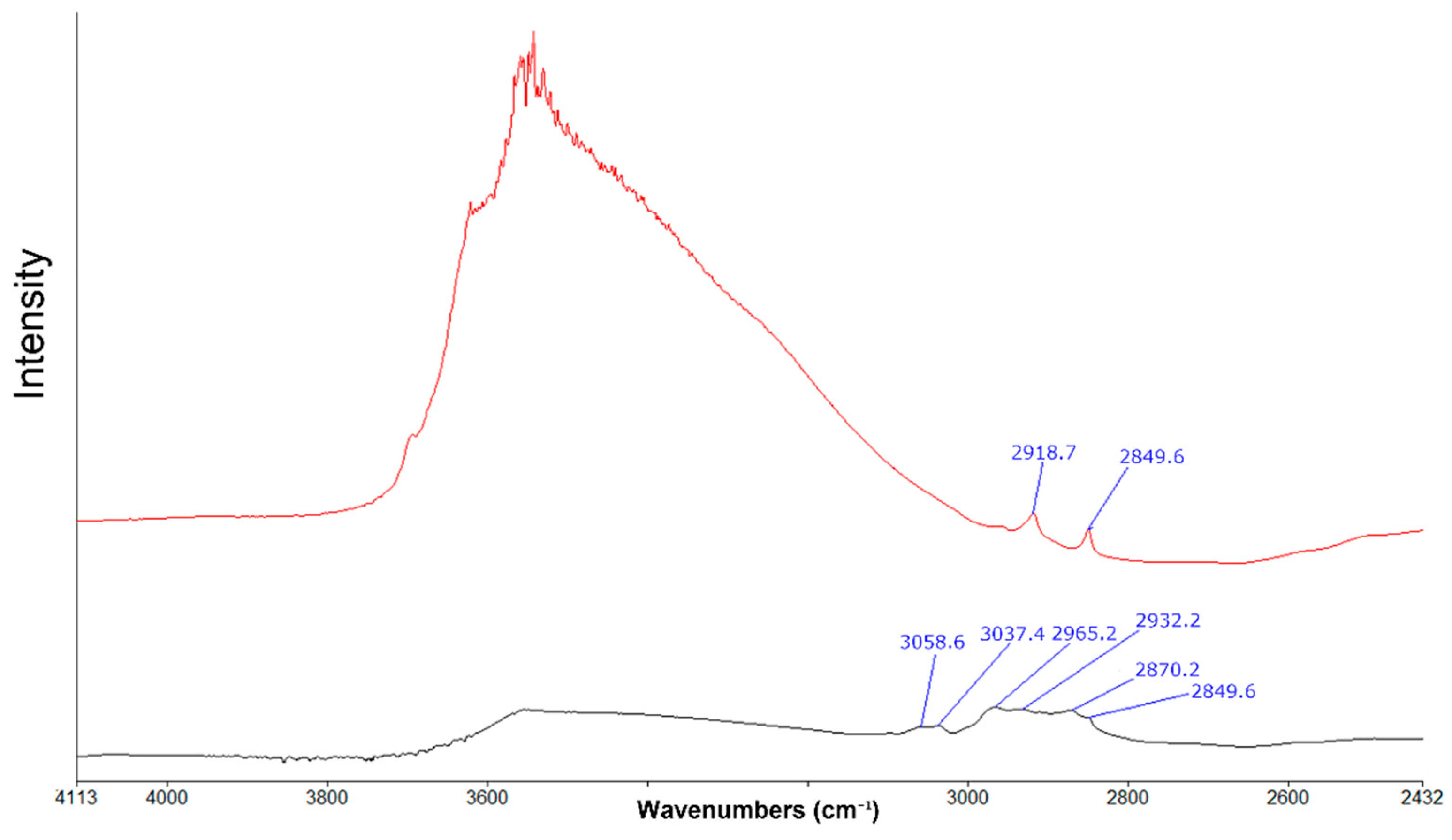
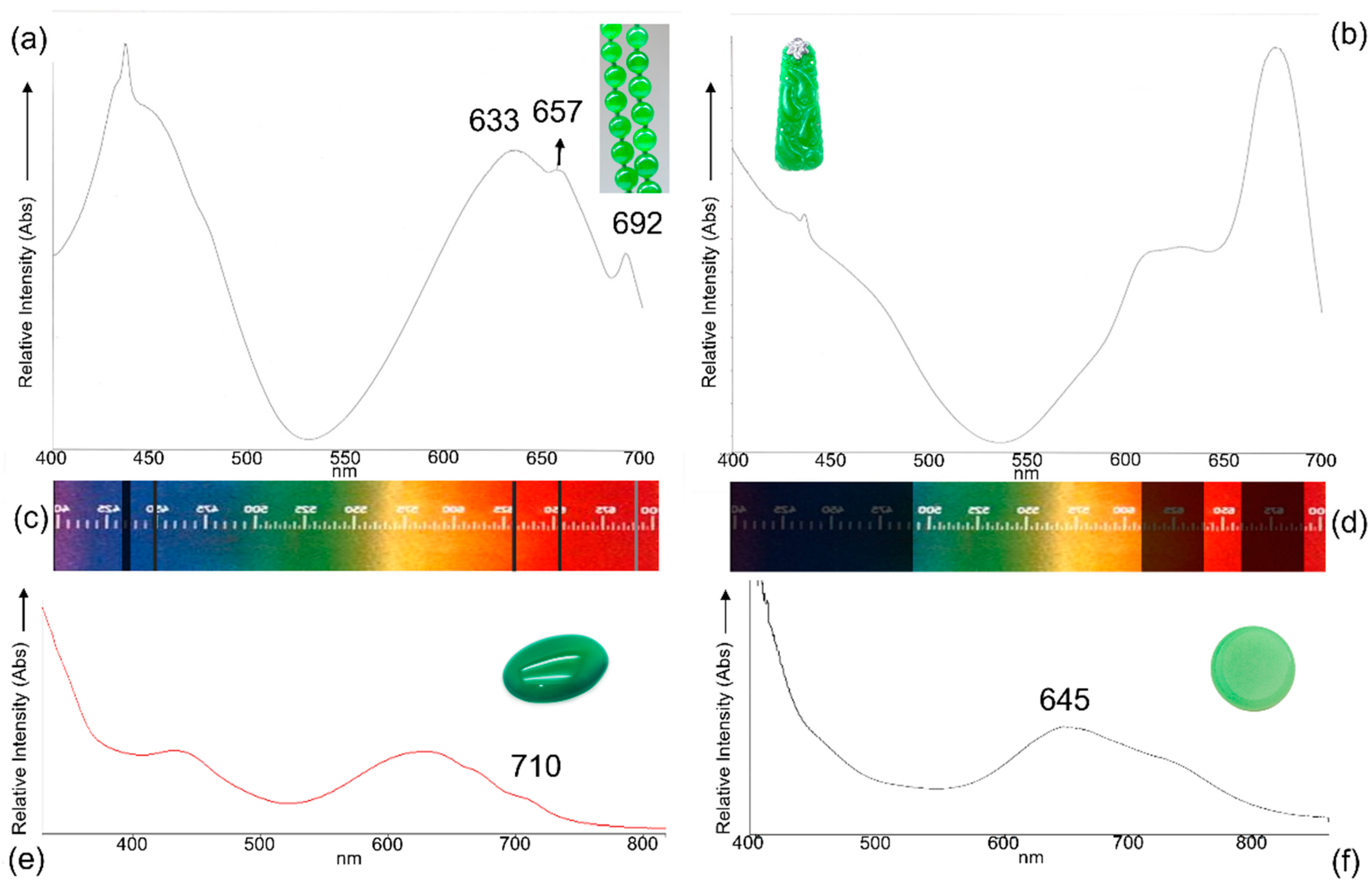

| Term | Definition |
|---|---|
| Jade | Trade name applicable to gem materials made only by jadeite or nephrite. |
| Jadeite | Gemstone Species–Mineralogical phase; piroxene mineral, columnar and/or granular crystals are aggregated. |
| Nephrite | Gemstone Species; a rock originated from a solid solution of the amphibole group minerals actinolite and tremolite, composed of an interlocking mass of granular and acicular crystals without a preferred orientation. |
| Fei Cui | Trade and historical name; traditionally translated as “hard jade”. |
| Maw-Sit-Sit | Gemstone Species; green rock constituted by a variable mineralogical association (i.e., albite, kosmochlor, chromite, eckermannite, natrolite, etc.) found in the Himalayan area. |
| Chrysoprase | Gemstone variety; “apple green” variety of chalcedony species—cryptocrystalline quartz. |
| Cr-chalcedony | Gemstone variety; green variety of chalcedony species—cryptocrystalline quartz. |
| Green Jasper | Gemstone variety; green variety of jasper species—microcrystalline quartz. |
| Green Quartzite | Rock (metamorphic), constituting mainly by quartz mineral associated with green phases (i.e., diopside, etc.). |
| Serpentine | Gemstone Species—Mineral polymorphic phase; three different polymorphs (minerals having the same chemical composition but different crystal structures) are identified: lizardite, antigorite, and chrysotile. |
| Californite | Gemstone variety; green massive variety of vesuvianite (idrocrase) species. |
| Diopside Omphacite Kosmochlor Pumpellyite | Gemstone Species–Mineralogical phases; piroxene minerals. |
| Hydrogrossular Grossular | Gemstone Species–Mineralogical phases; belonging to garnet group. |
| Species and Variety | General Description | RI | SG | Optical Character | Transparency | Fluorescence | Mohs Grade |
|---|---|---|---|---|---|---|---|
| Jadeite | Light to dark green, often with uneven coloration, mottling, or root-like markings. Vitreous to greasy polish luster with a dimpled polished surface, granular to splintery fracture with dull fracture luster. | 1.666 to 1.680 (+/−0.008) 1.66 spot reading common | 3.34 (+0.06/−0.09) | AGG | STP to O | LW: None to Faint White SW: None | 6 |
| Nephrite | Light to dark green, possibly with lighter or darker mottling. Vitreous to greasy polish luster, splintery to granular fracture with dull fracture luster. | 1.606 to 1.632 1.61 spot reading common | 2.95 (+0.15/−0.05) | AGG | TL to O | LW: None SW: None | 6–6½ |
| Serpentine | Usually yellowish green, green, or greenish yellow. Waxy to greasy polish luster, granular to uneven fracture, with dull to waxy fracture luster. | 1.560 to 1.570 (+0.004/−0.070) 1.56 spot reading common | 2.57 (+0.23/−0.13) | AGG | STL to O | LW: None to Med chalky (B, W, and G) SW: None | 2½–3½ |
| Maw-Sit-Sit | Opaque saturated green rock with characteristic dark green to black veining or mottling. Waxy to vitreous polish luster, granular fracture with dull fracture luster. | Spot readings around 1.53 to 1.74 (Multiple readings result from combination of different minerals) | 2.77 (+0.38/−0.31) | AGG | O | LW: None SW: None | 6 |
| Hydrogrossular (Garnet Group) | Green to bluish green. Might show black inclusions. Vitreous polish luster, uneven, granular, or splintery fracture with greasy to vitreous fracture luster. | 1.720 (+0.010/−0.050) 1.72 spot reading common | 3.47 (+0.08/−0.32) | AGG | TL to O | LW: None SW: None | 6–7½ |
| Vesuvianite (Idocrase), Californite variety | Yellowish green, green, brownish green. Vitreous to greasy polish luster, granular fracture with vitreous to dull fracture luster. | 1.713 to 1.718 (+0.003/−0.013) 1.70 or 1.71 spot reading common | 3.40 (+0.10/−0.15) | AGG | TL to O | LW: None SW: None | 6–7 |
| Diopside | Bluish green to yellowish green. Chrome diopside is intense green. Vitreous polish luster, conchoidal to uneven fracture with vitreous to resinous fracture luster. Massive and may show splintery to granular fracture. | 1.675 to 1.701 (+0.029/−0.010) | 3.29 (+0.11/−0.07) | DR (single crystal) AGG (massive) | TL to O (massive) | LW: None to Med Green SW: None | 5½–6½ |
| Omphacite | Green to dark green. Brittle tenancy with fracture uneven, conchoidal and luster vitreous, silky. | 1.662–1.723 1.68 or 1.71 spot reading common | 3.16 to 3.43 1 | AGG | O | LW: None to Med chalky (B, W, and G) SW: None | 5–6 |
| Pumpellyite Group | Blue–green, dark green to white fibrous to lamellar masses with glassy luster. | 1.674 to 1.764 | 3.20 to 3.30 1 | DR (single crystal) AGG (massive) | TL to O | LW: None SW: None | 5–6 |
| Chalcedony (chrome or chrysoprase variety) | Green to slightly bluish green. Greasy to vitreous polish luster, conchoidal fracture with dull to waxy fracture luster. | 1.535 to 1.539 (1.53 to 1.54 spot reading) | 2.60 (+0.10/−0.05) | AGG | STP to STL | LW: None SW: None | 6½–7 |
| Mineral Phase | Chemical Formula | Group/Family | Crystal System |
|---|---|---|---|
| Jadeite | NaAl[Si2O6] | Clinopyroxene | Monocline |
| Actinolite 1 | Ca2(Mg,Fe)5[Si2O6](OH)2 | Amphibole | Monocline |
| Tremolite 1 | Ca2Mg5[Si2O6](OH)2 | ||
| Lizardite 2 | Mg3[Si2O5](OH)4 | Trioctahedral Phyllosilicate | Trigonal |
| Antigorite 2 | Monocline | ||
| Chrysotile 2 | Orthorhombic | ||
| Albite 3 | Na[AlSi3O8] | Tectosilicate, Plagioclase Feldspar | Triclinic |
| Kosmochlor 3 | NaCr[Si2O6] | Pyroxene | Monocline |
| Chromite 3 | Fe2+Cr2O4 | Oxides, Spinels | Cubic |
| Eckermannite 3 | Na3Mg4Al[Si8O22](OH)2 | Amphibole | Monocline |
| Natrolite 3 | Na16[Al16Si24O80]·16H2O | Tectosilicate, Zeolite | Orthorhombic |
| Hydrogrossular | Ca3Al2(Si3O12)·(H2O)2-6 | Nesosilicate, Garnet | Cubic |
| Vesuvianite | (Ca,Na)19(Al,Mg,Fe)13(SiO4)10(Si2O7)4 (OH,F,O)10 | Sorosilicate | Tetragonal |
| Diopside | CaMgSi2O6 | Clinopyroxene | Monocline |
| Omphacite | (Ca,Na)(Mg,Fe2+,Fe3+)[AlSi3O8] | Clinopyroxene | Monocline |
| Pumpellyite | Ca2MgAl2(Si2O7)(SiO4)(OH)2·H2O | Sorosilicate | Monocline |
| Quartz 4 | SiO2 | Tectosilicate | Trigonal |
| Mineral Phase/Variety | Main Vibrations–Raman Shift (cm−1) | References | |||
|---|---|---|---|---|---|
| Jadeite | 1039, 990 | 700 | [96,120,121,122,123] | ||
| Nephrite | 1060, 1032 | 674 | [90,120,122] | ||
| (Actinolite) | 1062, 1027 | 669 | [90,124] | ||
| (Tremolite) | 1062, 1031 | 676 | [90,121] | ||
| Serpentine | 1044 | 683, 635 | 378 | [122] | |
| (Antigorite) | 1044 | 683, 635 | 375 | [122,125] | |
| (Lizardite) | 1096 b | 690 | 388 | [125] | |
| (Chrysotile) | 1105 | 692 | 389 | [125] | |
| Albite1 | 510, 482 | [120,122,123] | |||
| Kosmochlor 1 | 950 | 683 | (551−) 556, 522, 413 (−418) | 340 | [96,126,127] |
| Chromite 1 | 690 b | 560 b | [126,128] | ||
| Eckermannite 1 | 1025, 992 | 690 | [129] | ||
| Natrolite 1 | 535, 443 | [130] | |||
| Hydrogrossular | 871 | 361 | [131] | ||
| Vesuvianite | 930, 862 | 643 | [132] | ||
| Diopside | 1010 | 666 | [120,133] | ||
| Omphacite | 1024 | 685 | [92,122,123,134] | ||
| Pumpellyite | 990, 920 | 697 | [135] | ||
| Chalcedony | 503, 465 | [34,136,137] | |||
| Mineral Phase/Variety | Main Vibrations–Wavenumbers (cm−1) | References | |||
|---|---|---|---|---|---|
| Jadeite | 582 | 999 | 1090 | [122,123,141,142,143] | |
| Nephrite | 686, 756 | 950, 995 | 1064, 1102 | 3660, 3676 | [122,123,141,144] |
| (Actinolite) | 668, 756 | 918, 942, 992 | 1066 b | 3675 | [144] |
| (Tremolite) | 686, 758 | 920, 951, 990 | 1057 | 3673 | [145] |
| Serpentine | 471 | 990 | 1080 | 3671 | [122,146,147] |
| (Antigorite) | 436, 449 (−471), 617 | 962, (990–) 997 | 1084 | 3676 | [146,147] |
| (Lizardite) | 442, 621 | 976 | 1010, 1084 | 3687 | [146] |
| (Chrysotile) | 439, 609 | 949 | 1000, 1080 | 3649, 3685 | [146] |
| Albite 1 | 994 | 1040 | [123,148] | ||
| Kosmochlor 1 | 980 | 1114 | [96] | ||
| Chromite 1 | 520 b, 650 b | [149] | |||
| Hydrogrossular | 840, 910 | 3660 | [150] | ||
| Vesuvianite | 482 | 900, 980 | 1011 | 3570 | [151] |
| Diopside | 456 | 860, 960 | 1065 | [152] | |
| Omphacite | 448, 523 | 956 | 1060 | [122,126,142] | |
| Pumpellyite | 440 | 947 | 3406, 3556 | [153] | |
| Chalcedony | 450 | 780, 796 | 1067 | [154] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, M. The Importance of Multidisciplinary Analytical Strategies to Solve Identification and Characterization Challenges in Gemology: The Example of the “Green Stones”. Appl. Sci. 2022, 12, 7168. https://doi.org/10.3390/app12147168
Musa M. The Importance of Multidisciplinary Analytical Strategies to Solve Identification and Characterization Challenges in Gemology: The Example of the “Green Stones”. Applied Sciences. 2022; 12(14):7168. https://doi.org/10.3390/app12147168
Chicago/Turabian StyleMusa, Maya. 2022. "The Importance of Multidisciplinary Analytical Strategies to Solve Identification and Characterization Challenges in Gemology: The Example of the “Green Stones”" Applied Sciences 12, no. 14: 7168. https://doi.org/10.3390/app12147168
APA StyleMusa, M. (2022). The Importance of Multidisciplinary Analytical Strategies to Solve Identification and Characterization Challenges in Gemology: The Example of the “Green Stones”. Applied Sciences, 12(14), 7168. https://doi.org/10.3390/app12147168






